Effect of K2ZrF6 Concentration on the Two-Step PEO Coating Prepared on AZ91 Mg Alloy in Alkaline Silicate Solution
Abstract
:1. Introduction
2. Experimental Details
2.1. Specimens Pre-Treatment and Electrolytes
2.2. PEO Coating Deposition by Primary and Secondary Step
2.3. Coating Characterization
3. Result and Discussion
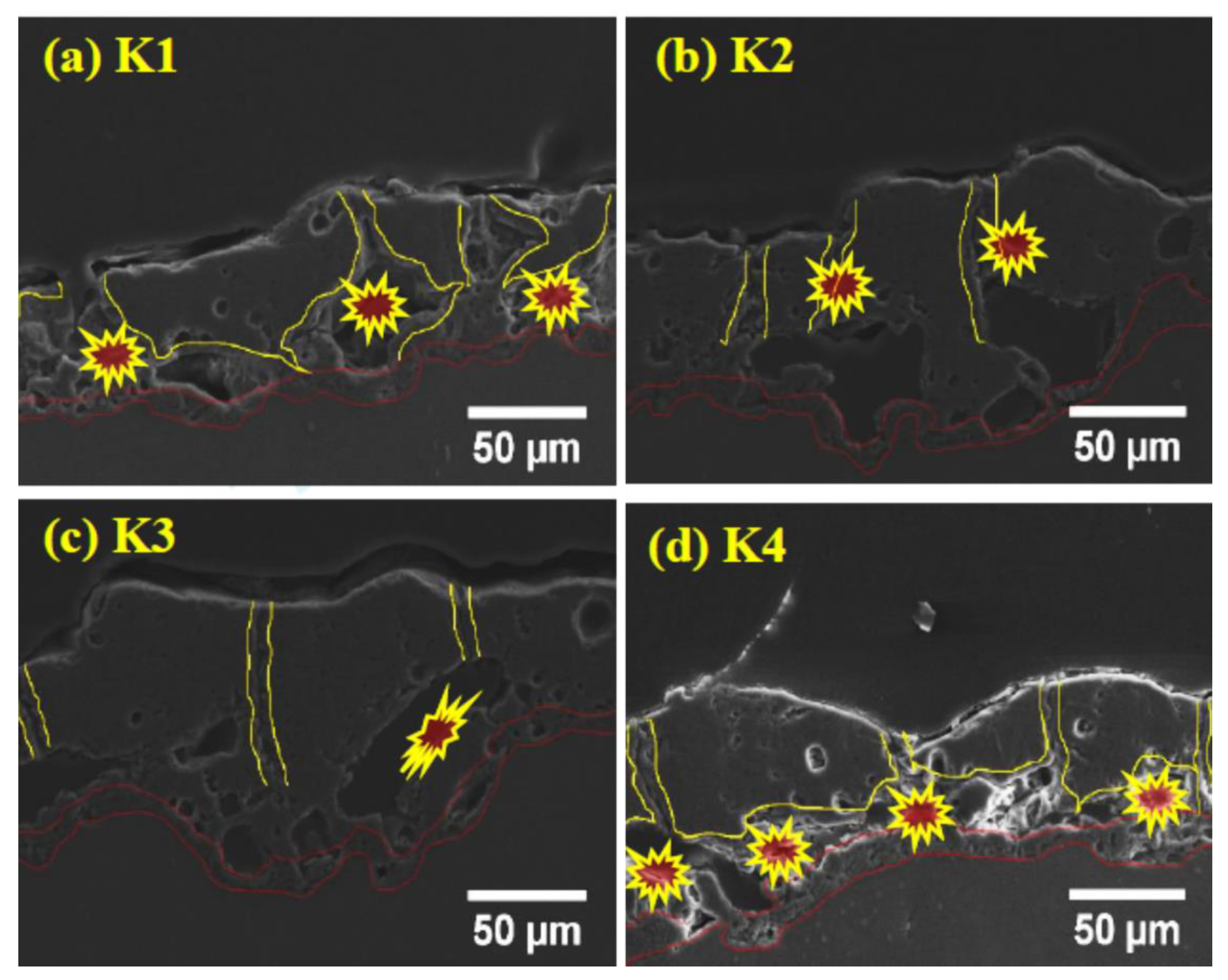
3.1. Microstructure
- Due to the finite solubility of K2ZrF6 at higher pH, a colloidal solution is formed, such dielectric particles of K2ZrF6 obstruct the path of the discharge channels.
- Due to the obstruction and presence of these particles in the discharge effective zones instead of long-time stable discharges, packets of intensive sparks occur randomly due to the breakdown of K2ZrF6-dominated local zones.
- Increasing the concentration could cause partial dissociation of the K2ZrF6 due to functionalization and electrophoretic processes, thus allowing the ZrF62- ion to play a part in the conductive transport of electrolyte constituents to the discharge channels and react with the cations thus, offering a minimum resistance and causing stable discharges.
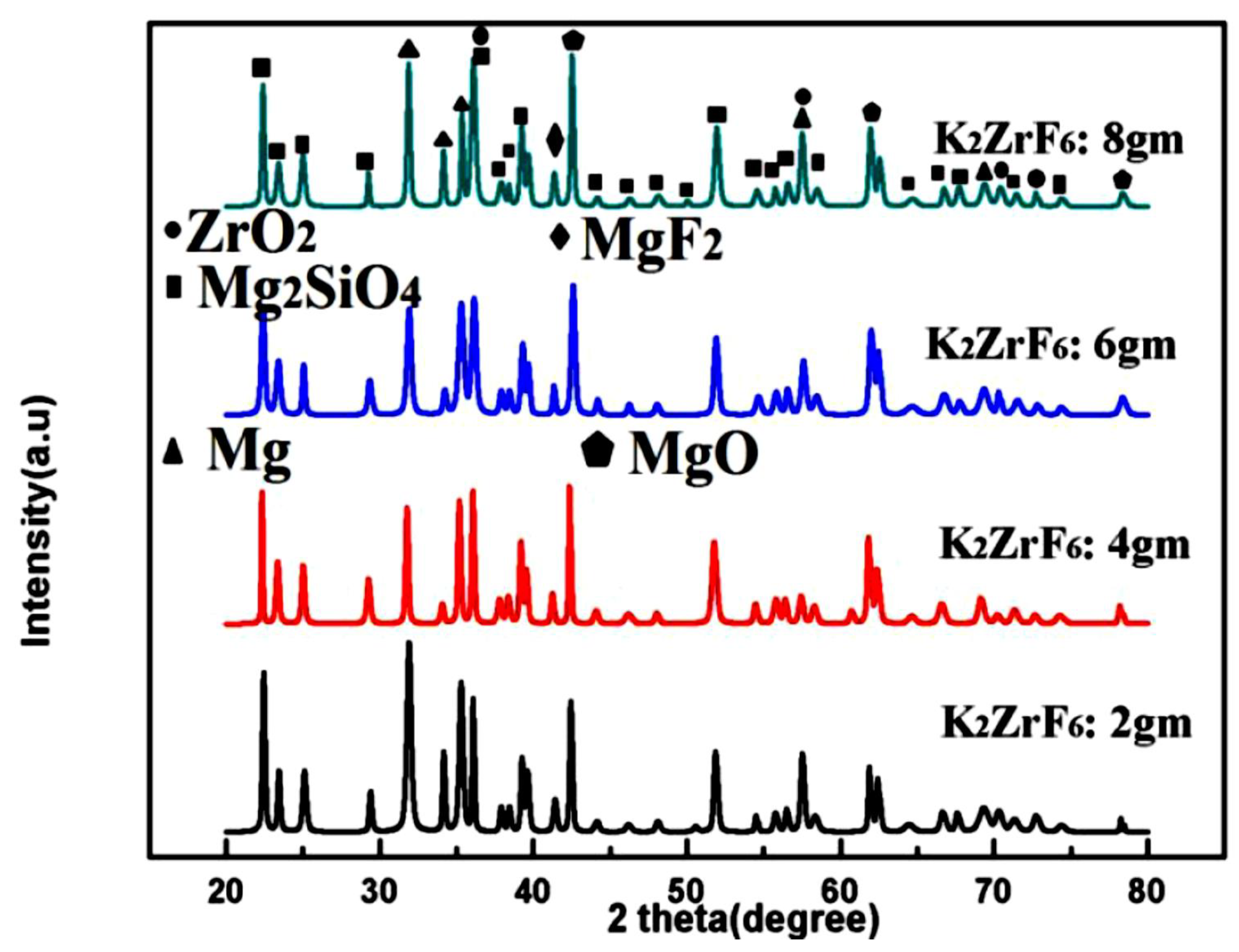
3.2. Phase Analysis
3.3. Hardness
3.4. Corrosion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohannad, M.S.; Bosta, A.; Kung-Jeng, M. Suggested mechanism for the MAO ceramic coating on aluminium substrates using bipolar current mode in the alkaline silicate electrolytes. Appl. Surf. Sci. 2014, 308, 121–138. [Google Scholar]
- Rehman, Z.U.; Koo, B.H. Combined effect of long processing time and Na2SiF6 on the properties of PEO coatings formed on AZ91D. J. Mater. Eng. Perform. 2016, 25, 3531–3537. [Google Scholar] [CrossRef]
- Shi, Z.; Song, G.; Atrens, A. Influence of Anodising Current on the Corrosion Resistance of Anodised AZ91D Magnesium Alloy. Corros. Sci. 2006, 48, 1939–1959. [Google Scholar] [CrossRef]
- Curran, J.A.; Clyne, T.W. Porosity in plasma electrolytic oxide coatings. Acta Mater. 2006, 54, 1985–1993. [Google Scholar] [CrossRef]
- Du, K.Q.; Guo, X.H.; Guo, Q.Z.; Wang, Y.; Wang, F.H.; Tian, Y. Effect of PEO Coating Microstructure on Corrosion of Al 2024. J. Electrochem. Soc. 2012, 159, C597–C606. [Google Scholar] [CrossRef]
- Hwanga, D.Y.; Kim, Y.M.; Park, D.Y.; Yoo, B.; Shin, D.H. Corrosion resistance of oxide layers formed on AZ91 Mg alloy in KMnO4 electrolyte by plasma electrolytic oxidation. Electrochim. Acta 2009, 54, 5479–5485. [Google Scholar] [CrossRef]
- Lv, G.H.; Chen, H.; Wang, X.Q.; Pang, H.; Zhang, G.L.; Zou, B.; Lee, H.J.; Yang, S.Z. Effect of additives on structure and corrosion resistance of plasma electrolytic oxidation coatings on AZ91D magnesium alloy in phosphate based electrolyte. Surf. Coat. Technol. 2010, 205, S36–S40. [Google Scholar] [CrossRef]
- Duan, H.P.; Yan, C.W.; Wang, F.H. Effect of electrolyte additives on performance of plasma electrolytic oxidation films formed on magnesium alloy AZ91D. Electrochim. Acta 2007, 52, 3785–3793. [Google Scholar] [CrossRef]
- Ko, Y.G.; Lee, E.S.; Shin, D.H. Influence of voltage waveform on anodic film of AZ91 Mg alloy via plasma electrolytic oxidation: Microstructural characteristics and electrochemical responses. J. Alloys Compd. 2014, 586, S357–S361. [Google Scholar] [CrossRef]
- Du, K.Q.; Guo, X.H.; Guo, Q.Z.; Wang, F.H.; Tian, Y. A monolayer PEO coating on 2024 Al alloy by transient self-feedback control mode. Mater. Lett. 2013, 91, 45–49. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Uzair, M.; Lim, H.T.; Koo, B.H. Structural and electrochemical properties of the catalytic CeO2 nanoparticles-based PEO ceramic coatings on AZ91 Mg alloy. J. Alloys Compd. 2017, 726, 284–294. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N.; Venkateswarlu, K.; Subrahmanyam, C.; Krishna, L.R.; Rao, K.P. Effect of K2TiF6 and Na2B4O7 as electrolyte additives on pore morphology and corrosion properties of plasma electrolytic oxidation coatings on ZM21 magnesium alloy. Surf. Coat. Technol. 2013, 222, 31–37. [Google Scholar] [CrossRef]
- Wang, L.Q.; Zhou, J.S.; Liang, J.; Chen, J.M. Corrosion Mechanism of Plasma Electrolytic Oxidation Coated Magnesium Alloy with Laser Surface Melting Pre-treatment. J. Electrochem. Soc. 2014, 161, C20–C24. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.M.; Zhou, Y.; Guo, L.X.; Ouyang, J.H. Microstructure and corrosion resistance of modified 2024 Al alloy using surface mechanical attrition treatment combined with microarc oxidation process. Corros. Sci. 2011, 53, 473–480. [Google Scholar] [CrossRef]
- Yang, W.; Xu, D.; Guo, Q.; Chen, T.; Chen, J. Influence of electrolyte composition on microstructure and properties of coatings formed on pure Ti substrate by micro arc oxidation. Surf. Coat. Technol. 2018, 349, 522–528. [Google Scholar] [CrossRef]
- Luo, H.; Cai, Q.; Wei, B.; Yu, B.; He, J.; Li, D. Study on the microstructure and corrosion resistance of ZrO2-containing ceramic coatings formed on magnesium alloy by plasma electrolytic oxidation. J. Alloys Compd. 2009, 474, 551–556. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Choi, D. Investigation of ZrO2 nanoparticles concentration and processing time effect on the localized PEO coatings formed on AZ91 alloy. J. Magnes. Alloys. 2019, 7, 555–565. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Ahn, B.H.; Jeong, Y.S.; Song, J.I.; Koo, B.H. The influence of various additives on the properties of PEO coatings formed on AZ31 Mg alloy. Surf. Rev. Lett. 2016, 23, 165006–165012. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Skeldon, P.; Thompson, G.E.; Pardo, A. Corrosion and Transport of Species during Plasma Electrolytic Oxidation of WE43-T6 Magnesium Alloy. J. Electrochem. Soc. 2008, 155, C101–C111. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Koo, B.H. Effect of Na2SiO3·5H2O concentration on the microstructure and corrosion properties of two-step PEO coatings formed on AZ91 alloy. Surf. Coat. Technol. 2017, 317, 117–124. [Google Scholar] [CrossRef]
- Yao, Z.; Su, P.; Shen, Q.; Ju, P.; Wu, C.; Zhai, Y.; Jiang, Z. Preparation of thermal control coatings on Ti alloy by plasma electrolytic oxidation in K2ZrF6 solution. Surf. Coat. Technol. 2015, 269, 273–278. [Google Scholar] [CrossRef]
- Dong, K.; Song, Y.; Shan, D.; Han, E.H. Corrosion behavior of a self-sealing pore micro-arc oxidation film on AM60 magnesium alloy. Corros. Sci. 2015, 100, 275–283. [Google Scholar] [CrossRef]
- Lee, K.M.; Ko, Y.G.; Shin, D.H. Microstructural characteristics of oxide layers formed on Mg–9 wt%Al–1 wt%Zn alloy via two-step plasma electrolytic oxidation. J. Alloys Compd. 2014, 615, S418–S422. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Aoki, Y.; Habazaki, H. Two-step plasma electrolytic oxidation of Ti–15V–3Al–3Cr–3Sn for wear-resistant and adhesive coating. Surf. Coat. Technol. 2011, 205, 4732–4740. [Google Scholar] [CrossRef] [Green Version]
- Einkhah, F.; Lee, K.M.; Sani, M.A.F.; Yoo, B.; Shin, D.H. Structure and corrosion behavior of oxide layer with Zr compounds on AZ31 Mg alloy processed by two-step plasma electrolytic oxidation. Surf. Coat. Technol. 2014, 238, 75–79. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. A spectroscopic and microstructural study of oxide coatings produced on a Ti–6Al–4V alloy by plasma electrolytic oxidation. Mater. Chem. Phys. 2012, 134, 484–492. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Matthews, A. DC plasma electrolytic oxidation of biodegradable cp-Mg: In-vitro corrosion studies. Surf. Coat. Technol. 2013, 234, 132–142. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Su, J.F.; Nie, X. A study of the interactive effects of hybrid current modes on the tribological properties of a PEO (plasma electrolytic oxidation) coated AM60B Mg-alloy. Surf. Coat. Technol. 2013, 215, 421–430. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Koo, B.H. Two-Step Plasma Electrolytic Oxidation Coatings on AZ91D Alloy in a K2ZrF6–Na2SiO3.10H2O Based Electrolyte Solution. Sci. Adv. Mater. 2018, 10, 109–114. [Google Scholar] [CrossRef]


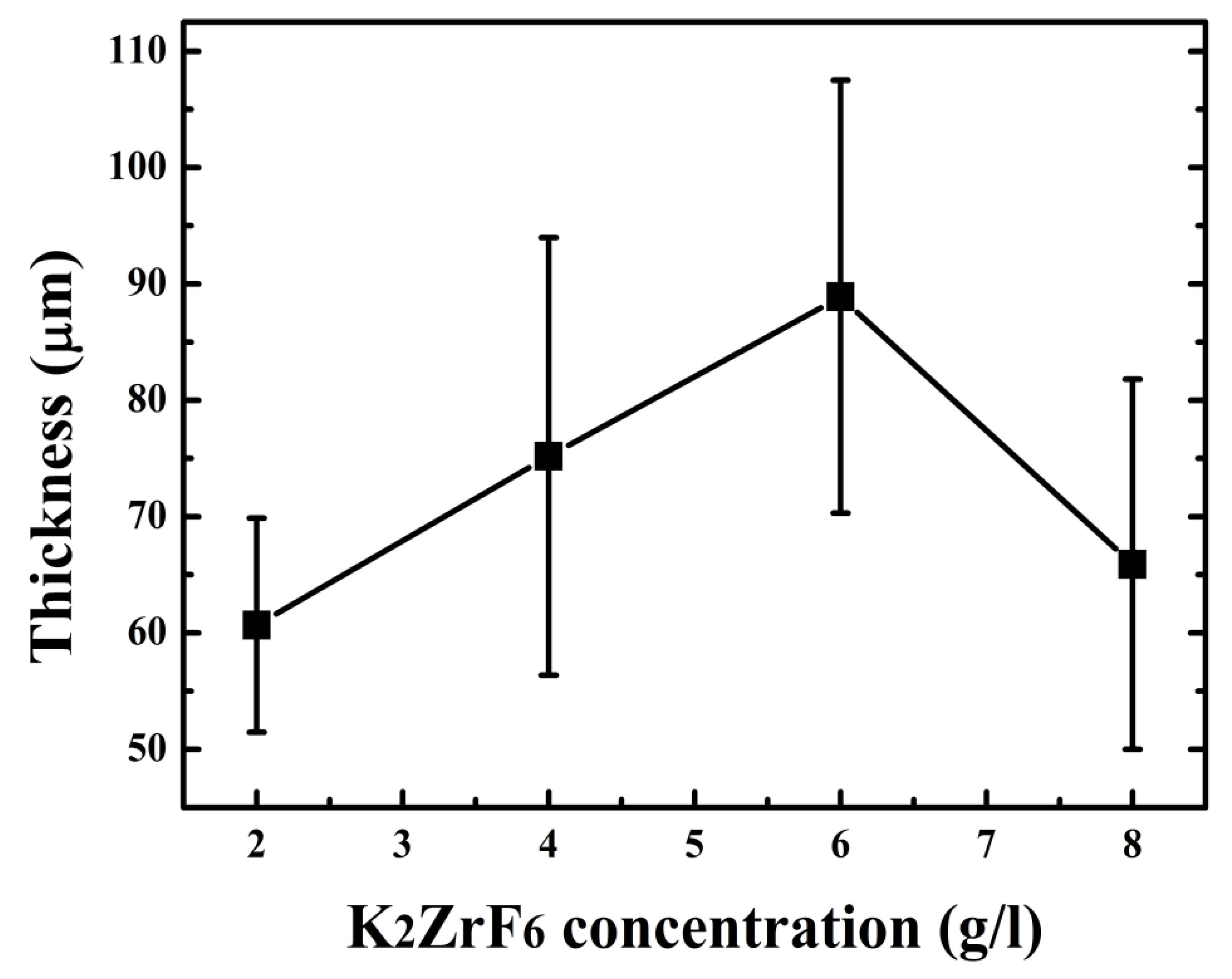
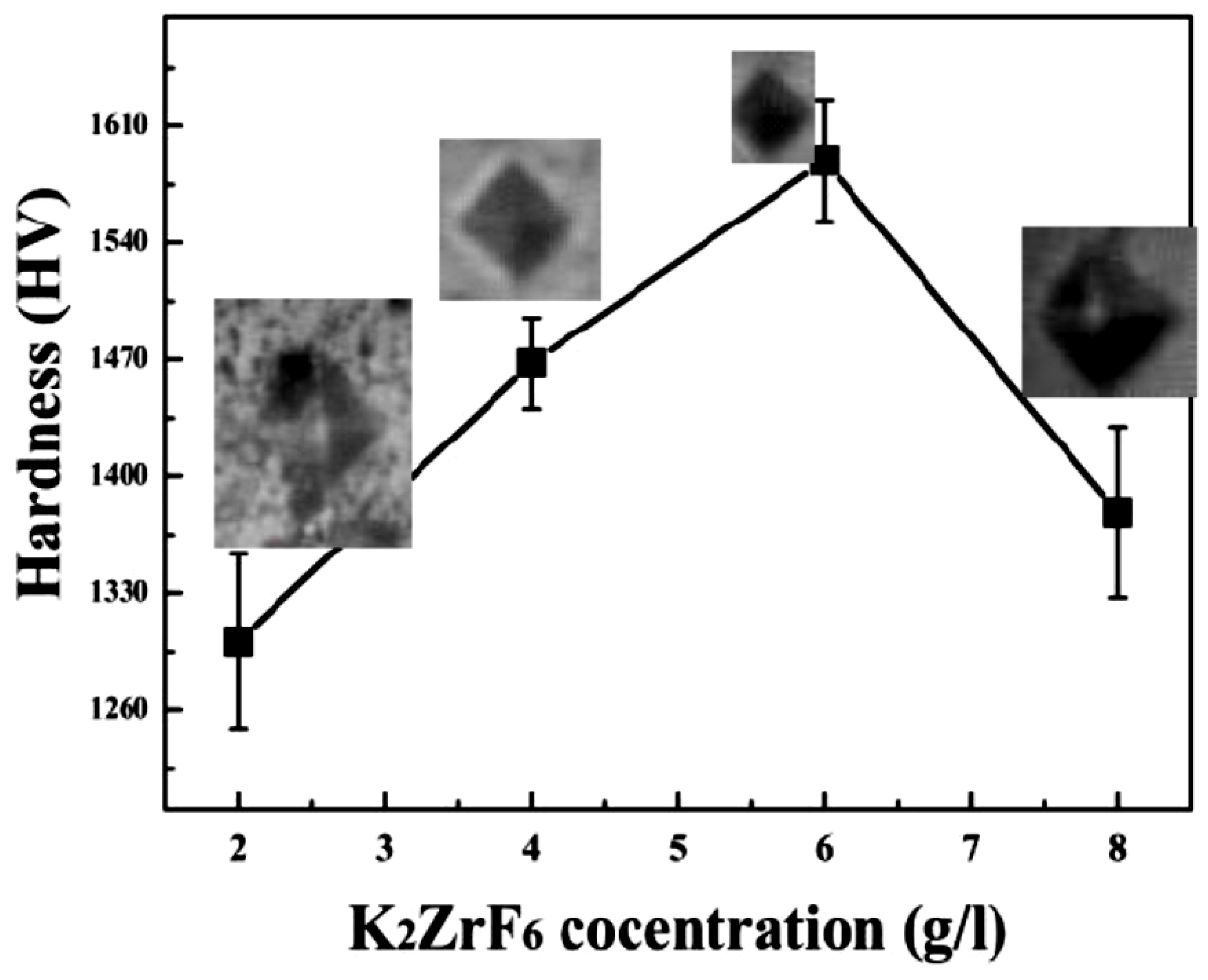
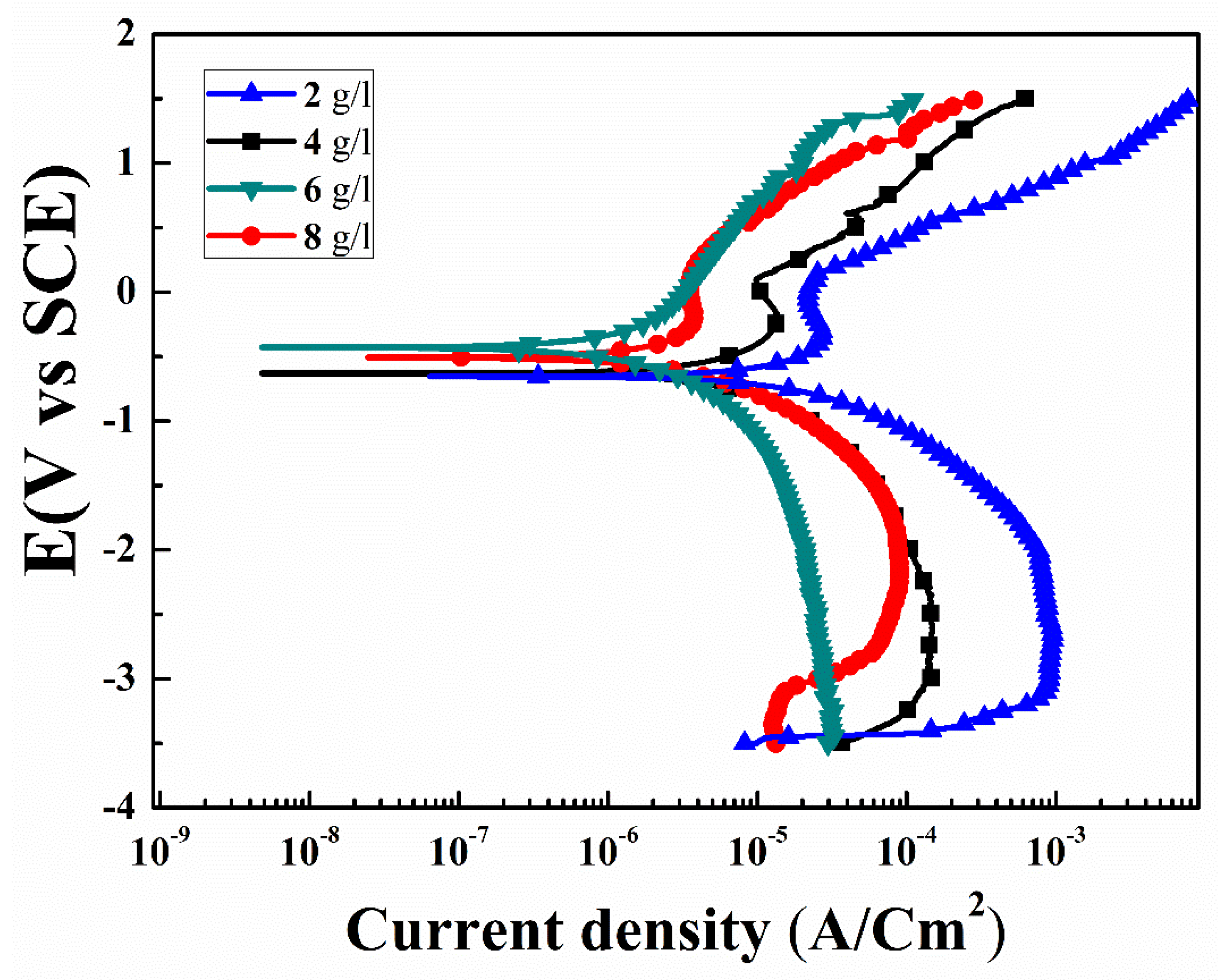
| Con (g/l): | βa (mV/d) | βc (mV/d) | Ecorr (V) | Icorr (A/cm2) | Rp (Ω.cm2) | Hardness (HV) |
|---|---|---|---|---|---|---|
| Primary | 47.3 | 67.4 | −0.96 | 28.1 × 10−8 | 42.9 | 875 |
| 2 | 45.6 | 46.4 | −0.65 | 94.1 × 10−8 | 10.6 × 103 | 1300.92 |
| 4 | 48.8 | 41.7 | −0.51 | 14.8×10−8 | 65.7 × 103 | 1467.82 |
| 6 | 41.2 | 57.5 | −0.28 | 2.08×10−8 | 386 × 103 | 1589.45 |
| 8 | 42.1 | 42.5 | −0.47 | 9.16×10−8 | 114 × 103 | 1378.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ur Rehman, Z.; Heun Koo, B.; Jung, Y.-G.; Lee, J.H.; Choi, D. Effect of K2ZrF6 Concentration on the Two-Step PEO Coating Prepared on AZ91 Mg Alloy in Alkaline Silicate Solution. Materials 2020, 13, 499. https://doi.org/10.3390/ma13030499
Ur Rehman Z, Heun Koo B, Jung Y-G, Lee JH, Choi D. Effect of K2ZrF6 Concentration on the Two-Step PEO Coating Prepared on AZ91 Mg Alloy in Alkaline Silicate Solution. Materials. 2020; 13(3):499. https://doi.org/10.3390/ma13030499
Chicago/Turabian StyleUr Rehman, Zeeshan, Bon Heun Koo, Yeon-Gil Jung, Je Hyun Lee, and Dongjin Choi. 2020. "Effect of K2ZrF6 Concentration on the Two-Step PEO Coating Prepared on AZ91 Mg Alloy in Alkaline Silicate Solution" Materials 13, no. 3: 499. https://doi.org/10.3390/ma13030499
APA StyleUr Rehman, Z., Heun Koo, B., Jung, Y.-G., Lee, J. H., & Choi, D. (2020). Effect of K2ZrF6 Concentration on the Two-Step PEO Coating Prepared on AZ91 Mg Alloy in Alkaline Silicate Solution. Materials, 13(3), 499. https://doi.org/10.3390/ma13030499




