Influence of Chloride Concentration on Stress Corrosion Cracking and Crevice Corrosion of Austenitic Stainless Steel in Saline Environments
Abstract
:1. Introduction
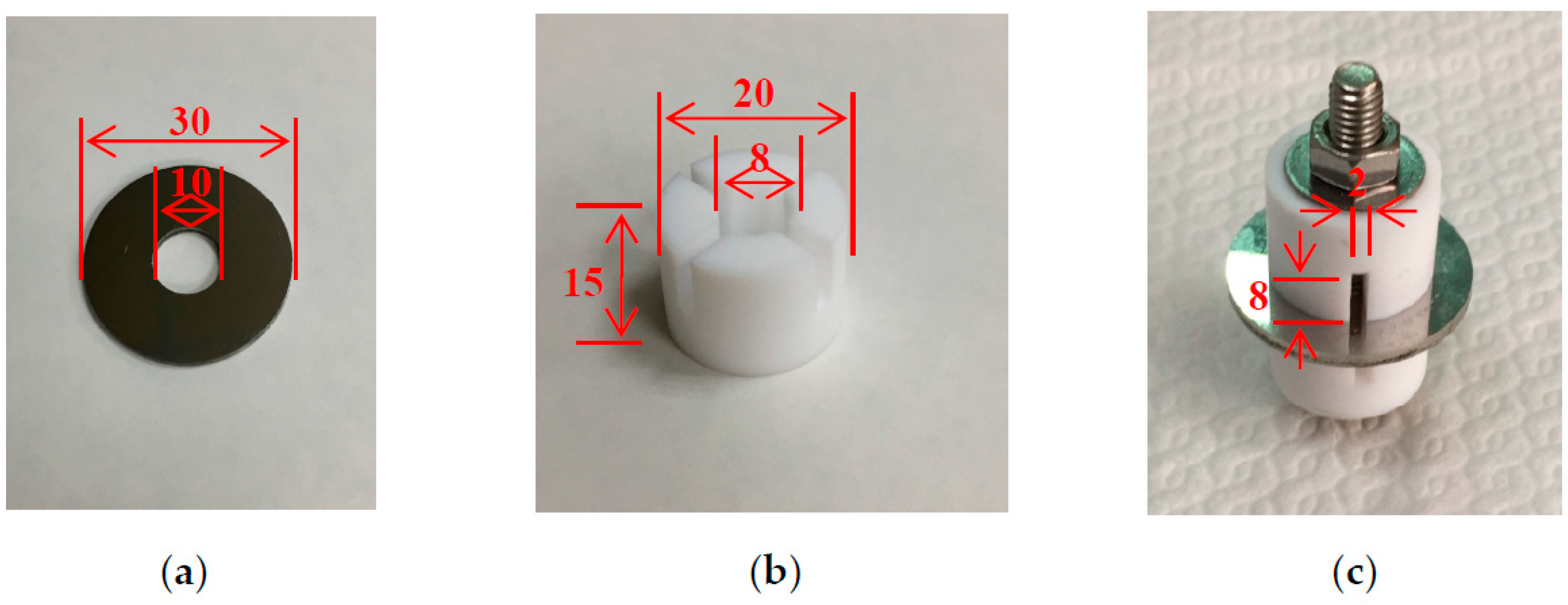
2. Experimental Procedure
3. Results and Discussion
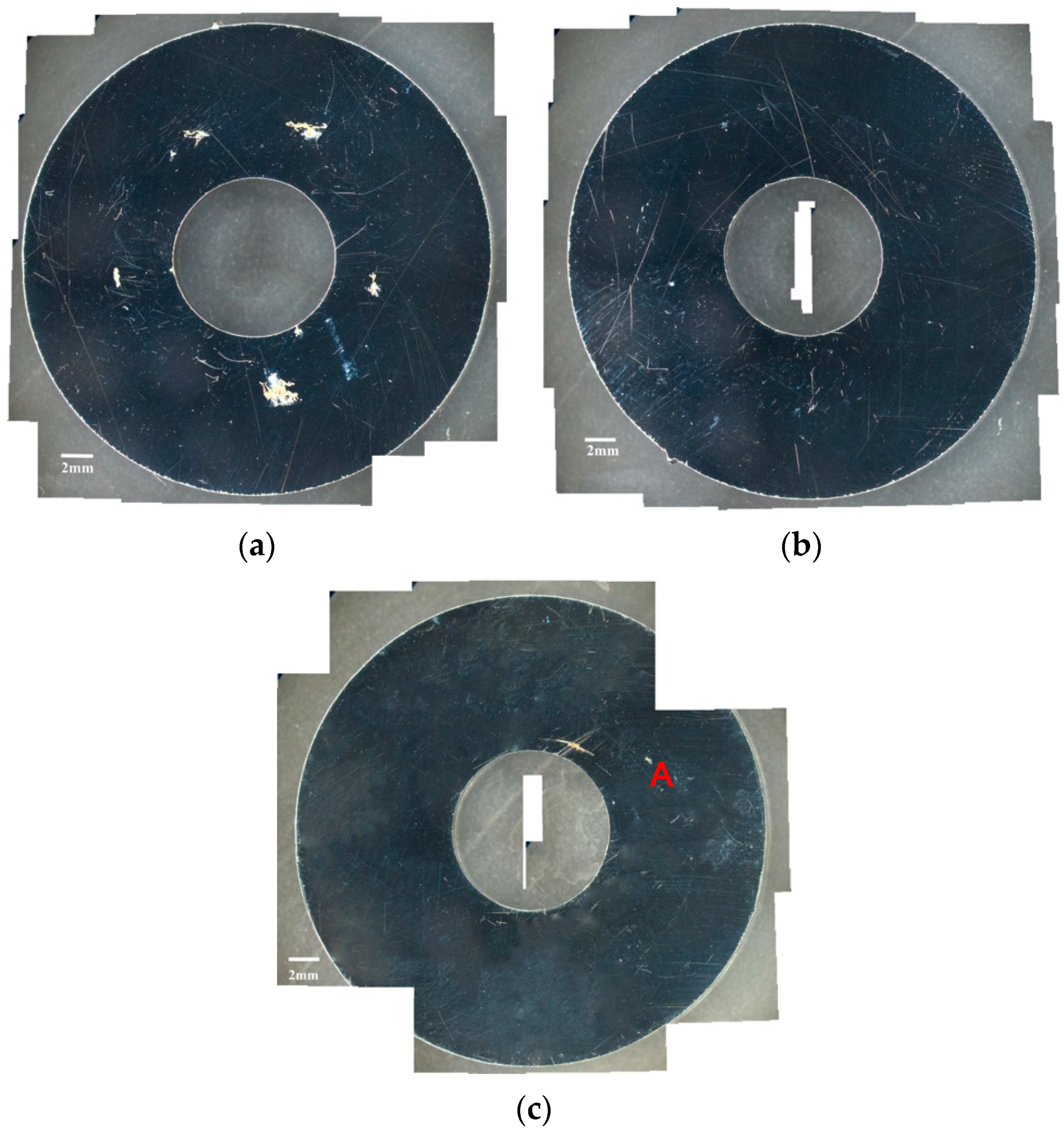
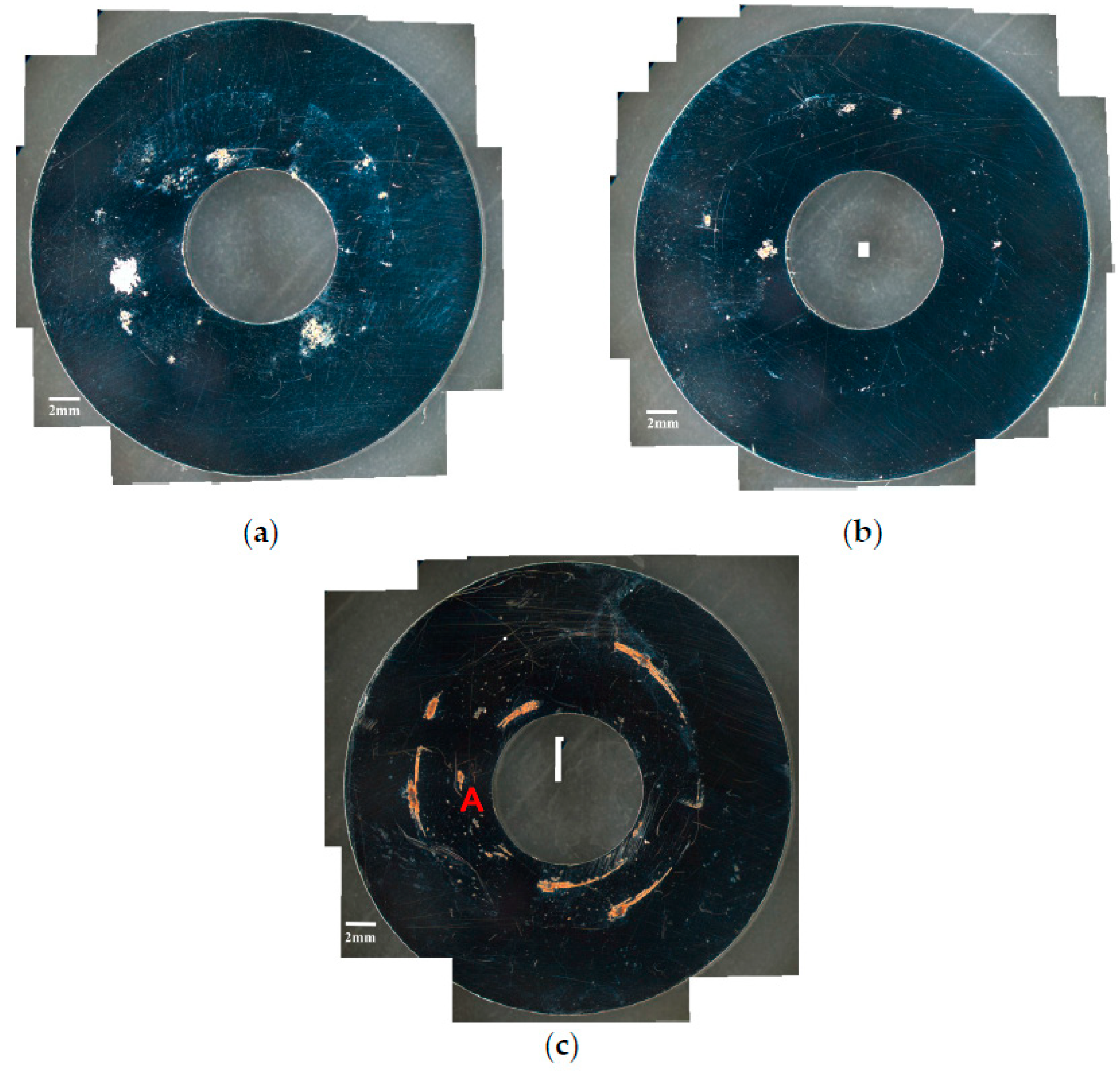
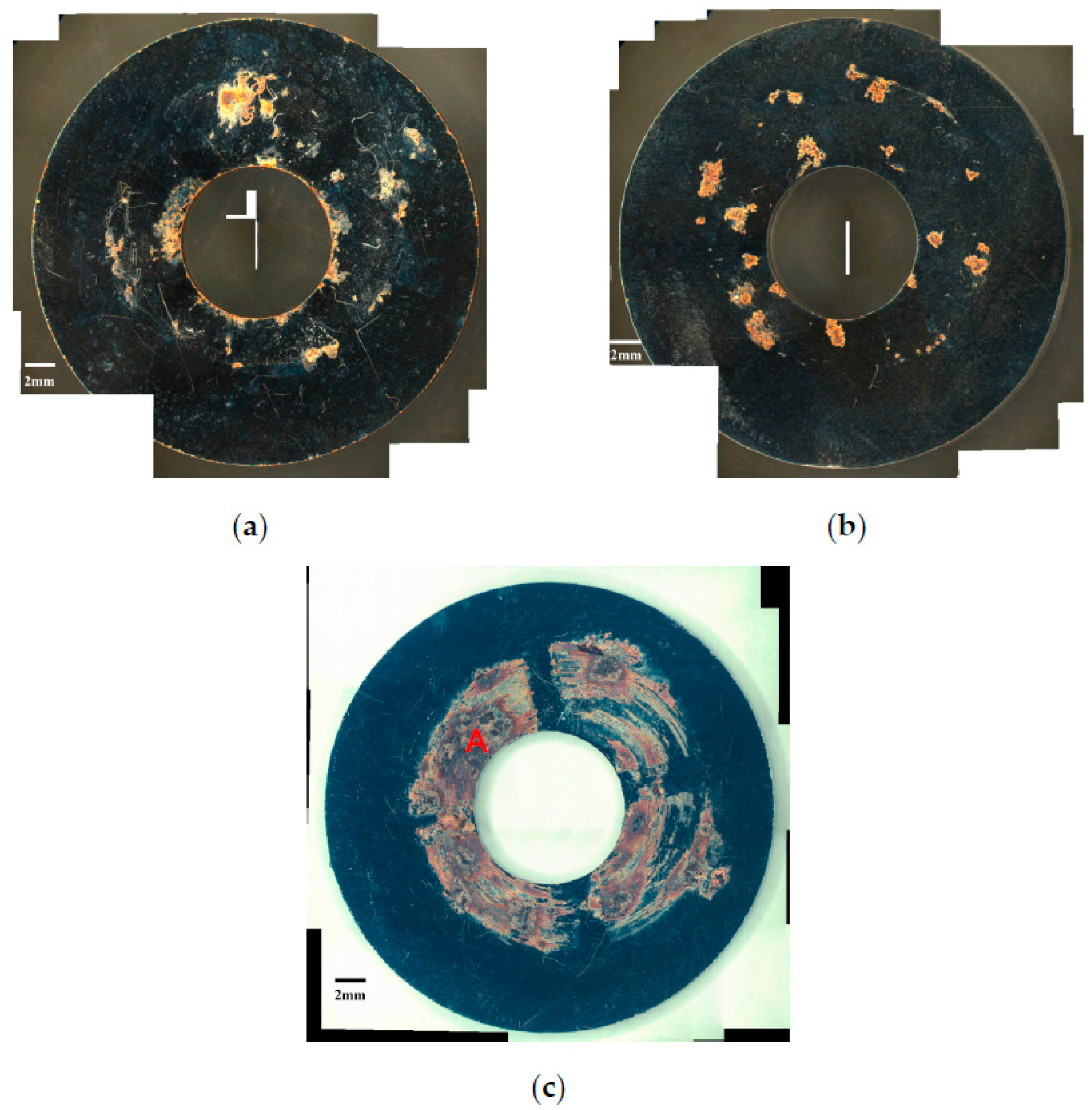
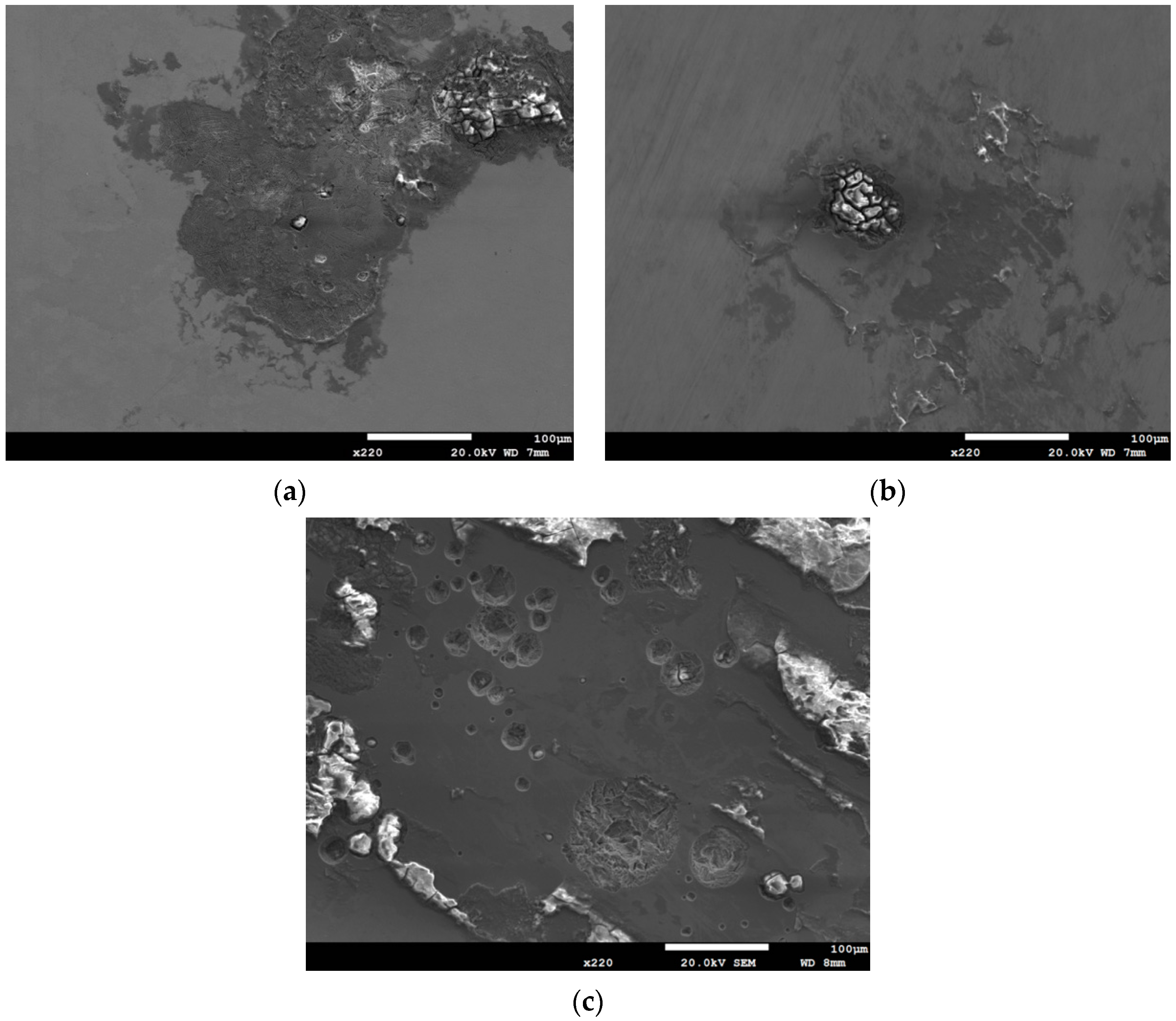
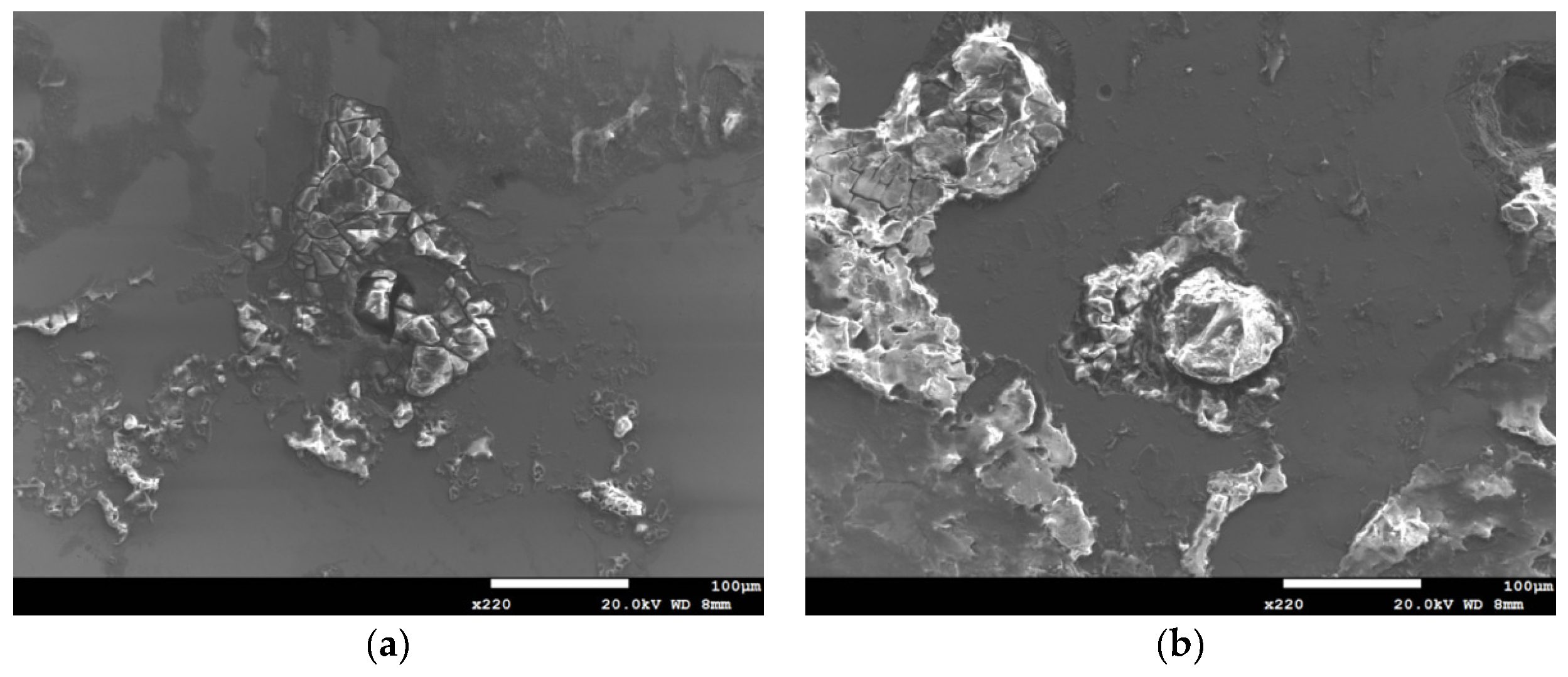
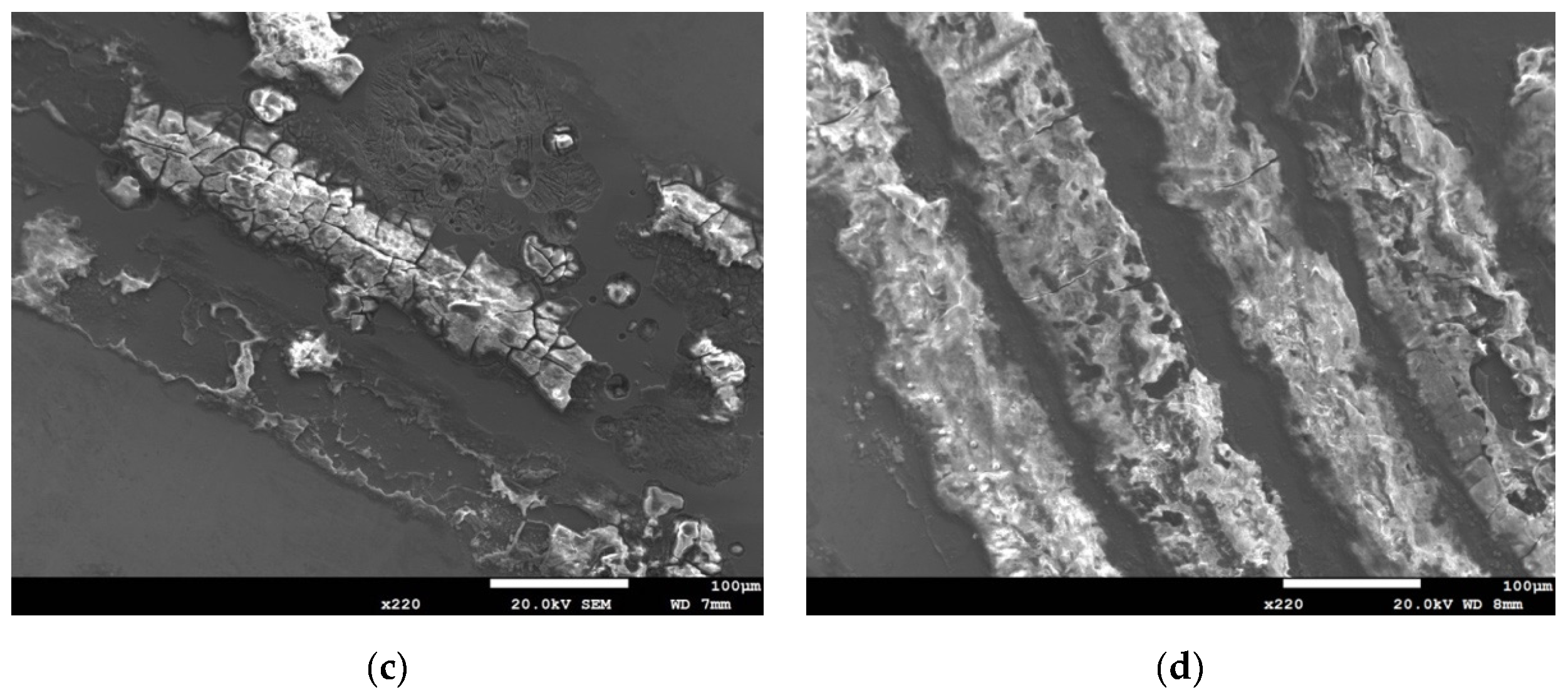
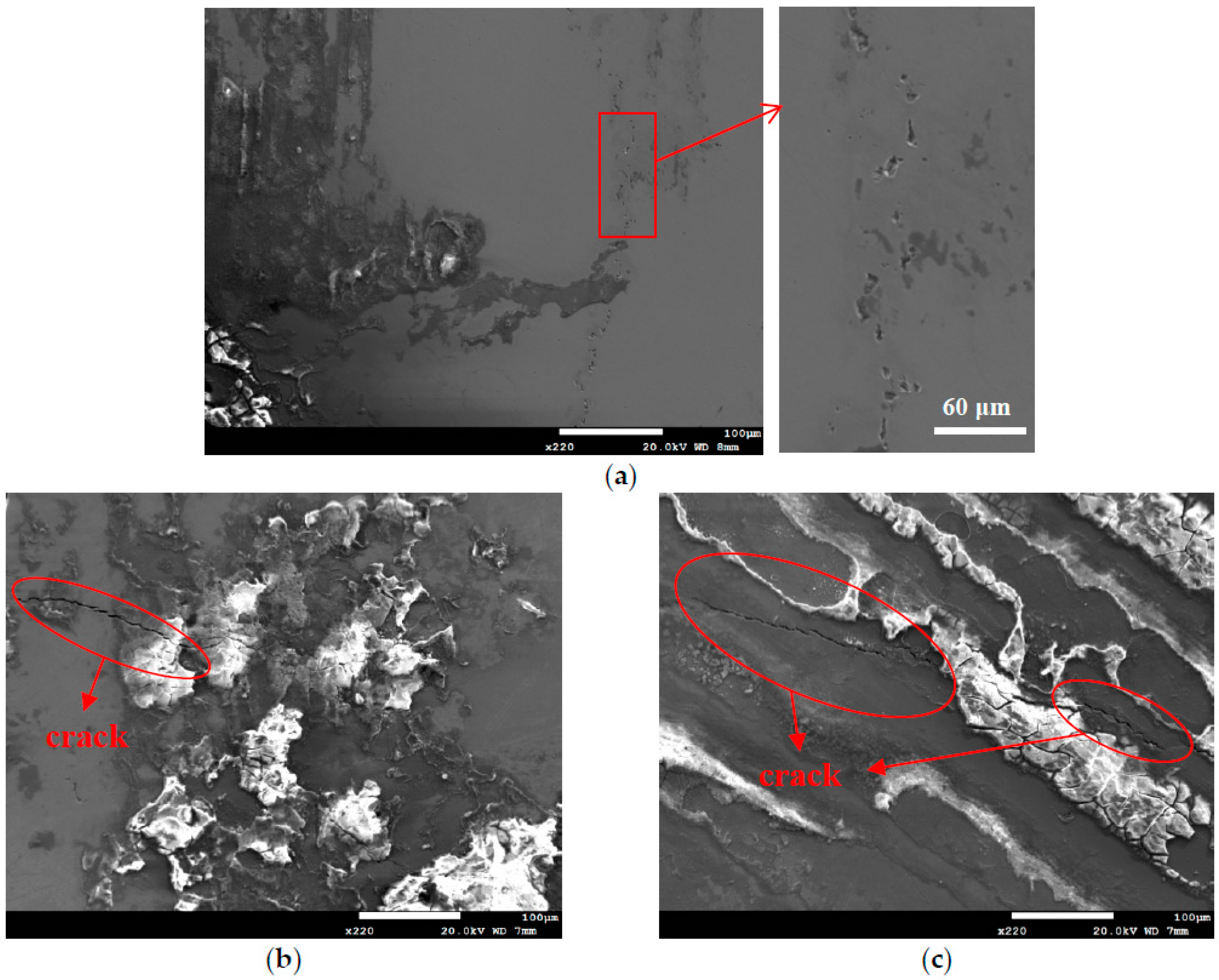
3.1. Analysis of Surface Morphology
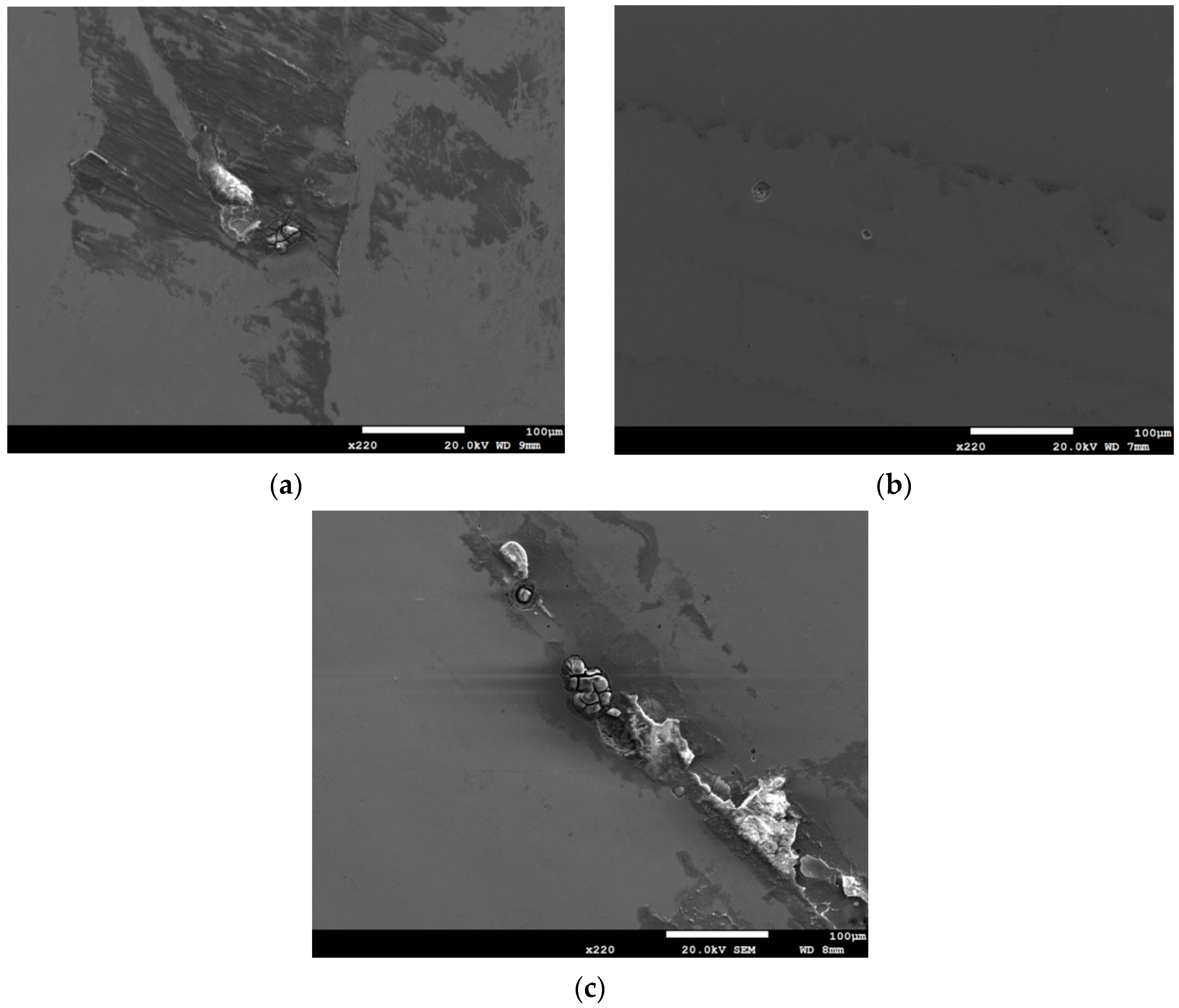
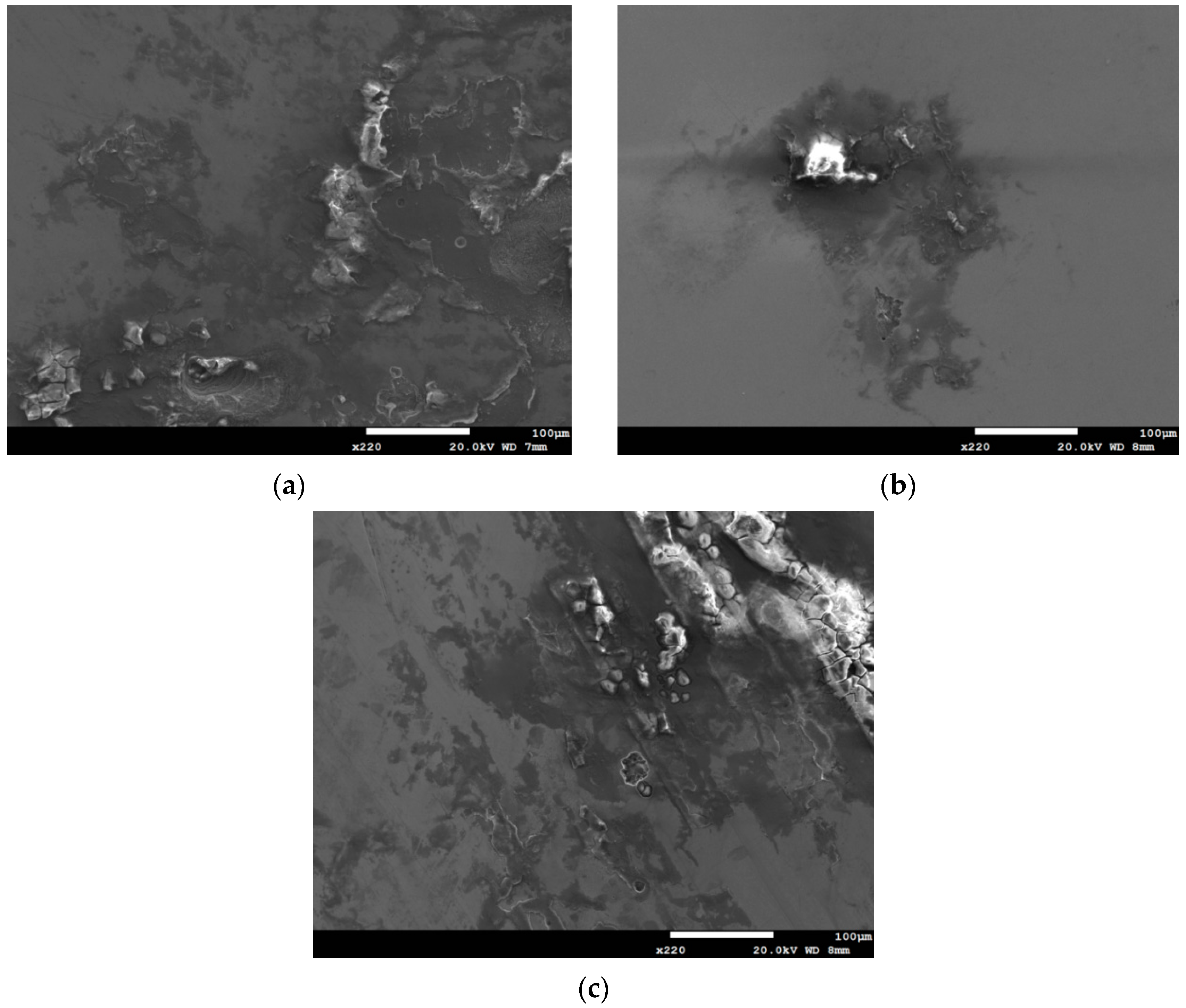
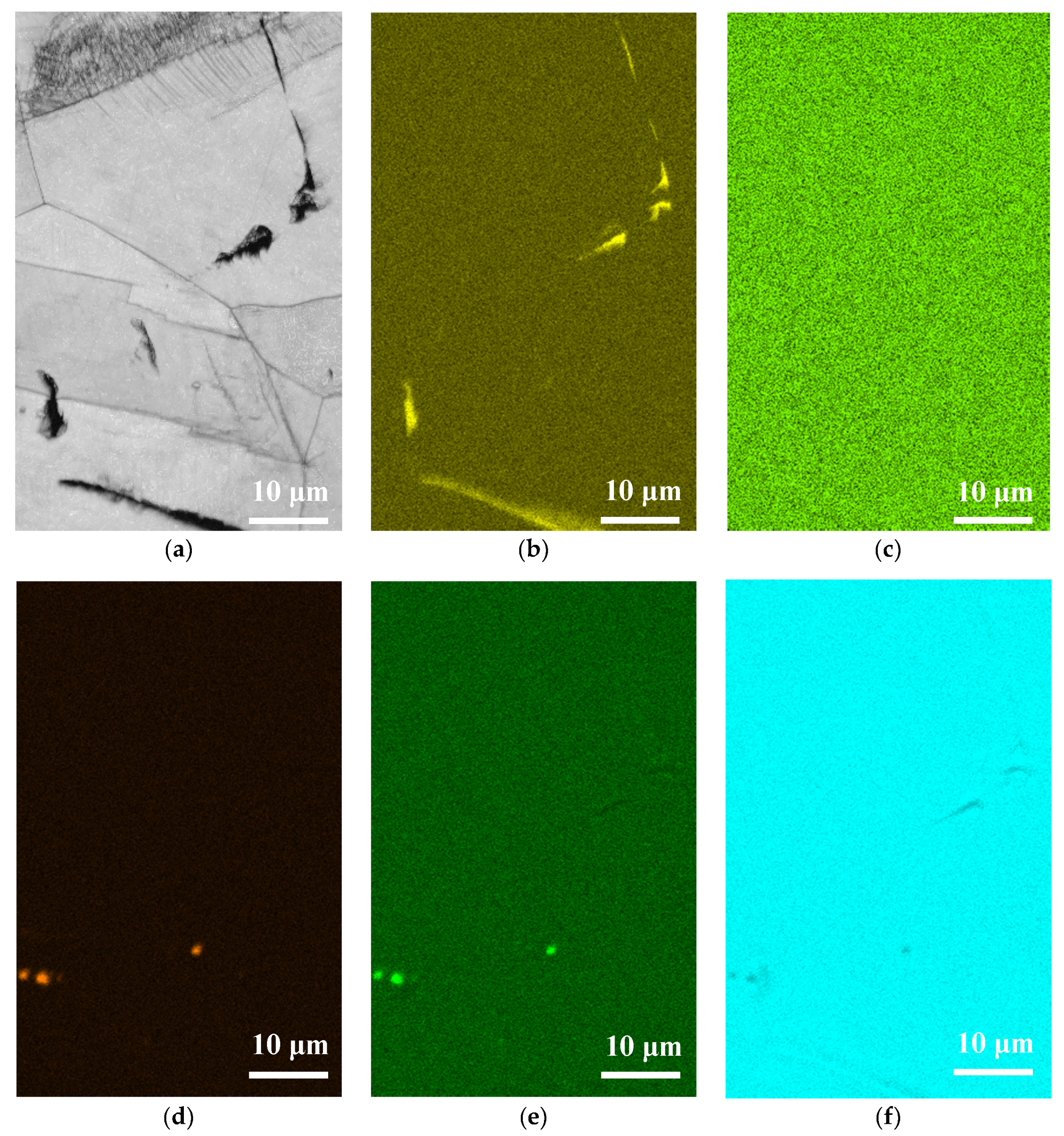
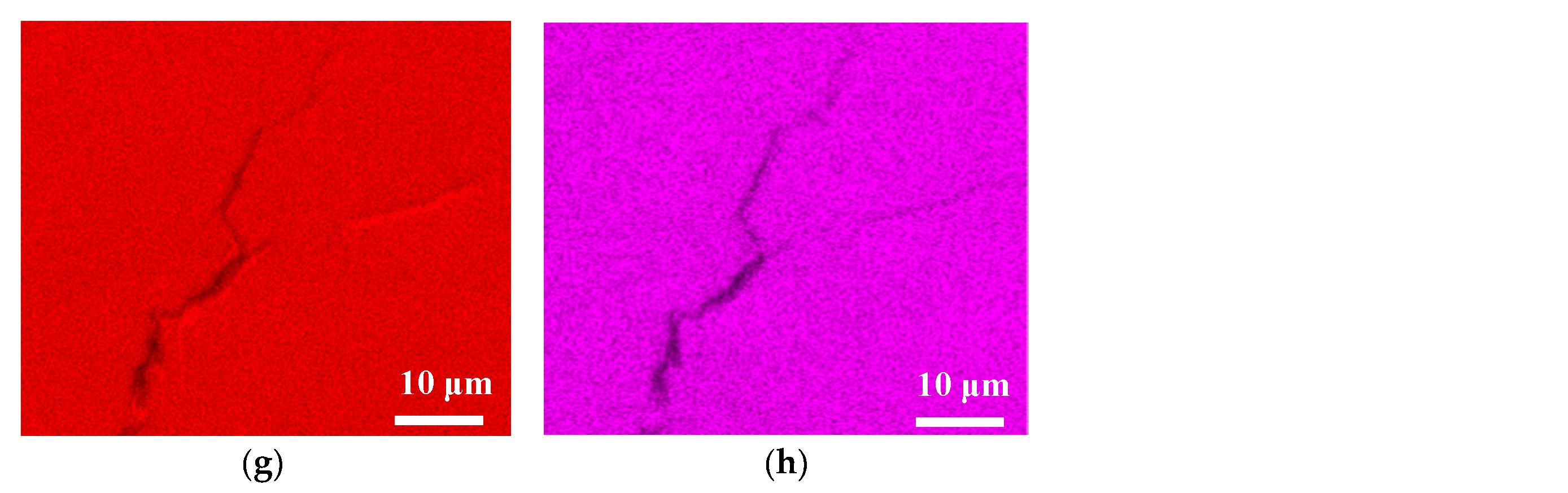
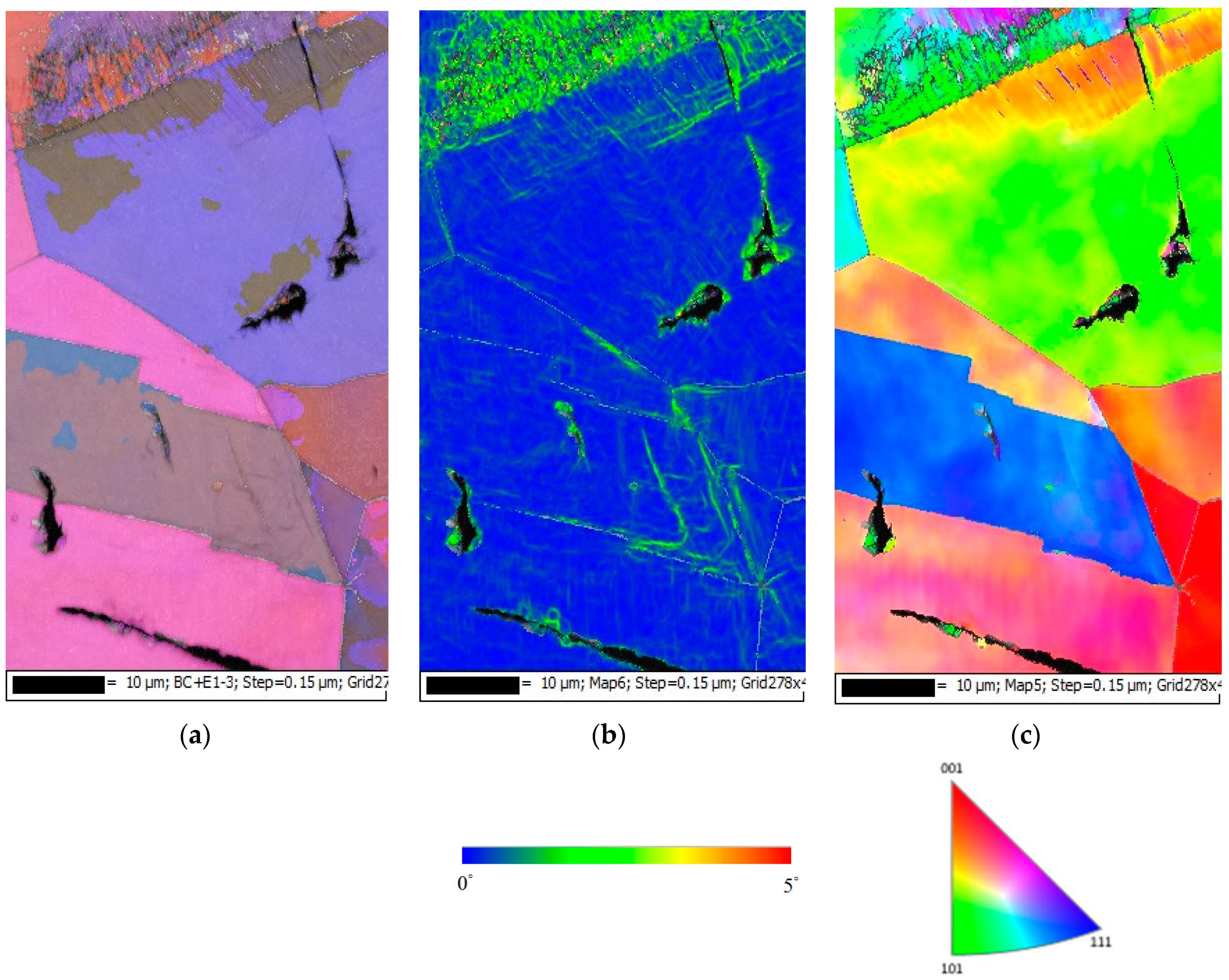
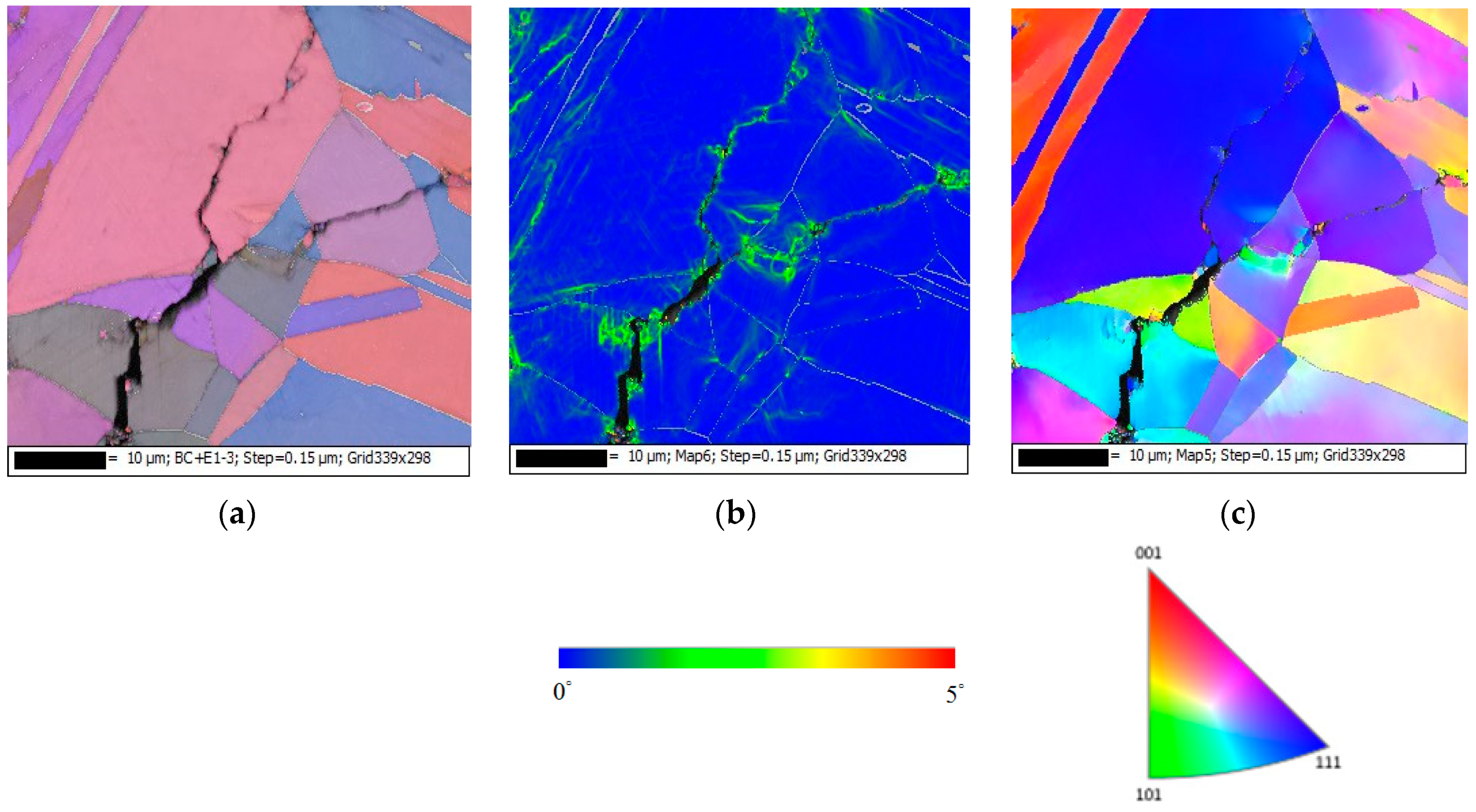
3.2. Analysis of EDS and EBSD
4. Conclusions
- (1)
- The specimens with chloride concentration 1 g/m2 tested for 400 h at 55% RH had minimum rusted areas. Nevertheless, there was little difference in the rusted areas of the specimens tested at 45% and 55% RH for 10,000 h, whereas the area of rust significantly increased when the RH increased to 70%. Some shallow corrosion beneath rust spots were found, but no cracks were observed in the corrosion region after 400-h testing, whereas SCC cracks were observed with the specimens tested for 10,000 h at all relative humidity levels. The discrete SCC cracks were observed with specimens exposed to 45% RH, but, on the other hand, distinct stress corrosion cracking cracks were found with those tested at 55% and 70% RH. This can be accounted for by a limited amount of chloride transported to the sites of crevice of the specimens with chloride concentration of 1 g/m2 exposed to 45% RH, the lowest RH. It may be necessary to have a higher RH environment to facilitate transportation of chloride to the crevice sites, because of the low chloride concentration of the specimens. The observations that the chlorine concentration at the crack regions increases with increasing the relative humidity give evidence to substantiate the aforementioned argument.
- (2)
- The results of the 10,000-h tests of this research suggest that the chloride concentration threshold for the initiation of SCC in 304L stainless steel at 45 °C is between 0.1 g/m2 and 1 g/m2.
- (3)
- Corrosion bands were exclusively observed on the specimens with chloride concentration of 0.1 g/m2 and 1 g/m2 tested at 70% RH. The width of corrosion band increased with increasing chloride concentration. Moreover, corrosion bands were analyzed to have higher chlorine contents.
- (4)
- The 304L austenitic stainless steel specimens with chloride concentration of 1 g/m2 tested at 45 °C were cracked with a trans-granular SCC mode, which was proved by the results of EBSD.
Author Contributions
Funding
Conflicts of Interest
References
- Schoell, R.; Xi, L.; Zhao, Y.; Wu, X.; Yu, Z.; Kenesei, P.; Almer, J.; Shayer, Z.; Kaoumi, D. In situ synchrotron X-ray tomography of 304 stainless steels undergoing chloride-induced stress corrosion cracking. Corros. Sci. 2020, 170, 108687. [Google Scholar] [CrossRef]
- Dastgerdi, A.A.; Brenna, A.; Ormellese, M.; Pedeferri, M.; Bolzoni, F. Experimental design to study the influence of temperature, pH, and chloride concentration on the pitting and crevice corrosion of UNS S30403 stainless steel. Corros. Sci. 2019, 159, 108160. [Google Scholar] [CrossRef] [Green Version]
- Yeom, H.; Dabney, T.; Pocquette, N.; Ross, K.; Pfefferkorn, F.E.; Sridharan, K. Cold spray deposition of 304L stainless steel to mitigate chloride-induced stress corrosion cracking in canisters for used nuclear fuel storage. J. Nucl. Mater. 2020, 538, 152254. [Google Scholar] [CrossRef]
- Wu, X. On residual stress analysis and microstructural evolution for stainless steel type 304 spent nuclear fuel canisters weld joint: Numerical and experimental studies. J. Nucl. Mater. 2020, 534, 152131. [Google Scholar] [CrossRef]
- Guo, L.; Street, S.R.; Mohammed-Ali, H.B.; Ghahari, M.; Mi, N.; Glanvill, S.; Plessis, A.D.; Reinhard, C.; Rayment, T.; Davenport, A.J. The effect of relative humidity change on atmospheric pitting corrosion of stainless steel 304L. Corros. Sci. 2019, 150, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Saegusa, T.; Yagawa, G.; Aritomi, M. Topics of research and development on concrete cask storage of spent nuclear fuel. Nucl. Eng. Des. 2008, 238, 1168–1174. [Google Scholar] [CrossRef]
- Aoyama, T.; Sugawara, Y.; Muto, I.; Hara, N. In situ monitoring of crevice corrosion morphology of type 316L stainless steel and repassivation behavior induced by sulfate ions. Corros. Sci. 2017, 127, 131–140. [Google Scholar] [CrossRef]
- Li, Y.Z.; Wang, X.; Zhang, G.A. Corrosion behavior of 13Cr stainless steel under stress and crevice in 3.5wt.% NaCl solution. Corros. Sci. 2020, 163, 108290. [Google Scholar] [CrossRef]
- Han, D.; Jiang, Y.; Shi, C.; Deng, B.; Li, J. Effect of temperature, chloride ion and pH on the crevice corrosion behavior of SAF 2205 duplex stainless steel in chloride solutions. J. Mater. Sci. 2012, 47, 1018–1025. [Google Scholar] [CrossRef]
- Meyer, R.M.; Pardini, A.; Cuta, J.; Adkins, H.; Casella, A.; Qiao, A.; Larche, M.R.; Diaz, A.; Doctor, S.R. NDE to Manage. Atmospheric SCC in Canister for Dry Storage of Spent Fuel: An Assessment; PNNL-22495 401001060; Pacific Northwest National Laboratory: Richland, WA, USA, 1 September 2013. [Google Scholar]
- Tani, J.I.; Mayuzumi, M.; Hara, N. Stress corrosion cracking of stainless-steel canister for concrete cask storage of spent fuel. J. Nucl. Mater. 2008, 379, 42–47. [Google Scholar] [CrossRef]
- Brossia, C.; Kelly, R. Occluded solution chemistry control and the role of alloy sulfur on the initiation of crevice corrosion in type 304ss. Corros. Sci. 1998, 40, 1851–1871. [Google Scholar] [CrossRef]
- Machuca, L.L.; Bailey, S.I.; Gubner, R.; Watkin, E.L.; Ginige, M.P.; Kaksonen, A.H.; Heidersbach, K. Effect of oxygen and biofilms on crevice corrosion of UNS S31803and UNS N08825 in natural seawater. Corros. Sci. 2013, 67, 242–255. [Google Scholar] [CrossRef]
- Pickering, H.W. The significance of the local electrode potential within pits, crevices and cracks. Corros. Sci. 1989, 29, 325–341. [Google Scholar] [CrossRef]
- Cai, B.P.; Liu, Y.H.; Tian, X.J.; Wang, F.; Li, H.; Ji, R.J. An experimental study of crevice corrosion behavior of 316L stainless steel in artificial seawater. Corros. Sci. 2010, 52, 3235–3242. [Google Scholar] [CrossRef]
- Cho, K.; Pickering, H.W. The role of chloride ions in the IR& IR* criterion for crevice corrosion in iron. J. Electrochem. Soc. 1991, 138, L56–L58. [Google Scholar]
- Shojaei, E.; Mirjalili, M.; Moayed, M.H. The influence of the crevice induced IR drop on polarization measurement of localized corrosion behavior of 316L stainless steel. Corros. Sci. 2019, 156, 96–105. [Google Scholar] [CrossRef]
- Scatigno, G.G.; Dong, P.; Ryan, M.P.; Wenman, M.R. The effects of salt loading on chloride-induced stress corrosion cracking of 304L austenitic stainless steel under atmospheric conditions. Materialia 2019, 8, 100509. [Google Scholar] [CrossRef]
- Shoji, S.; Ohnaka, N.; Furutani, Y.; Saitoh, T. Effects of relative humidity on atmospheric stress corrosion cracking of stainless steels. Corros. Eng. 1986, 35, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Prosek, T.; Iverson, A.; Taxsen, C.; Thierry, D. Low temperature on stress corrosion cracking of stainless steels in the atmosphere in the presence of chloride deposits. Corrosion 2009, 65, 105–117. [Google Scholar] [CrossRef]
- Cook, A.B.; Lyon, S.B.; Stevens, N.P.C.; Gunther, M.; McFiggans, G.; Newman, R.C.; Engelberg, D.L. Assessing the risk of under-deposit chloride-induced stress corrosion cracking in austenitic stainless steel nuclear waste containers. Corros. Eng. Sci. Technol. 2014, 49, 529–534. [Google Scholar] [CrossRef]
- Albores-Silca, O.E.; Charles, E.A.; Padovani, C. Effect of chloride deposition on stress corrosion cracking of 316L stainless steel used for intermediate level radioactive waste containers. Corros. Eng. Sci. Technol. 2011, 46, 124–128. [Google Scholar] [CrossRef]
- Yeh, C.P.; Tsai, K.C.; Huang, J.Y. Effects of relative humidity on crevice corrosion behavior of 304L stainless-steel nuclear material in a chloride environment. Metals 2019, 9, 1185. [Google Scholar] [CrossRef] [Green Version]
- Masuda, H. SKFM observation of SCC on SUS304 stainless steel. Corros. Sci. 2007, 49, 120–129. [Google Scholar] [CrossRef]
- Qiao, L.J.; Gao, K.W.; Volinsky, A.A.; Li, X.Y. Discontinuous surface cracks during stress corrosion cracking of stainless steel single crystal. Corros. Sci. 2011, 53, 3509–3514. [Google Scholar] [CrossRef]
- He, X.; Mintz, T.S.; Pabalan, R.; Miller, L.; Oberson, G. Assessment of Stress Corrosion Cracking Susceptibility for Austenitic Stainless Steels Exposed to Atmospheric Chloride and Non-Chloride Salts; NUREG/CR-7170; U.S. Nuclear Regulatory Commission: Montgomery, MD, USA, 2014. [Google Scholar]
- Li, J.X.; Chu, W.Y.; Wang, Y.B.; Qiao, L.J. In situ TEM study of stress corrosion cracking of austenitic stainless steel. Corros. Sci. 2003, 45, 1355–1365. [Google Scholar] [CrossRef]
- Zhu, L.K.; Yu, Y.; Li, J.X.; Qiao, L.J.; Volinsky, A.A. Stress corrosion cracking under low stress: Continuous or discontinuous cracks? Corros. Sci. 2014, 80, 350–358. [Google Scholar] [CrossRef]
- Nash, B.K.; Kelly, R.G. Characterization of the crevice solution chemistry of 304stainless-steel. Corros. Sci. 1993, 35, 817–825. [Google Scholar] [CrossRef]








| Element | C | S | Si | Ni | Cr | Mn | Fe |
|---|---|---|---|---|---|---|---|
| wt.% | 0.017 | 0.0290 | 0.450 | 9.000 | 18.000 | 1.540 | Bal. |
| Composition | NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 | KBr | SrCl2 | H3BO3 | NaF |
|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 58.490 | 26.460 | 9.750 | 2.765 | 1.645 | 0.477 | 0.238 | 0.095 | 0.071 | 0.007 |
| Location | O | Na | Mg | S | Cl | Ca | Cr | Mn | Fe | Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.73 | 0.11 | 0.04 | 0.00 | 0.00 | 0.04 | 18.78 | 1.74 | 70.46 | 8.10 |
| B | 33.2 | 0.98 | 0.30 | 0.24 | 3.11 | 0.06 | 8.67 | 0.38 | 51.77 | 1.28 |
| C | 16.59 | 1.64 | 0.60 | 0.06 | 0.31 | 0.08 | 16.83 | 1.69 | 56.05 | 6.14 |
| D | 30.31 | 1.26 | 0.62 | 0.18 | 2.98 | 0.17 | 13.48 | 0.87 | 48.51 | 1.63 |
| E | 27.74 | 0.36 | 0.45 | 0.16 | 2.40 | 0.09 | 6.47 | 0.96 | 57.95 | 3.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-P.; Tsai, K.-C.; Huang, J.-Y. Influence of Chloride Concentration on Stress Corrosion Cracking and Crevice Corrosion of Austenitic Stainless Steel in Saline Environments. Materials 2020, 13, 5640. https://doi.org/10.3390/ma13245640
Yeh C-P, Tsai K-C, Huang J-Y. Influence of Chloride Concentration on Stress Corrosion Cracking and Crevice Corrosion of Austenitic Stainless Steel in Saline Environments. Materials. 2020; 13(24):5640. https://doi.org/10.3390/ma13245640
Chicago/Turabian StyleYeh, Chun-Ping, Kun-Chao Tsai, and Jiunn-Yuan Huang. 2020. "Influence of Chloride Concentration on Stress Corrosion Cracking and Crevice Corrosion of Austenitic Stainless Steel in Saline Environments" Materials 13, no. 24: 5640. https://doi.org/10.3390/ma13245640
APA StyleYeh, C.-P., Tsai, K.-C., & Huang, J.-Y. (2020). Influence of Chloride Concentration on Stress Corrosion Cracking and Crevice Corrosion of Austenitic Stainless Steel in Saline Environments. Materials, 13(24), 5640. https://doi.org/10.3390/ma13245640




