Enhancing Electrical Conductivity of Composites of Single-Walled Carbon Nanotubes and Ethyl Cellulose with Water Vapor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Compounds and Materials
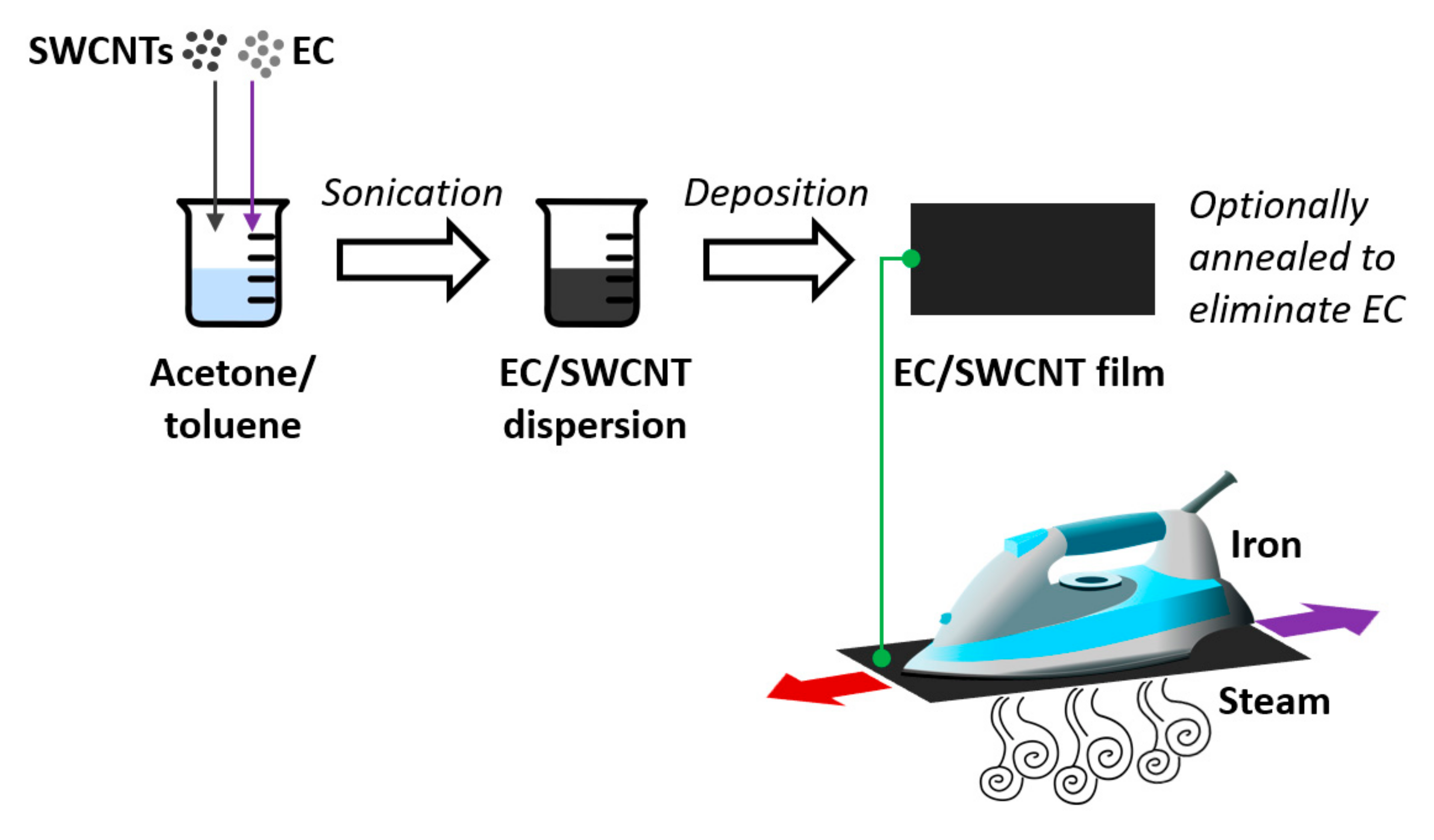
2.2. Preparation of EC/SWCNT and SWCNT Films
2.3. Doping of EC/SWCNT and SWCNT Films with Water Vapor
2.4. Characterization
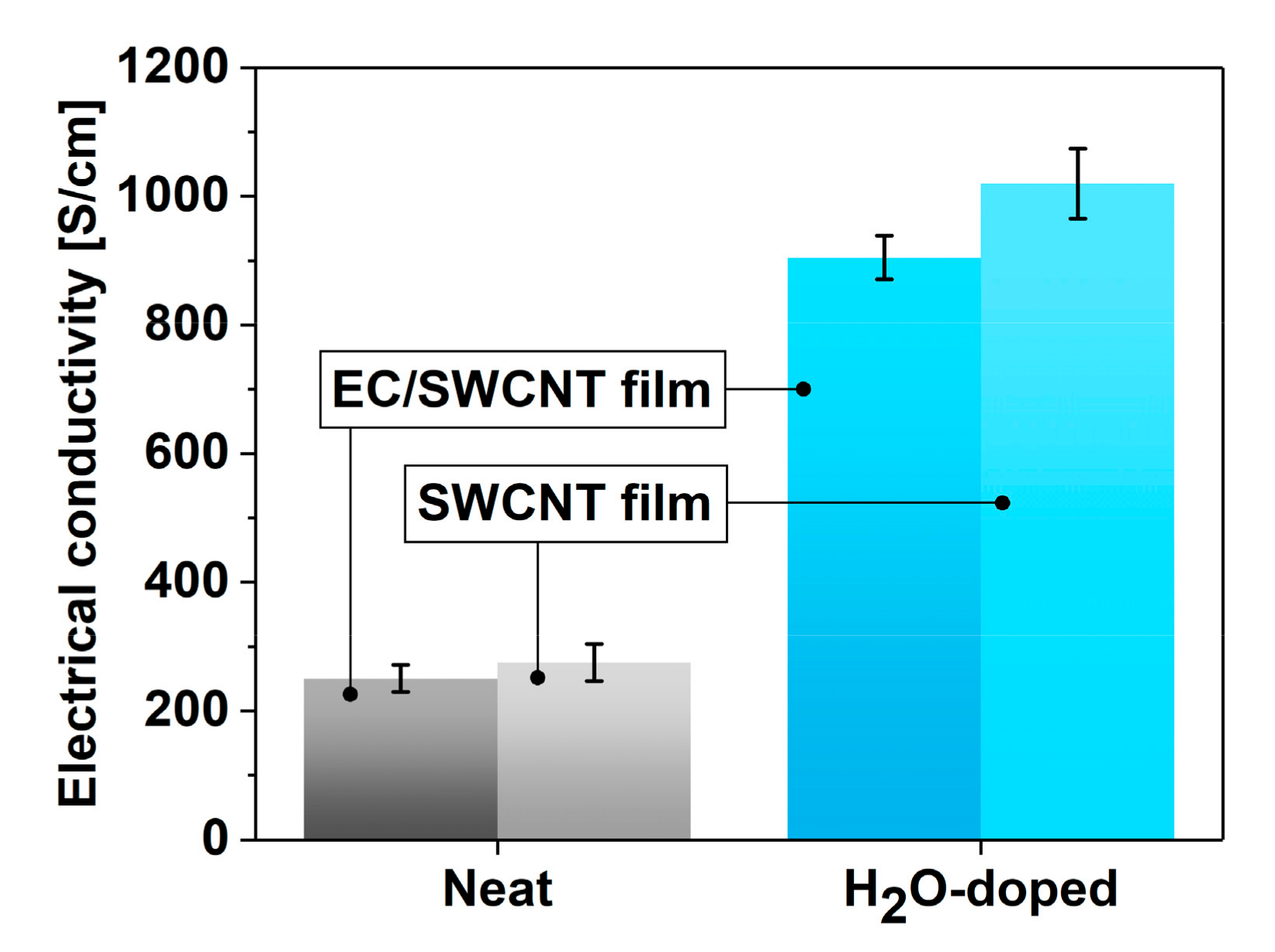
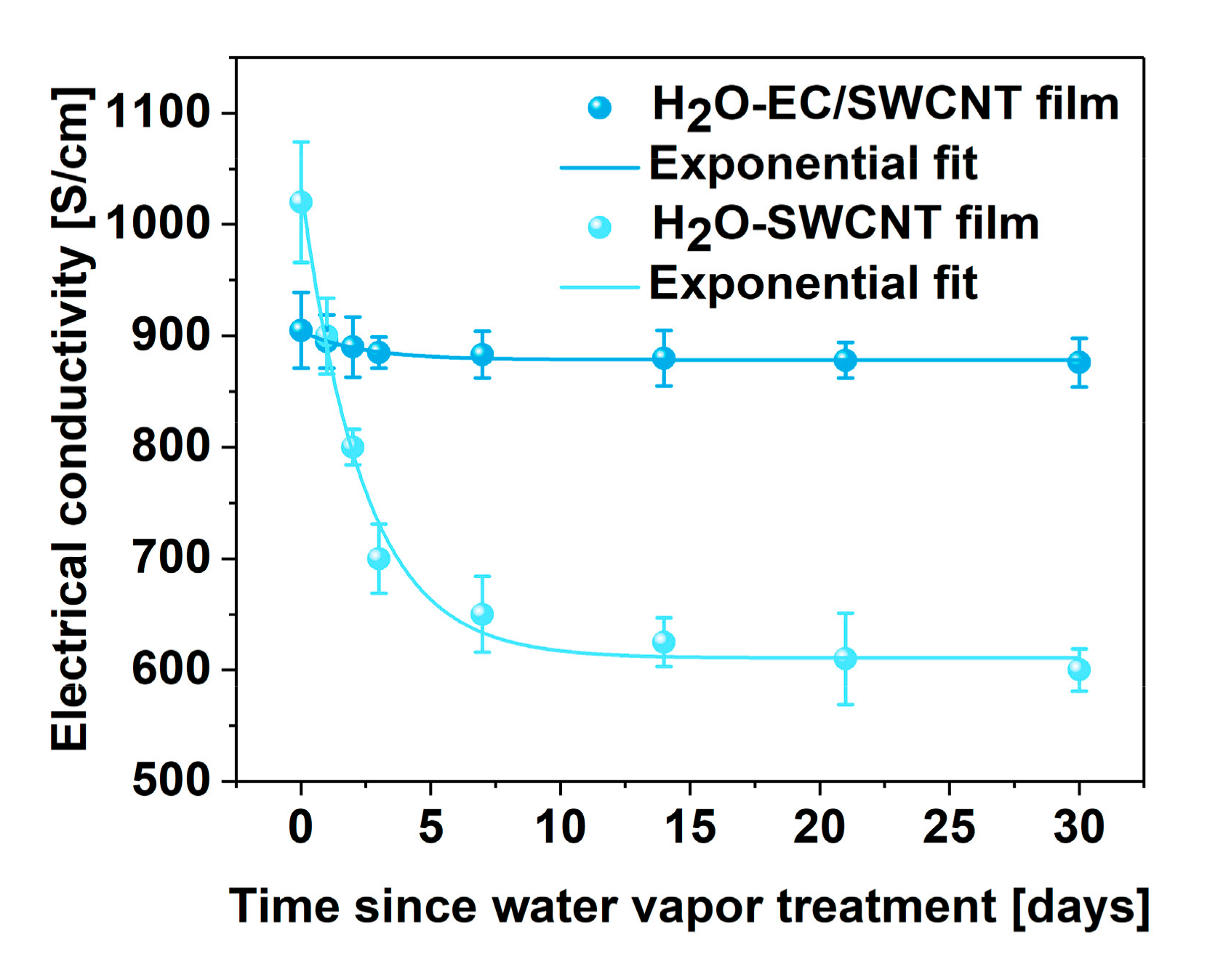
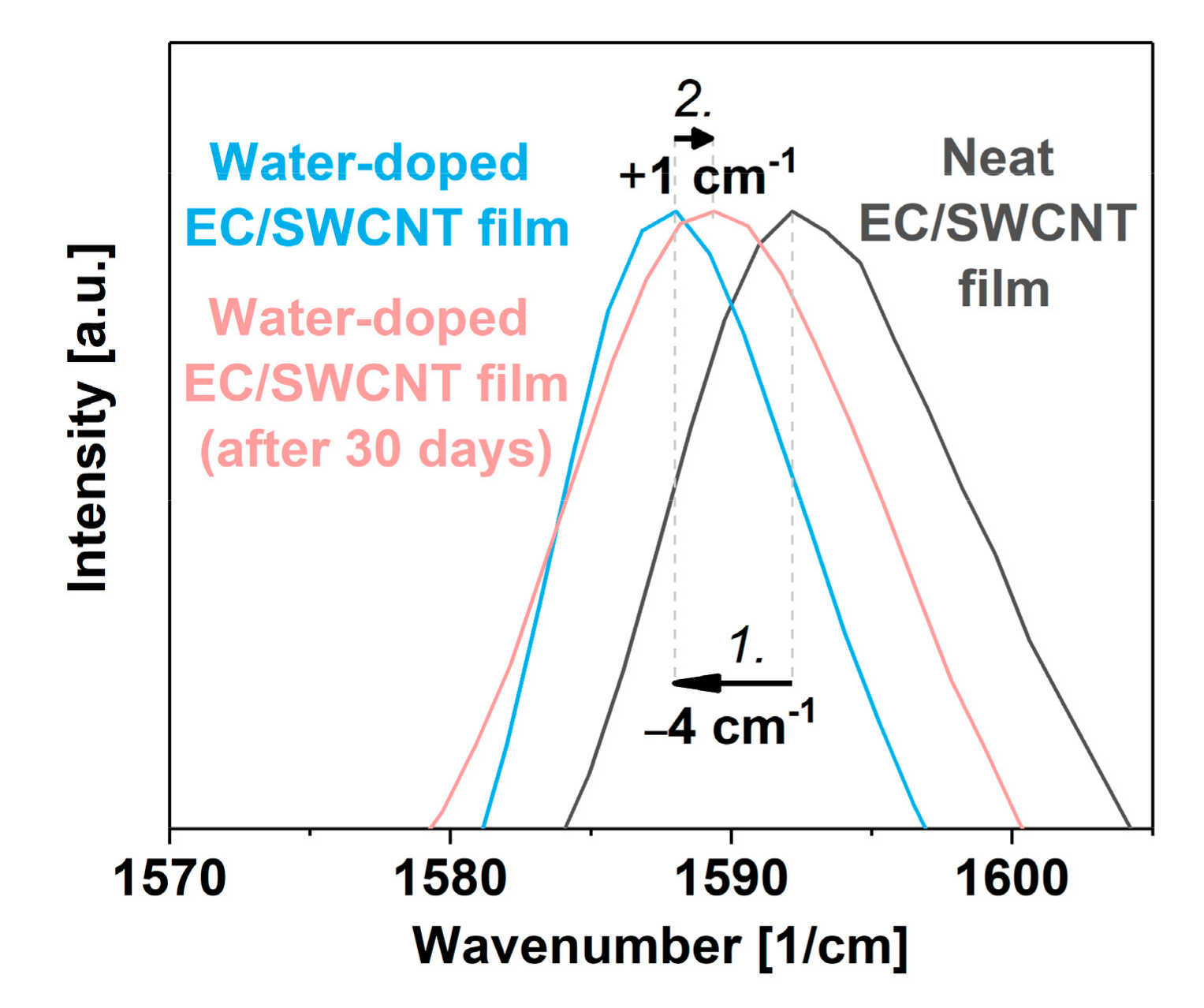
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Energy; Our World in Data: Oxford, UK, 2014. [Google Scholar]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Thermoelectric materials: Carbon-nanotube-based thermoelectric materials and devices. Adv. Mater. 2018, 30, 1870072. [Google Scholar] [CrossRef] [Green Version]
- Feynman, R.P. There’s plenty of room at the bottom. Calif. Inst. Technol. Eng. Sci. Mag. 1960, 4, 23–36. [Google Scholar]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudha, P.N.; Sangeetha, K.; Vijayalakshmi, K.; Barhoum, A. Chapter 12—Nanomaterials History, Classification, Unique Properties, Production and Market. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Barhoum, A., Makhlouf, A.S.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341–384. [Google Scholar]
- Yang, C.C.; Mai, Y.-W. Thermodynamics at the nanoscale: A new approach to the investigation of unique physicochemical properties of nanomaterials. Mater. Sci. Eng. R Rep. 2014, 79, 1–40. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, J.E. Chemical Doping of Single-Wall Carbon Nanotubes. Acc. Chem. Res. 2002, 35, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, P.R. Electrical Properties and Applications of Carbon Nanotube Structures. J. Nanosci. Nanotechnol. 2007, 7, 1239–1267. [Google Scholar] [CrossRef] [PubMed]
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks: A review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef] [Green Version]
- Che, J.; Çagin, T.; Goddard, W.A., III. Thermal conductivity of carbon nanotubes. Nanotechnology 2000, 11, 65. [Google Scholar] [CrossRef]
- Brozena, A.H.; Kim, M.; Powell, L.R.; Wang, Y. Controlling the optical properties of carbon nanotubes with organic colour-centre quantum defects. Nat. Rev. Chem. 2019, 3, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Tabakman, S.M.; Welsher, K.; Hong, G.; Dai, H. Optical Properties of Single-Walled Carbon Nanotubes Separated in a Density Gradient: Length, Bundling, and Aromatic Stacking Effects. J. Phys. Chem. C 2010, 114, 19569–19575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boncel, S.; Sundaram, R.M.; Windle, A.H.; Koziol, K.K.K. Enhancement of the Mechanical Properties of Directly Spun CNT Fibers by Chemical Treatment. ACS Nano 2011, 5, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Hong, S.; Myung, S. A flexible approach to mobility. Nat. Nanotechnol. 2007, 2, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Hazeghi, A.; Takei, K.; Chen, H.; Member, S.; Chan, P.C.H.; Javey, A.; Wong, H.-S.P. Low-resistance electrical contact to carbon nanotubes with graphitic interfacial layer. IEEE Trans. Electron. Devices 2011, 59, 12–19. [Google Scholar] [CrossRef]
- Bulmer, J.S.; Lekawa-Raus, A.; Rickel, D.G.; Balakirev, F.F.; Koziol, K.K. Extreme Magneto-transport of Bulk Carbon Nanotubes in Sorted Electronic Concentrations and Aligned High Performance Fiber. Sci. Rep. 2017, 7, 12193. [Google Scholar] [CrossRef]
- Zhou, W.; Vavro, J.; Nemes, N.M.; Fischer, J.E.; Borondics, F.; Kamaras, K.; Tanner, D. Charge transfer and Fermi level shift in p-doped single-walled carbon nanotubes. Phys. Rev. B 2005, 71, 205423. [Google Scholar] [CrossRef] [Green Version]
- Kamarás, K.; Pekker, Á.; Botka, B.; Hu, H.; Niyogi, S.; Itkis, M.E.; Haddon, R.C. The effect of nitric acid doping on the optical properties of carbon nanotube films. Phys. Status Solidi B 2010, 247, 2754–2757. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, J.; Vajtai, R.; Ajayan, P.M.; Barrera, E.V. Iodine doped carbon nanotube cables exceeding specific electrical conductivity of metals. Sci. Rep. 2011, 1, 83. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.S.; Kim, H.J.; Fischer, J.E.; Thess, A.; Smalley, R.E. Conductivity enhancement in single-walled carbon nanotube bundles doped with K and Br. Nature 1997, 388, 255–257. [Google Scholar] [CrossRef]
- Klinke, C.; Chen, J.; Afzali, A.; Avouris, P. Charge Transfer Induced Polarity Switching in Carbon Nanotube Transistors. Nano Lett. 2005, 5, 555–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, P.; Arenal, R.; Rümmeli, M.; Rubio, A.; Pichler, T. The doping of carbon nanotubes with nitrogen and their potential applications. Carbon 2010, 48, 575–586. [Google Scholar] [CrossRef]
- Schmid, M.; Goze-Bac, C.; Krämer, S.; Roth, S.; Mehring, M.; Mathis, C.; Petit, P. Metallic properties of Li-intercalated carbon nanotubes investigated by NMR. Phys. Rev. B 2006, 74, 073416. [Google Scholar] [CrossRef]
- Lekawa-Raus, A.; Kurzepa, L.; Kozlowski, G.; Hopkins, S.C.; Wozniak, M.; Lukawski, D.; Glowacki, B.A.; Koziol, K.K. Influence of atmospheric water vapour on electrical performance of carbon nanotube fibres. Carbon 2015, 87, 18–28. [Google Scholar] [CrossRef]
- Na, P.S.; Kim, H.; So, H.-M.; Kong, K.-J.; Chang, H.; Ryu, B.H.; Choi, Y.; Lee, J.-O.; Kim, B.-K.; Kim, J.-J.; et al. Investigation of the humidity effect on the electrical properties of single-walled carbon nanotube transistors. Appl. Phys. Lett. 2005, 87, 093101. [Google Scholar] [CrossRef]
- Tsai, J.T.H.; Lu, C.-C.; Li, J.G. Fabrication of humidity sensors by multi-walled carbon nanotubes. J. Exp. Nanosci. 2010, 5, 302–309. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, X.; Chen, W.; Li, T. Carbon nanotubes humidity sensor based on high testing frequencies. Sens. Actuators A Phys. 2011, 168, 10–13. [Google Scholar] [CrossRef]
- Podlesny, B.; Kumanek, B.; Borah, A.; Yamaguchi, R.; Shiraki, T.; Fujigaya, T.; Janas, D. Thermoelectric Properties of Thin Films from Sorted Single-Walled Carbon Nanotubes. Materials 2020, 13, 3808. [Google Scholar] [CrossRef]
- Bulmer, J.S.; Gspann, T.S.; Barnard, J.S.; Elliott, J.A. Chirality-independent characteristic crystal length in carbon nanotube textiles measured by Raman spectroscopy. Carbon 2017, 115, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Danish, M.; Luo, S. A New Route to Enhance the Packing Density of Buckypaper for Superior Piezoresistive Sensor Characteristics. Sensors 2020, 20, 2904. [Google Scholar] [CrossRef] [PubMed]
- Tersoff, J. Contact resistance of carbon nanotubes. Appl. Phys. Lett. 1999, 74, 2122–2124. [Google Scholar] [CrossRef]
- Trinh, P.V.; Anh, N.N.; Tam, N.T.; Hong, N.T.; Hong, P.N.; Minh, P.N.; Thang, B.H. Influence of defects induced by chemical treatment on the electrical and thermal conductivity of nanofluids containing carboxyl-functionalized multi-walled carbon nanotubes. RSC Adv. 2017, 7, 49937–49946. [Google Scholar] [CrossRef] [Green Version]
- Algharagholy, L.A. Defects in Carbon Nanotubes and their Impact on the Electronic Transport Properties. J. Elec Mater. 2019, 48, 2301–2306. [Google Scholar] [CrossRef]
- Kumanek, B.; Wasiak, T.; Stando, G.; Stando, P.; Łukowiec, D.; Janas, D. Simple Method to Improve Electrical Conductivity of Films Made from Single-Walled Carbon Nanotubes. Nanomaterials 2019, 9, 1113. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.; Souza Filho, A.G.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-Palen, E.; Kruszynska, M.; Bachmatiuk, A.; Kalenczuk, R. Characterization of carbon nanotubes by Raman spectroscopy. Mater. Sci. Pol. 2008, 26, 433–441. [Google Scholar]
- Janas, D. Powerful doping of chirality-sorted carbon nanotube films. Vacuum 2018, 149, 48–52. [Google Scholar] [CrossRef]
- Newcomb, B.A.; Chae, H.G.; Gulgunje, P.V.; Gupta, K.; Liu, Y.; Tsentalovich, D.E.; Pasquali, M.; Kumar, S. Stress transfer in polyacrylonitrile/carbon nanotube composite fibers. Polymer 2014, 55, 2734–2743. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose—A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rdest, M.; Janas, D. Enhancing Electrical Conductivity of Composites of Single-Walled Carbon Nanotubes and Ethyl Cellulose with Water Vapor. Materials 2020, 13, 5764. https://doi.org/10.3390/ma13245764
Rdest M, Janas D. Enhancing Electrical Conductivity of Composites of Single-Walled Carbon Nanotubes and Ethyl Cellulose with Water Vapor. Materials. 2020; 13(24):5764. https://doi.org/10.3390/ma13245764
Chicago/Turabian StyleRdest, Monika, and Dawid Janas. 2020. "Enhancing Electrical Conductivity of Composites of Single-Walled Carbon Nanotubes and Ethyl Cellulose with Water Vapor" Materials 13, no. 24: 5764. https://doi.org/10.3390/ma13245764




