Barium-Strontium Titanate/Porous Glass Structures for Microwave Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis and Characterization
2.2. Electrical Characterization
3. Results and Discussion
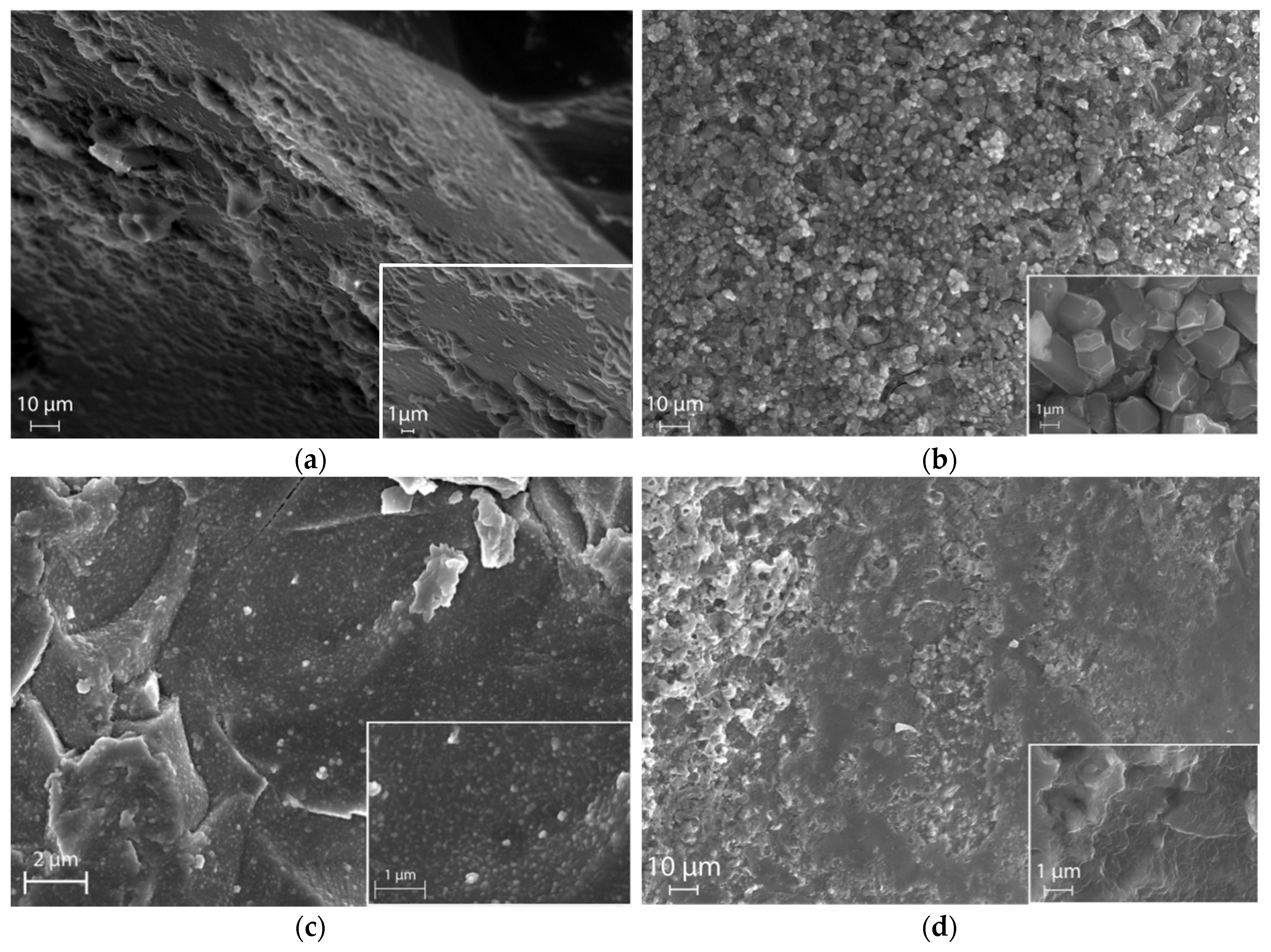
3.1. Structural and Morphologic Characterization of Porous Glasses
3.2. Characterization of BST in Porous Space of Glass
3.3. Electrical Characterization of BST-Glass Structures
3.4. Porous Matrix Filling Estimation
4. Conclusions
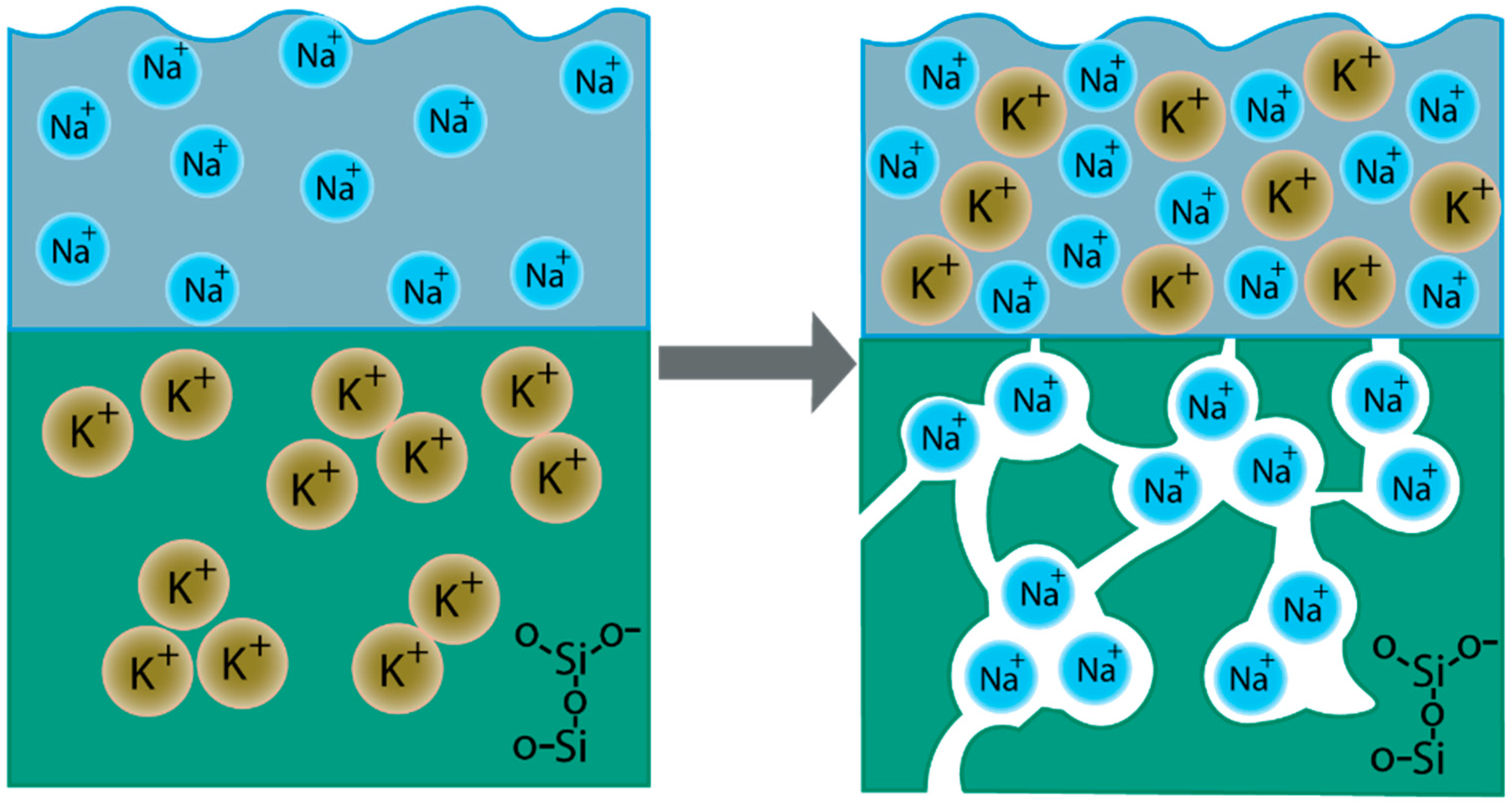
- The method of obtaining porous glasses due to ion exchange between KFS glass and LiNO3 and NaNO3 melts allowed us to control a wide range of pore sizes and made it possible to form glass porous structures with pores of the required size. The ion exchange treatment between the cations of potassium-containing glass and NaNO3 and LiNO3 melts leads to the formation of porous structures with significantly different pore size distributions, which affects the efficiency of the barium-strontium titanate insertion in a porous space and, consequently, the electrical characteristics of the structures.
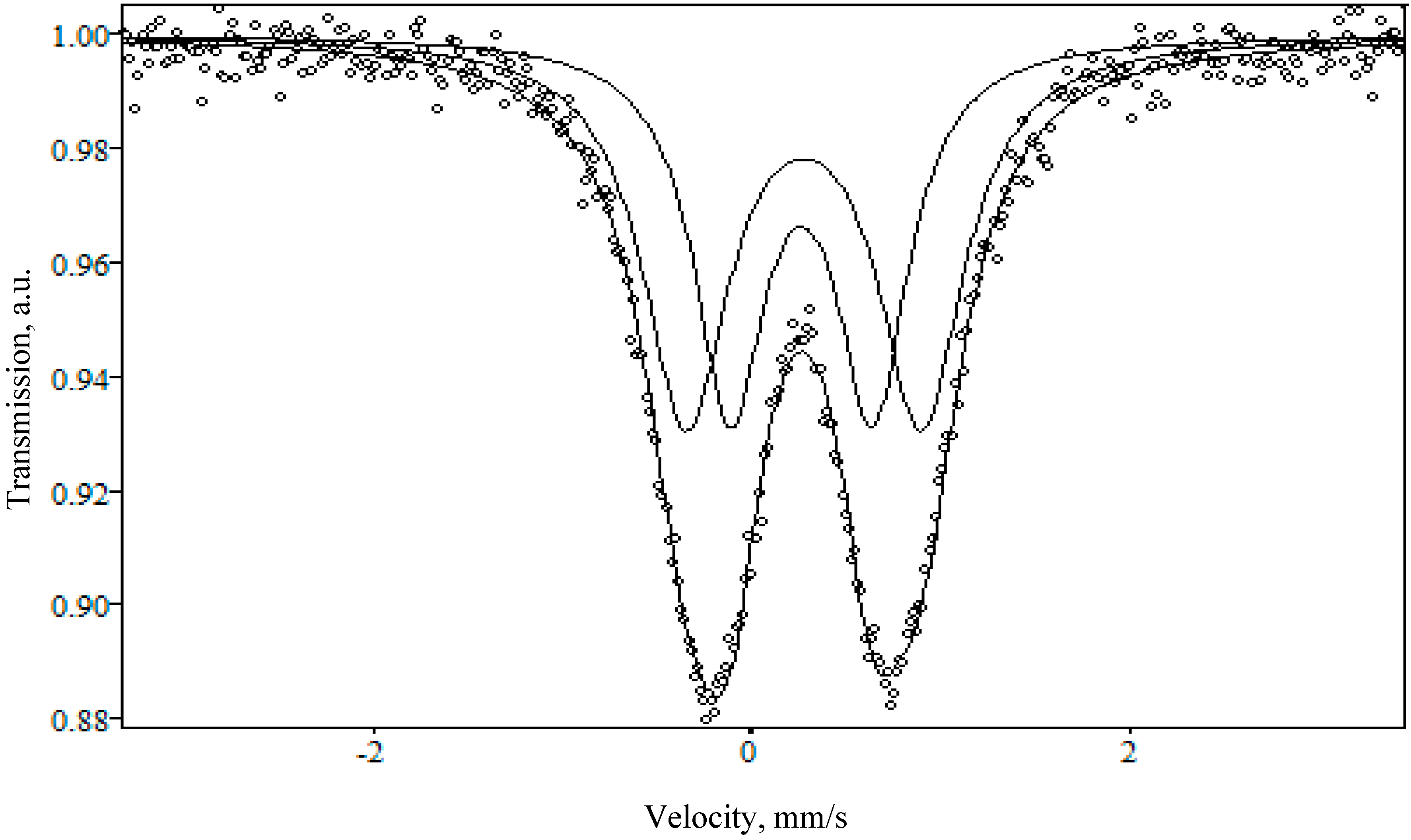
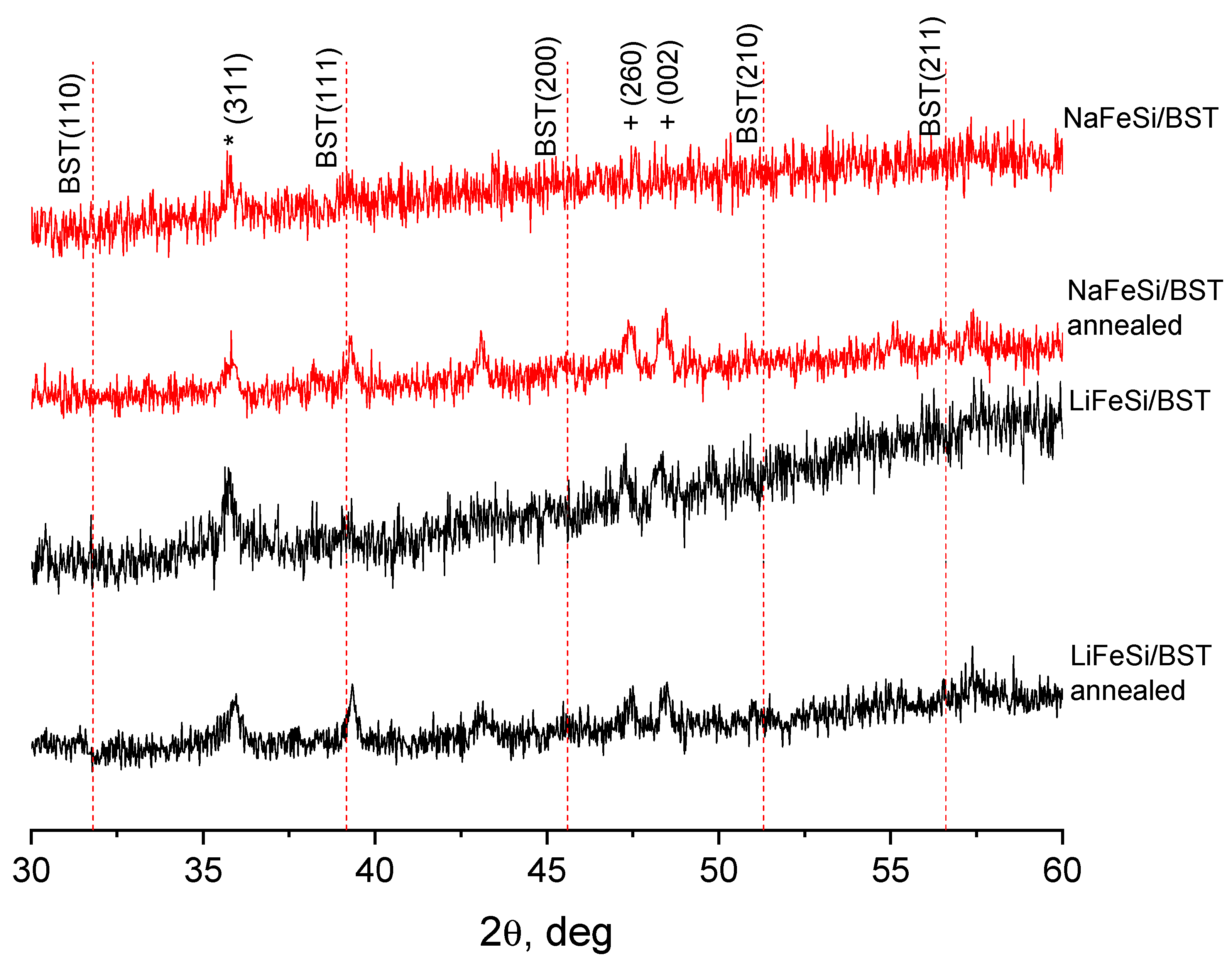
- XRD data indicate the presence of crystalline iron oxide, both in the initial glass and in the porous glass after ion exchange. The ion exchange procedure leads to a change in the phase composition and crystal structure of iron oxide. The ion exchange in the NaNO3 melt leads to a decrease in the content of the crystalline phase of iron oxide in the NaFeSi glass. On the contrary, the presence of rather intense reflexes of iron oxide in LiFeSi porous glass indicates a redistribution of the crystalline phases of iron oxide as a result of ion exchange. The charge state of iron in porous glass, and, consequently, the type of iron oxide—γ-Fe2O3 was determinated by Mössbauer spectroscopy.
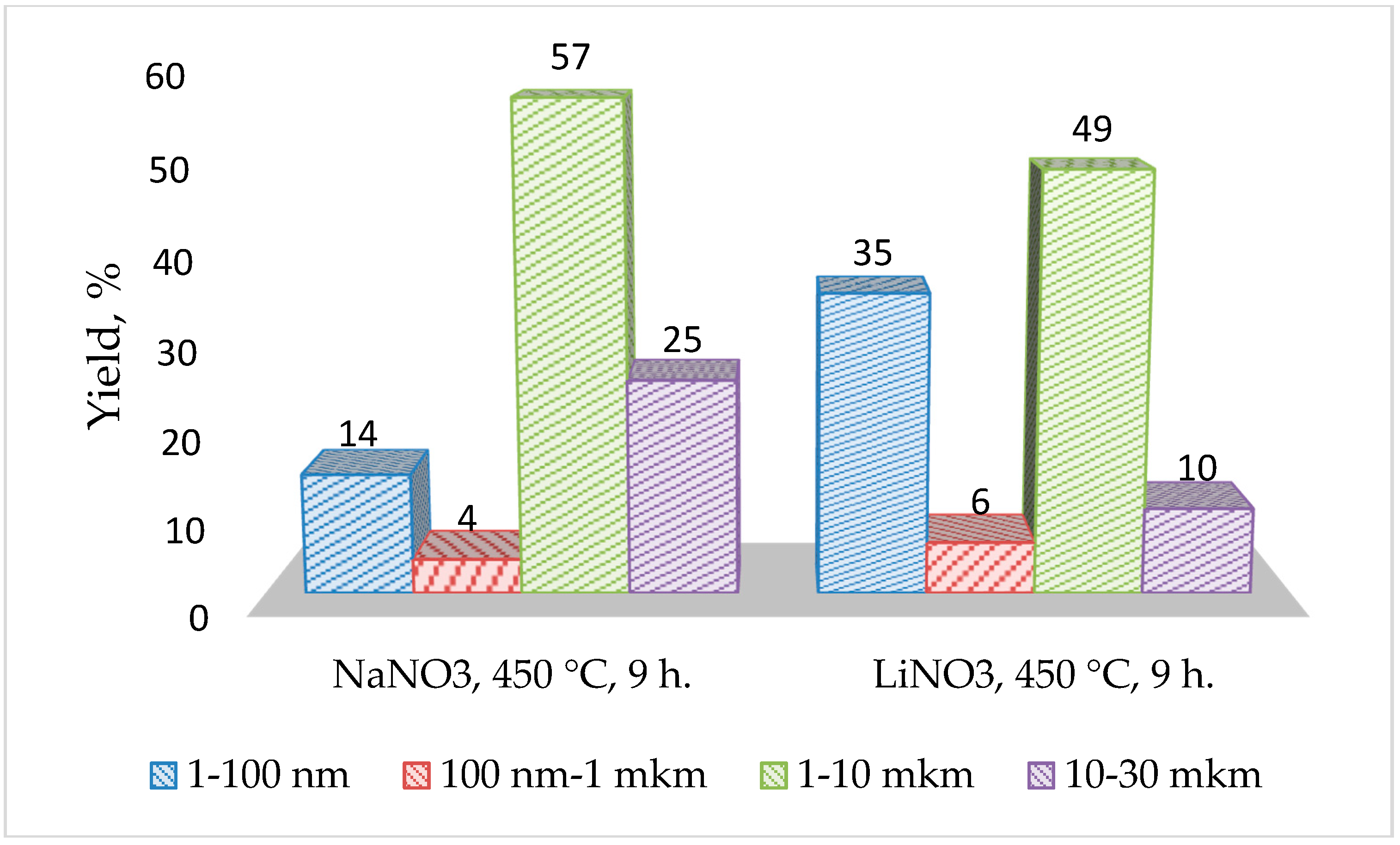
- Based on the porometric data and the results of electron microscopy, it can be assumed that the glass subjected to ion exchange in the LiNO3 melt shows a more developed porous structure, which has a positive effect on the efficiency of introducing barium-strontium titanate into it. The impregnation of porous glasses with BST sol leads to uniform pore filling and the formation of a rather uniform surface without significant cavities.
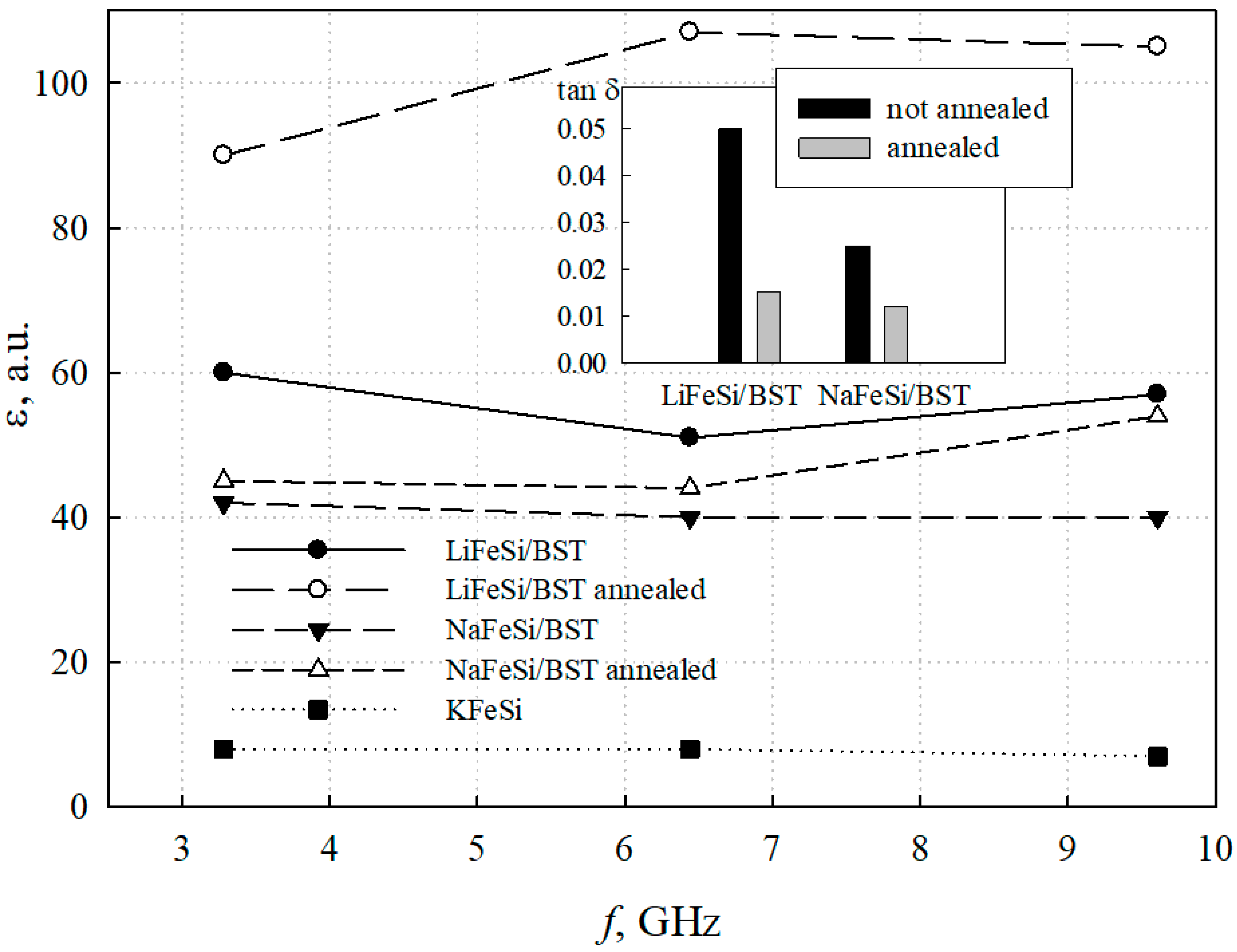
- Based on porous silicate glasses obtained by ion exchange, glass-ceramic materials containing a solid solution of barium-strontium titanate with a dielectric constant of more than 100 at microwaves were synthesized for the first time. The resulting glass-ceramic structures were characterized by dielectric permittivity and losses, depending on the method of obtaining porous glass and high-temperature processing in an oxygen environment. An increase in the intensity of BST peak (111), an increase in permittivity, and a decrease in losses of glass-ceramic samples after annealing in oxygen confirm the presence of crystalline BST in the pore space of NaFeSi and LiFeSi glasses.
- Annealing of glass-ceramic structures in an oxygen environment has a positive effect on their electrical characteristics. NaFeSi/BST glass-ceramic structures demonstrate a slight increase in the permittivity and a decrease in losses from 0.025 until 0.01 after the high-temperature treatment in oxygen. LiFeSi/BST structures look more preferable: the permittivity increases from 60 to 100, while the losses reduce by more than three times, from 0.05 to 0.02 as a result of annealing.
Author Contributions
Funding
Conflicts of Interest
References
- Borderon, C.; Ginestar, S.; Gundel, H.W.; Haskou, A.; Nadaud, K.; Renoud, R.; Sharaiha, A. Design and Development of a tunable ferroelectric microwave surface mounted device. IEEE Trans. Ultrason. Ferroelectr. Freq. Contr. 2020, 67, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Ranaa, L.; Sharmad, A.; Freundorfer, A.P.; Sayer, M.; Tomar, M.; Gupta, V. High frequency coplanar microwave resonator using ferroelectric thin film for wireless communication applications. Mater. Today Proc. 2018, 5, 15395–15398. [Google Scholar] [CrossRef]
- Romanofsky, R.R.; Toonen, R.C. Past, present and future of ferroelectric and multiferroic thin films for array antennas. Multidimens. Syst. Signal Process. 2018, 29, 475–487. [Google Scholar] [CrossRef]
- Nguyen, Q.M.; Anthony, T.K.; Zaghloul, A.I. Free-Space-Impedance-Matched composite dielectric metamaterial with high refractive index. IEEE Antennas Wirel. Propag. Lett. 2019, 18, 2751–2755. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Oh, J.A.S.; Wu, T. Electrical impedance matching based on piezoelectric ceramics for energy harvesting application. Mater. Technol. 2020, 35, 650–655. [Google Scholar] [CrossRef]
- Gowdhaman, P.; Annamalai, V.; Thakur, O.P. Piezo, ferro and dielectric properties of ceramic-polymer composites of 0-3 connectivity. Ferroelectrics 2016, 493, 120–129. [Google Scholar] [CrossRef]
- Zhou, W.; Fan, J.; Xin, Z.; You, G. Micro-Structure modelling and electrical properties analysis of PZT matrix ferroelectric composites. Materials 2020, 13, 448. [Google Scholar] [CrossRef]
- He, Y.; Peng, Y.; Xu, Y. The anomalous increase in tunability in Ba0.55Sr0.45TiO3-MgO-Mg2SiO4 composite ceramics. J. Am. Ceram. 2019, 102, 2706–2717. [Google Scholar] [CrossRef]
- Nenasheva, E.A.; Kartenko, N.F.; Gaidamaka, I.M.; Redozubov, S.S.; Kozyrev, A.B.; Kanareykin, A.D. Low permittivity ferroelectric composite ceramics for tunable applications. Ferroelectrics 2017, 506, 174–183. [Google Scholar] [CrossRef]
- Koroleva, E.Y.; Burdin, D.Y.; Kumzerov, Y.A.; Sysoeva, A.A.; Filimonov, A.V.; Vakhrushev, S.B. Dielectric properties of magnetic-ferroelectric CoO–NaNO2–porous glass nanocomposite. Phys. Solid State 2017, 59, 2036–2044. [Google Scholar] [CrossRef]
- Rysiakiewicz-Pasek, E.; Antropova, T.; Polyakova, I.; Pshenko, O.; Ciżman, A. New Insight into phase transitions of porous glass-based ferroelectric nanocomposites. Materials 2020, 13, 3698. [Google Scholar] [CrossRef] [PubMed]
- Belysheva, D.N.; Sinel’shchikova, O.Y.; Tyurnina, N.G.; Tyurnina, Z.G.; Sviridov, S.I.; Tumarkin, A.V.; Zlygostov, M.V.; Ugolkov, V.L. Glycine–Nitrate synthesis of solid solutions of barium–strontium metatitanate. Phys. Solid State 2019, 61, 2371–2375. [Google Scholar] [CrossRef]
- Tumarkin, A.V.; Al’myashev, V.I.; Razumov, S.V.; Gaidukov, M.M.; Gagarin, A.G.; Altynnikov, A.G.; Kozyrev, A.B. Structural properties of barium strontium titanate films grown under different technological conditions. Phys. Solid State 2015, 57, 553–557. [Google Scholar] [CrossRef]
- Wu, J.-M.; Huang, H.-L. Microwave properties of zinc, barium and lead borosilicate glasses. J. Non-Cryst. Solids 1999, 260, 116–124. [Google Scholar] [CrossRef]
- Letz, M. Microwave Materials and Applications; Sebastian, M.T., Jantunen, H., Ubic, R., Eds.; Wiley: New York, NY, USA, 2017; Volume 1, p. 345. [Google Scholar]
- Kreisberg, V.A.; Antropova, T.V.; Kalinina, S.V. Effect of the composition and conditions of the synthesis of porous glass on their micro- and mesoporous structures. Glass Phys Chem. 2014, 40, 501–512. [Google Scholar] [CrossRef]
- Jones, J.R.; Ahir, S.; Hench, L.L. Large-Scale production of 3D bioactive glass macroporous scaffolds for tissue engineering. J. Sol-Gel Sci. Technol. 2004, 29, 179–188. [Google Scholar] [CrossRef]
- Yatskovskaya, O.V.; Baklanova, O.N.; Gulyaeva, T.I.; Drozdov, V.A.; Gorbunov, V.A. The effect of polyethylene glycol molecular weight on characteristics of the porous structure of silica materials. Prot. Met. Phys. Chem. Surf. 2013, 49, 216–221. [Google Scholar] [CrossRef]
- Tallia, F.; Gallo, M.; Pontiroli, L.; Baino, F.; Fiorilli, S.; Onida, B.; Anselmetti, G.C.; Manca, A.; Vitale-Brovarone, C. Zirconia-containing radiopaque mesoporous bioactive glasses. Mater. Lett. 2014, 130, 281–284. [Google Scholar] [CrossRef]
- Sviridov, S.I.; Tyrnina, Z.G.; Tyrnina, N.G.; Kryuchkova, L.Y.; Vlasenko, N.S. Ion-exchange formation of alkali silicate glass with a porous structure. Glass Phys. Chem. 2017, 43, 28–33. [Google Scholar] [CrossRef]
- Sviridov, S.I.; Eliseeva, N.P. Interaction of glasses with nitrate melts in the systems containing Na+, K+, and Ba2+. Glass Phys. Chem. 1999, 25, 163–171. [Google Scholar]
- Costa, F.; Borgese, M.; Degiorgi, M.; Monorchio, A. Electromagnetic characterization of materials by using Transmission/Reflection (T/R) devices. Electronics 2017, 6, 95. [Google Scholar] [CrossRef]
- Tyurnina, Z.G.; Tyurnina, N.G.; Sviridov, S.I.; Vlasenko, N.S. Ion-Exchange Formation of Magnetic Iron-Containing Glass with a Porous Structure. Glass Phys. Chem. 2020, 46, 305–311. [Google Scholar] [CrossRef]
- Siegrist, T. Crystallographica—A software toolkit for crystallography. J. Appl. Crystallogr. 1997, 30, 418–419. [Google Scholar] [CrossRef]
- Lebedeva, S.M.; Bykov, V.N. Valent and structural state of iron ions in basalt glasses of different crystallinity according to the data of Mössbauer spectroscopy. Geochem. Int. 2008, 46, 511–516. [Google Scholar] [CrossRef]
- Ryu, S.S.; Lee, S.K.; Yoon, D.H. Synthesis of fine Ca-doped BaTiO3 powders by solid-state reaction method—Part I: Mechanical activation of starting materials. J. Electroceramics 2007, 18, 243–250. [Google Scholar] [CrossRef]
- Roy, A.C.; Mohanta, D. Structural and ferroelectric properties of solid-state derived carbonate-free barium titanate (BaTiO3) nanoscale particles. Scr. Mater. 2009, 61, 891–894. [Google Scholar] [CrossRef]
- Altynnikov, A.G.; Gagarin, A.G.; Gaidukov, M.M.; Tumarkin, A.V.; Petrov, P.K.; Alford, N.; Kozyrev, A.B. Suppression of slow capacitance relaxation phenomenon in Pt/Ba0.3Sr0.7TiO3/Pt thin film ferroelectric structures by annealing in oxygen atmosphere. Appl. Phys. Lett. 2014, 104, 042903. [Google Scholar] [CrossRef]
- Tumarkin, A.; Gagarin, A.; Altynnikov, A.; Gaidukov, M.; Odinets, A.; Razumov, S.; Kozyrev, A. Effect of annealing in oxygen atmosphere on structure and microwave properties of multilayered tunable (Ba,Sr)TiO3 capacitors. Thin Solid Film. 2015, 593, 189–192. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, X.M.; Meng, H.; Lv, P.; Zhang, J.X. Migration and redistribution of oxygen vacancy in barium titanate ceramics. Appl. Phys. Lett. 2006, 89, 071916. [Google Scholar] [CrossRef]
- Perry, N.H.; Kim, J.J.; Tuller, H.L. Oxygen surface exchange kinetics measurement by simultaneous optical transmission relaxation and impedance spectroscopy: Sr(Ti, Fe)O3-x thin film case study. Sci. Technol. Adv. Mater. 2018, 19, 130–141. [Google Scholar] [CrossRef]
- Ascienzo, D.; Kurt, O.; Greenbaum, S.; Bayer, T.J.; Maier, R.A.; Randall, C.A.; Ren, Y. Formation of structural defects and strain in electrodegraded Fe-doped SrTiO3 crystals due to oxygen vacancy migration. J. Am. Ceram. Soc. 2018, 101, 2545–2561. [Google Scholar] [CrossRef]
- Rai, H.M.; Singh, P.; Saxena, S.K.; Mishra, V.; Warshi, M.K.; Kumar, R.; Rajput, P.; Sagdeo, A.; Choudhuri, I.; Patha, B.; et al. Room-temperature magneto-dielectric effect in LaGa0.7Fe0.3O3+γ; origin and impact of excess oxygen. Inorg. Chem. 2017, 56, 3809–3819. [Google Scholar] [CrossRef] [PubMed]
- Fomin, A.A.; Fomina, M.A.; Rodionov, I.V.; Koshuro, V.A.; Poshivalova, E.Y.; Shchelkunov, A.Y.; Skaptsov, A.A.; Zakharevich, A.M.; Atkin, V.S. Superhard oxide coatings formed on titanium treated by high-frequency currents. Tech. Phys. Lett. 2015, 41, 909–911. [Google Scholar] [CrossRef]
- Adilov, S.R.; Afanaciev, V.P.; Kashkul, I.N.; Kumekov, S.E.; Mukhin, N.V.; Terukov, E.I. Studying the composition and structure of films obtained by thermal oxidation of copper. Glass Phys. Chem. 2017, 43, 272–275. [Google Scholar] [CrossRef]
- Mukhin, N.; Afanasjev, V.; Sokolova, I.; Chigirev, D.; Kastro, R.; Rudaja, L.; Lebedeva, G.; Oseev, A.; Tumarkin, A. Heat-Resistant Ferroelectric-Polymer Nanocomposite with High Dielectric Constant. Materials 2018, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, Y.; Gong, M.; Liu, X.; Cui, Z.-K.; Pei, Q.; Gu, J.; Huang, C.; Zhuang, Q. Enhanced dielectric performance of PDMS-based three-phase percolative nanocomposite films incorporating a high dielectric constant ceramic and conductive multi-walled carbon nanotubes. J. Mater. Chem. C 2018, 6, 10829–10837. [Google Scholar] [CrossRef]
- Barrow, D.A.; Petroff, T.E.; Tandon, R.P.; Sayer, M. Characterization of thick lead zirconate titanate films fabricated using a new sol gel based process. J. Appl. Phys. 1997, 81, 876–881. [Google Scholar] [CrossRef]
- Yamada, T.; Ueda, T.; Kitayama, T. Piezoelectricity of a high-content lead zirconate titanate/polymer composite. J. Appl. Phys. 1982, 53, 4328–4332. [Google Scholar] [CrossRef]
- Yoon, D.-H.; Zhang, J.; Lee, B.I. Dielectric constant and mixing model of BaTiO3 composite thick films. Mater. Res. Bull. 2003, 38, 765–772. [Google Scholar] [CrossRef]
- Tang, Z.; Scriven, L.E.; Davis, H.T. A three-component model of the electrical double layer. J. Chem. Phys. 1992, 97, 494–503. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, D.; Xiong, G.; Zhou, Z. A simplified model for the dielectric function of three-component composite materials. Phys. A Stat. Mech. Appl. 2000, 275, 256–261. [Google Scholar] [CrossRef]
- Staskov, N.I.; Sotskaya, L.I. Three-Component Model of an Effective Medium for Determining the Composition of Layers on Silicon Wafers. J. Appl. Spectrosc. 2017, 84, 764–769. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M.; Pitaevskii, L.P.; Sykes, J.B.; Kearsley, M.J. Statistical Physics. Part I, 3rd ed.; Lifshitz, E.M., Pitaevskii, L.P., Eds.; Elsevier/Butterworth Heinemann: Amsterdam, The Netherlands; Boston, MA, USA, 1980; ISBN 9780750633727. [Google Scholar]
- Ben Abdallah, N.; Degond, P.; Deluzet, F.; Latocha, V.; Talaalout, R.; Vignal, M.H. Diffusion Limits of Kinetic Models. In Hyperbolic Problems: Theory, Numerics, Applications; Hou, T.Y., Tadmor, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 3–17. ISBN 978-3-642-62929-7. [Google Scholar]



| Sample | # | G (mm/s) | IS (mm/s) | QS (mm/s) | % |
|---|---|---|---|---|---|
| NaFeSi | 1 | 0.514 ± 0.025 | 0.249 ± 0.004 | 1.163 ± 0.040 | 64.00 |
| 2 | 0.351 ± 0.031 | 0.232 ± 0.004 | 0.714 ± 0.024 | 36.00 | |
| LiFeSi | 1 | 0.550 ± 0.024 | 0.266 ± 0.003 | 1.242 ± 0.036 | 55.88 |
| 2 | 0.453 ± 0.026 | 0.254 ± 0.003 | 0.753 ± 0.027 | 44.12 |
| Sample | Annealing | Concentration of Pores, cm3/g | εeff (9.7 GHz) | 2h/H |
|---|---|---|---|---|
| LiFeSi/BST | No | 0.041 | 58 | 0.84…0.94 |
| Yes | 0.041 | 110 | 0.92…0.98 | |
| NaFeSi/BST | No | 0.033 | 40 | 0.82…0.93 |
| Yes | 0.033 | 55 | 0.91…0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumarkin, A.; Tyurnina, N.; Tyurnina, Z.; Mukhin, N.; Sinelshchikova, O.; Gagarin, A.; Sviridov, S.; Drozdovsky, A.; Sapego, E.; Mylnikov, I. Barium-Strontium Titanate/Porous Glass Structures for Microwave Applications. Materials 2020, 13, 5639. https://doi.org/10.3390/ma13245639
Tumarkin A, Tyurnina N, Tyurnina Z, Mukhin N, Sinelshchikova O, Gagarin A, Sviridov S, Drozdovsky A, Sapego E, Mylnikov I. Barium-Strontium Titanate/Porous Glass Structures for Microwave Applications. Materials. 2020; 13(24):5639. https://doi.org/10.3390/ma13245639
Chicago/Turabian StyleTumarkin, Andrey, Natalya Tyurnina, Zoya Tyurnina, Nikolay Mukhin, Olga Sinelshchikova, Alexander Gagarin, Sergey Sviridov, Andrey Drozdovsky, Eugeny Sapego, and Ivan Mylnikov. 2020. "Barium-Strontium Titanate/Porous Glass Structures for Microwave Applications" Materials 13, no. 24: 5639. https://doi.org/10.3390/ma13245639
APA StyleTumarkin, A., Tyurnina, N., Tyurnina, Z., Mukhin, N., Sinelshchikova, O., Gagarin, A., Sviridov, S., Drozdovsky, A., Sapego, E., & Mylnikov, I. (2020). Barium-Strontium Titanate/Porous Glass Structures for Microwave Applications. Materials, 13(24), 5639. https://doi.org/10.3390/ma13245639






