Sustainable Rabbit Skin Glue to Produce Bioactive Nanofibers for Nonactive Wound Dressings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
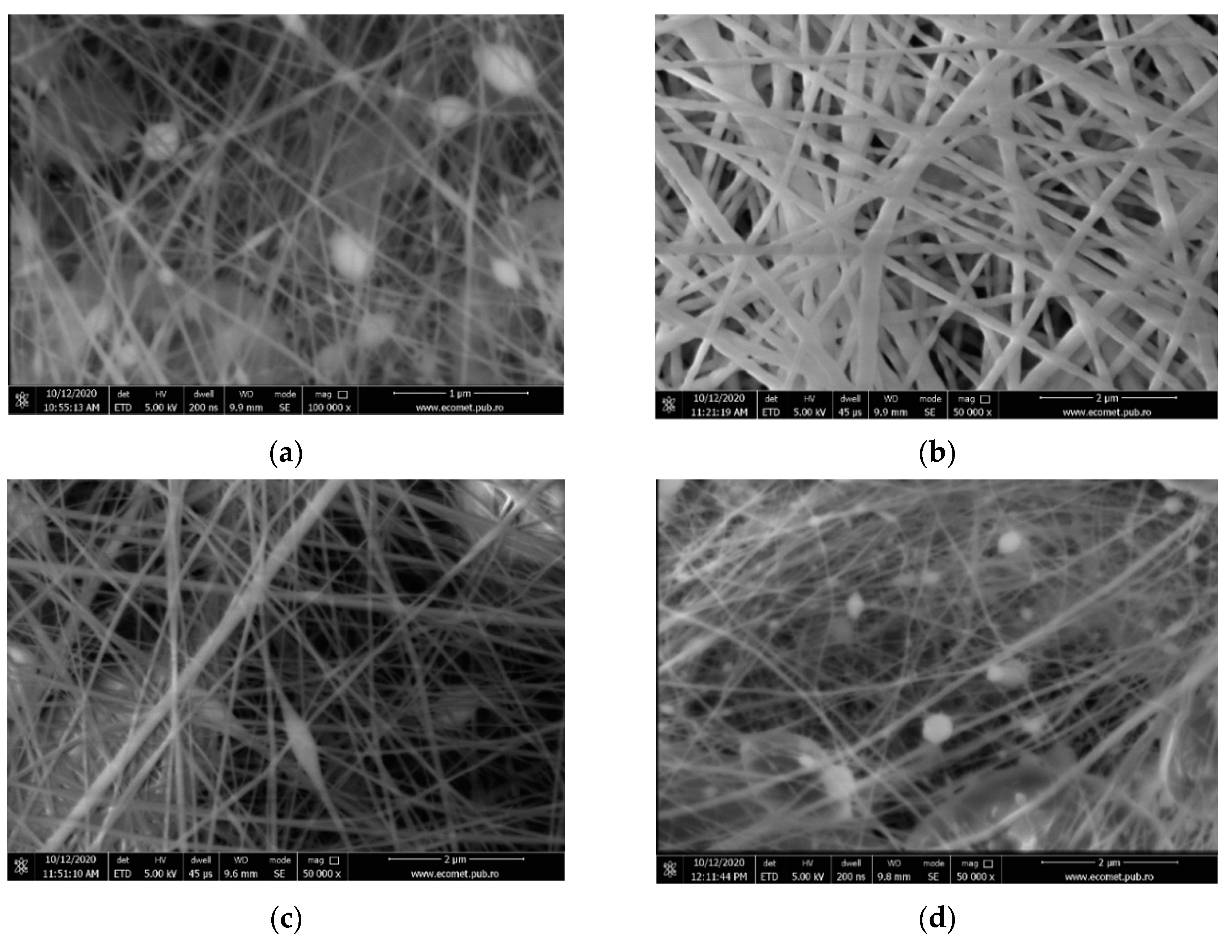
- (a)
- Water-based dispersant of ZnO NPs, with a concentration of 50 wt.% ZnO NPs, particle size <100 nm (by TEM measurement), ≤40 nm (aerodynamic particle sizer, APS), pH = 7.5 ± 1.5 supplied from Sigma Aldrich, Darmstadt, Germany;
- (b)
- Water-based dispersant of titanium dioxide nanoparticles (TiO2 NPs) in the form of anatase, doped with nitrogen and silver nanoparticles (TiPE Nanotechnology in life, Shanghai, China) (TiO2-N-Ag NPs), with a particle size of 6–8 nm, pH = 7–10, concentrations of 0.72% Ti and 0.86% Ag, with antibacterial, antifungal, and antiviral properties, without toxicity (oral LD50 ≥ 10,000 mg/kg).
- (c)
- Chitosan [(C6H11O4N)n] high viscosity is described by a viscosity of 1267 MPaxs and a sulfated ash content of 0.2% (Sigma-Aldrich, Darmstadt, Germany).
2.2. Preparing of Electrospinning Solution
2.3. Obtaining of the Collagen/Antimicrobial Agent Nanofibers
2.4. Investigation Methods
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Fourier-Transform Infrared Spectroscopy—Attenuated Total Reflectance (FTIR–ATR)
2.4.3. Antioxidant Activity
2.4.4. Biocompatibility Test
2.4.5. Assessment of Antimicrobial Activity
2.4.6. Statistical Analysis
3. Results
3.1. SEM Analysis
3.2. Structural Analysis by ATR-FTIR
3.3. ABTS Radical Scavenging Activity
3.4. Cell Viability Assay
3.5. Antimicrobial Activity
4. Discussion
5. Conclusions
6. Patent
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, M.; Ma, L.; Yu, W.; Zhang, X.; Shen, Y.; Zhang, Y. Research on rapid gelatinization of rabbit skin collagen as effect of acid treatment. Food Hydrocoll. 2018, 77, 945–951. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, L.; Yu, Y.; Zhou, H.; Guo, T.; Dai, H.; Zhang, Y. Physico-mechanical and antioxidant properties of gelatin film from rabbit skin incorporated with rosemary acid. Food Packag. 2019, 19, 121–130. [Google Scholar] [CrossRef]
- Elert, K.; García Sánchez, R.M.; Benavides-Reyes, C.; Linares Ordóñez, F. Influence of animal glue on mineralogy, strength and weathering resistance of lime plasters. Constr. Build. Mater. 2019, 226, 625–635. [Google Scholar] [CrossRef]
- Manzano, E.; Romero-Pastor, J.; Navas, N.; Rodríguez-Simón, L.R.; Cardell, C. A study of the interaction between rabbit glue binder and blue copper pigment under UV radiation: A spectroscopic and PCA approach. Vib. Spectrosc. 2010, 53, 260–268. [Google Scholar] [CrossRef]
- Ghezzi, L.; Duce, C.; Bernazzani, L.; Bramanti, E.; Colombini, M.P.; Tiné, M.R.; Bonaduce, I. Interactions between inorganic pigments and rabbit skin glue in reference paint reconstructions. J. Therm. Anal. Calorim. 2015, 122, 315–322. [Google Scholar] [CrossRef]
- Râpă, M.; Gaidău, C.; Stefan, L.M.; Matei, E.; Niculescu, M.; Berechet, M.D.; Stanca, M.; Tablet, C.; Tudorache, M.; Gavrilă, R.; et al. New Nanofibers Based on Protein By-Products with Bioactive Potential for Tissue Engineering. Materials 2020, 13, 3149. [Google Scholar] [CrossRef]
- Pellegrini, D.; Duce, C.; Bonaduce, I.; Biagi, S.; Colombini, M.P.; Tine, M.R.; Bramanti, E. Fourier transform infrared spectroscopic study of rabbit glue/inorganic pigments mixtures in fresh and aged reference paint reconstruction. Microchem. J. 2015, 134, 31–35. [Google Scholar] [CrossRef]
- Séon-Lutz, M.; Couffin, A.C.; Vignoud, S.; Schlatter, G.; Hébraud, A. Electrospinning in water and in situ crosslinking of hyaluronic acid/cyclodextrin nanofibers: Towards wound dressing with controlled drug release. Carbohydr. Polym. 2019, 207, 276–287. [Google Scholar] [CrossRef]
- Toriello, M.; Afsari, M.; Shon, H.K.; Tijing, L.D. Progress on the Fabrication and Application of Electrospun Nanofiber Composites. Membranes 2020, 10, 204. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial Biohybrid Nanofibers for Wound Dressings. Acta Biomater. 2020. [CrossRef]
- Khan, M.Q.; Kharaghani, D.; Shahzad, A.; Saito, Y.; Yamamoto, T.; Ogasawara, H.; Kim, I.S. Fabrication of antibacterial electrospun cellulose acetate/silver-sulfadiazine nanofibers composites for wound dressings applications. Polym. Test. 2019, 74, 39–44. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Yu, D.G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C 2020, 110805. [Google Scholar] [CrossRef] [PubMed]
- Najafiasl, M.; Osfouri, S.; Azin, R.; Zaeri, S. Alginate-based electrospun core/shell nanofibers containing dexpanthenol performed well in-vitro: A candidate for wound dressing. J. Drug Deliv. Sci. Technol. 2020, 101708. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Shan, Y.; Xiong, J.; Hu, Z.; Zhang, Y.; Gao, J. In vivo study of silk fibroin/gelatin electrospun nanofiber dressing loaded with astragaloside IV on the effect of promoting wound healing and relieving scar. J. Drug Deliv. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.F.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X.B. In vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test. 2019, 106298. [Google Scholar] [CrossRef]
- Mofidfar, M.; Kim, E.S.; Larkin, E.L.; Long, L.; Jennings, W.D.; Ahadian, S.; Ghannoum, M.A.; Wnek, G.E. Antimicrobial Activity of Silver Containing Crosslinked Poly(Acrylic Acid) Fibers. Micromachines 2019, 28, 829. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M.Q. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomed. 2014, 9, 2399–2407. [Google Scholar]
- McCann, M.T.; Gilmore, B.F.; Gorman, S.P. Staphylococcus epidermidis device related infections: Pathogenesis and clinical management. J. Pharm. Pharmacol. 2008, 60, 1551–1571. [Google Scholar] [CrossRef] [Green Version]
- LaRiviere, C.A.; Goldin, A.B.; Avansino, J. Silver Toxicity with the Use of Silver-Impregnated Dressing and Wound Vacuum-Assisted Closure in an Immunocompromised Patient. J. Am. Coll. Certif. Wound Spec. 2011, 3, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Devi Sekar, A.; Kumar, V.; Muthukumar, H.; Gopinath, P.; Matheswaran, M. Electrospinning of Fe-doped ZnO nanoparticles incorporated polyvinyl alcohol nanofibers for its Antibacterial treatment and cytotoxic studies. Eur. Polym. J. 2019, 118, 27–35. [Google Scholar] [CrossRef]
- Sun, L.; Han, J.; Liu, Z.; Wei, S.; Su, X.; Zhang, G. The facile fabrication of wound compatible anti-microbial nanoparticles encapsulated collagenous chitosan matrices for effective inhibition of poly-microbial infections and wound repairing in burn injury care: Exhaustive in vivo evaluations. J. Photochem. Photobiol. B Biol. 2019, 111539. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.W. Polyurethane nanofibers incorporated with ZnAg composite nanoparticles for antibacterial wound dressing applications. Compos. Commun. 2020, 19, 103–107. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Kim, I.S.; Ni, Q.Q. Cellulose acetate nanofibers embedded with AgNPs anchored TiO2 nanoparticles for long term excellent antibacterial applications. Carbohydr. Polym. 2019, 207, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Bonan, R.F.; Mota, M.F.; da Costa Farias, R.M.; Silva, S.D.; Bonan, P.R.F.; Diesel, L.; Menezesb, R.R.; da Cruz Perez, D.E. In vitro antimicrobial and anticancer properties of TiO2 blow-spun nanofibers containing silver nanoparticles. Mater. Sci. Eng. C 2019, 109876. [Google Scholar] [CrossRef]
- Jeckson, T.A.; Neo, Y.P.; Sisinthy, S.P.; Gorain, B. Delivery of therapeutics from layer-by-layer electrospun nanofiber matrix for wound healing: An update. J. Pharm. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zeng, R.; Hu, L.; Maffucci, K.G.; Ren, X.; Qu, Y. In vivo wound healing and in vitro antioxidant activities of Bletilla striata phenolic extracts. Biomed. Pharmacother. 2017, 93, 451–461. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. A simple quantitative procedure using monolayer cultures for cytotoxicity assays. J. Tissue Cult. Meth. 1984, 9, 7–9. [Google Scholar] [CrossRef]
- Andonegi, M.; Peñalba, M.; de la Caba, K.; Guerrero, P. ZnO nanoparticle-incorporated native collagen films with electroconductive properties. Mater. Sci. Eng. C 2020, 108, 110394. [Google Scholar] [CrossRef]
- Kang, L.; Jia, W.; Li, M.; Wang, Q.; Wang, C.; Liu, Y.; Wang, X.; Jina, L.; Jiang, J.; Gua, G.; et al. Hyaluronic acid oligosaccharide-modified collagen nanofibers as vascular tissue-engineered scaffold for promoting endothelial cell proliferation. Carbohydr. Polym. 2019, 223, 115106. [Google Scholar] [CrossRef]
- Soubhagya, A.S.; Moorthi, A.; Prabaharan, M. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef]
- Drobota, M.; Gradinaru, L.M.; Vlad, S.; Bargan, A.; Butnaru, M.; Angheloiu, M.; Aflori, M. Preparation and Characterization of Electrospun Collagen Based Composites for Biomedical Applications. Materials 2020, 13, 3961. [Google Scholar] [CrossRef] [PubMed]
- Sethmann, I.; Volkel, S.; Pfeifer, F.; Kleebe, H.J. Development of Phosphatized Calcium Carbonate Biominerals Calcium Carbonate Biominerals as Bioactive Bone Graft Substitute Materials, Part II: Functionalization with Antibacterial Silver Ions. J. Funct. Biomater. 2018, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamvar, R.; Pemmaraja, S.C.; Sharma, A.K.; Pruthi, V. Efficacy of ferulic acid encapsulated chitosan nanoparticles against Candida albicans biofilm. Microb. Pathog. 2016, 95, 21–31. [Google Scholar]
- Becker, U.; Fietzek, P.P.; Timpl, R.; Furthmays, H. Non-helical sequences of rabbit collagen. Eur. J. Biochem. 1975, 54, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, X.; Ma, L.; Li, H.; He, Z.; Zhang, Y. Preparation, characterisation and structure of rabbit (Hyla rabbit) skin gelatin. Int. J. Food Sci. Technol. 2016, 51, 574–580. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharm. 2019, 566, 307–328. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Carlson, B.; Gombart, A.; Reilly, D.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.S. Recent Advances in Nanomaterial-based Wound-Healing Therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, L.; He, Y.; Wang, Z.; Xu, J.; Ma, H. Hydrolysis kinetics and antioxidant activity of collagen under simulated gastrointestinal digestion. J. Funct. Foods 2014, 11, 493–499. [Google Scholar] [CrossRef]
- Medina-Medrano, J.R.; Quiñones-Muñoz, T.A.; Arce-Ortız, A.; Torruco-Uco, J.G.; Hernandez-Martınez, R.; Lizardi-Jimenez, M.A.; Varela-Santos, E. Antioxidant Activity of Collagen Extracts Obtained from the Skinand Gills of Oreochromissp. J. Med. Food 2019, 22, 1–7. [Google Scholar] [CrossRef]
- Tashakori, M.; Rakhshan, K.; Ramez, M.; Asgarian, S.; Janzadeh, A.; Azizi, Y.; Seifalian, A.; Ramezani, F. Conductive carbon nanofibers incorporated into collagen bio-scaffold assists myocardial injury repair. Int. J. Biol. Macromol. 2020, 163, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Manuja, A.; Raguvaran, R.; Kumar, B.; Kalia, A.; Tripathi, B.N. Accelerated healing of full thickness excised skin wound in rabbits using single application of alginate/acacia based nanocomposites of ZnO nanoparticles. Int. J. Biol. Macromol. 2020, 155, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Danilczuk, M.; Lund, A.; Saldo, J.; Yamada, H.; Michalik, J. Conduction electron spin resonance of small silver particles. Spectrochim. Acta A 2006, 63, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.L.; Caetano, B.L.; Chiari-Andréo, B.G.; Linhari Rodrigues Pietro, R.C.; Chiavacci, L.A. Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf. B 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, H.-I. Characterization and antibacterial properties of genipin-crosslinked chitosan/poly(ethylene glycol)/ZnO/Ag nanocomposites. Carbohydr. Polym. 2012, 89, 111–116. [Google Scholar] [CrossRef]
- Cruz-Romero, M.C.; Murphy, T.; Morris, M.; Cummins, E.; Kerry, J.P. Antimicrobial activity of chitosan, organic acids and nano-sized solubilisates for potential use in smart antimicrobially-active packaging for potential food applications. Food Control 2013, 34, 393–397. [Google Scholar] [CrossRef]
- Fernandez-Saiz, P.; Lagaron, J.M.; Hernandez-Muñoz, P.; Ocio, M.J. Characterization of antimicrobial properties on the growth of S. aureus of novel renewable blends of gliadins and chitosan of interest in food packaging and coating applications. Int. J. Food Microbiol. 2008, 124, 13–20. [Google Scholar] [CrossRef]
- Young, D.H.; Kohle, H.; Kauss, H. Effect of chitosan on membrane permeability of suspension cultured Glycine max and Phaseolus vulgaris cells. Plant Physiol. 1982, 70, 1449–1454. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, S.; Vaseeharan, B. Antibiofilm, anti cancer and ecotoxicity properties of collagen based ZnO nanoparticles. Adv. Powder Technol. 2018, 29, 2331–2345. [Google Scholar] [CrossRef]
- Khan, T.A.; Peh, K.K.; Ch’ng, H.S. Mechanical, Bioadhesive Strength and Biological Evaluations of Chitosan films for Wound Dressing. J. Pharm. Pharm. Sci. 2000, 3, 303–311. [Google Scholar]
- Nseir, N.; Regev, O.; Kaully, T.; Blumenthal, J.; Levenberg, S.; Zussman, E. Biodegradable scaffold fabricated of electrospun albumin fibers: Mechanical and biological characterization. Tissue Eng. Part C Methods 2013, 19, 257–264. [Google Scholar] [CrossRef] [PubMed]











| Property | Col | Col/ZnO NPs | Col/TiO2-N-Ag NPs | Col/CS |
|---|---|---|---|---|
| Viscosity (cP) | 147.3 ± 2.5 | 302 ± 10 | 257 ± 5 | 512 ± 2.10 |
| Torque (%) | 58.9 | 80 | 51.4 | 51.2 |
| Agitation rate (rpm) | 200 | 50 | 100 | 50 |
| Temperature (°C) | 20.5 | 21.5 | 21.6 | 21.5 |
| Shear stress (dyne/cm2) | 273.9 | 372.9 | 239 | 238.1 |
| Shear rate-SR (s−1) | 186 | 46.5 | 93.1 | 46.5 |
| Conductivity (μS/cm) | 272 | 353 | 271 | 245 |
| pH at 27 °C (pH units) | 3.05 | 3.83 | 2.91 | 2.81 |
| Parameters | Col | Col/ZnO NPs | Col/TiO2-N-Ag NPs | Col/CS |
|---|---|---|---|---|
| Flow rate (mL/h) | 0.7 | 0.4 | 0.5 | 0.6 |
| Voltage supply (kV) | 24.35 | 22.71 | 24.27 | 24.35 |
| Collector distance (mm) | 90 | 140 | 140 | 90 |
| Element | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) |
|---|---|---|---|---|---|---|---|---|
| Col | Col/ZnO NPs | Col/TiO2-N-Ag NPs | Col/CS | |||||
| Carbon | 46.32 | 51.93 | 29.97 | 44.70 | 43.45 | 49.05 | 46.81 | 52.31 |
| Nitrogen | 24.20 | 23.26 | 9.72 | 12.43 | 27.04 | 26.17 | 25.70 | 24.63 |
| Oxygen | 29.48 | 24.81 | 31.15 | 34.88 | 29.12 | 24.68 | 27.50 | 23.07 |
| Zinc | 29.16 | 7.99 | ||||||
| Silver | 0.08 | 0.01 | ||||||
| Titanium | 0.31 | 0.09 | ||||||
| β-Sheets (%) | Random Coils (%) | α-Helix (%) | Turns (%) | |
|---|---|---|---|---|
| Col | 26.96 | 32.08 | 27.98 | 12.96 |
| Col/ZnO NPs | 21.79 | 31.19 | 31.19 | 15.81 |
| Col/TiO2-N-Ag NPs | 62.63 | 19.28 | 16.88 | 1.19 |
| Col/CS | 56.29 | 2.3 | 27.16 | 14.22 |
| Microorganism/Electrospun Sample | Log10 Reduction | |||
|---|---|---|---|---|
| 2 Days | 7 Days | 14 Days | 28 Days | |
| Collagen nanofibers | ||||
| Escherichia coli | 0.74 | 0.60 | – | No increase |
| Staphylococcus aureus | 3.39 | 0.79 | – | No increase |
| Candida albicans | 4.44 | 0.49 | 0.44 | No increase |
| Collagen/ZnO NPs nanofibers | ||||
| Escherichia coli | 3.17 | 3.17 | – | No increase |
| Staphylococcus aureus | 3.17 | 3.17 | – | No increase |
| Candida albicans | 2.40 | 2.80 | 1.65 | No increase |
| Collagen/TiO2-N-Ag NPs nanofibers | ||||
| Escherichia coli | 3.17 | 3.17 | – | No increase |
| Staphylococcus aureus | 3.39 | 3.39 | – | No increase |
| Candida albicans | 4.44 | 3.45 | 2.10 | No increase |
| Collagen/CS nanofibers | ||||
| Escherichia coli | 3.17 | 1.5 | – | No increase |
| Staphylococcus aureus | 3.39 | 3.10 | – | No increase |
| Candida albicans | 4.44 | 2.94 | 2.15 | No increase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, E.; Gaidau, C.; Râpă, M.; Constantinescu, R.; Savin, S.; Berechet, M.D.; Predescu, A.M.; Berbecaru, A.C.; Coman, G.; Predescu, C. Sustainable Rabbit Skin Glue to Produce Bioactive Nanofibers for Nonactive Wound Dressings. Materials 2020, 13, 5388. https://doi.org/10.3390/ma13235388
Matei E, Gaidau C, Râpă M, Constantinescu R, Savin S, Berechet MD, Predescu AM, Berbecaru AC, Coman G, Predescu C. Sustainable Rabbit Skin Glue to Produce Bioactive Nanofibers for Nonactive Wound Dressings. Materials. 2020; 13(23):5388. https://doi.org/10.3390/ma13235388
Chicago/Turabian StyleMatei, Ecaterina, Carmen Gaidau, Maria Râpă, Roxana Constantinescu, Simona Savin, Mariana Daniela Berechet, Andra Mihaela Predescu, Andrei Constantin Berbecaru, George Coman, and Cristian Predescu. 2020. "Sustainable Rabbit Skin Glue to Produce Bioactive Nanofibers for Nonactive Wound Dressings" Materials 13, no. 23: 5388. https://doi.org/10.3390/ma13235388
APA StyleMatei, E., Gaidau, C., Râpă, M., Constantinescu, R., Savin, S., Berechet, M. D., Predescu, A. M., Berbecaru, A. C., Coman, G., & Predescu, C. (2020). Sustainable Rabbit Skin Glue to Produce Bioactive Nanofibers for Nonactive Wound Dressings. Materials, 13(23), 5388. https://doi.org/10.3390/ma13235388







