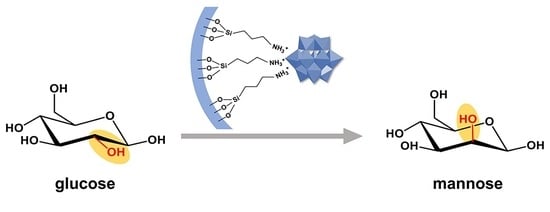
H3PMo12O40 Immobilized on Amine Functionalized SBA-15 as a Catalyst for Aldose Epimerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalysts
2.3. Catalyst Characterization
2.4. Catalytic Tests
3. Results and Discussion
3.1. Catalyst Characterization
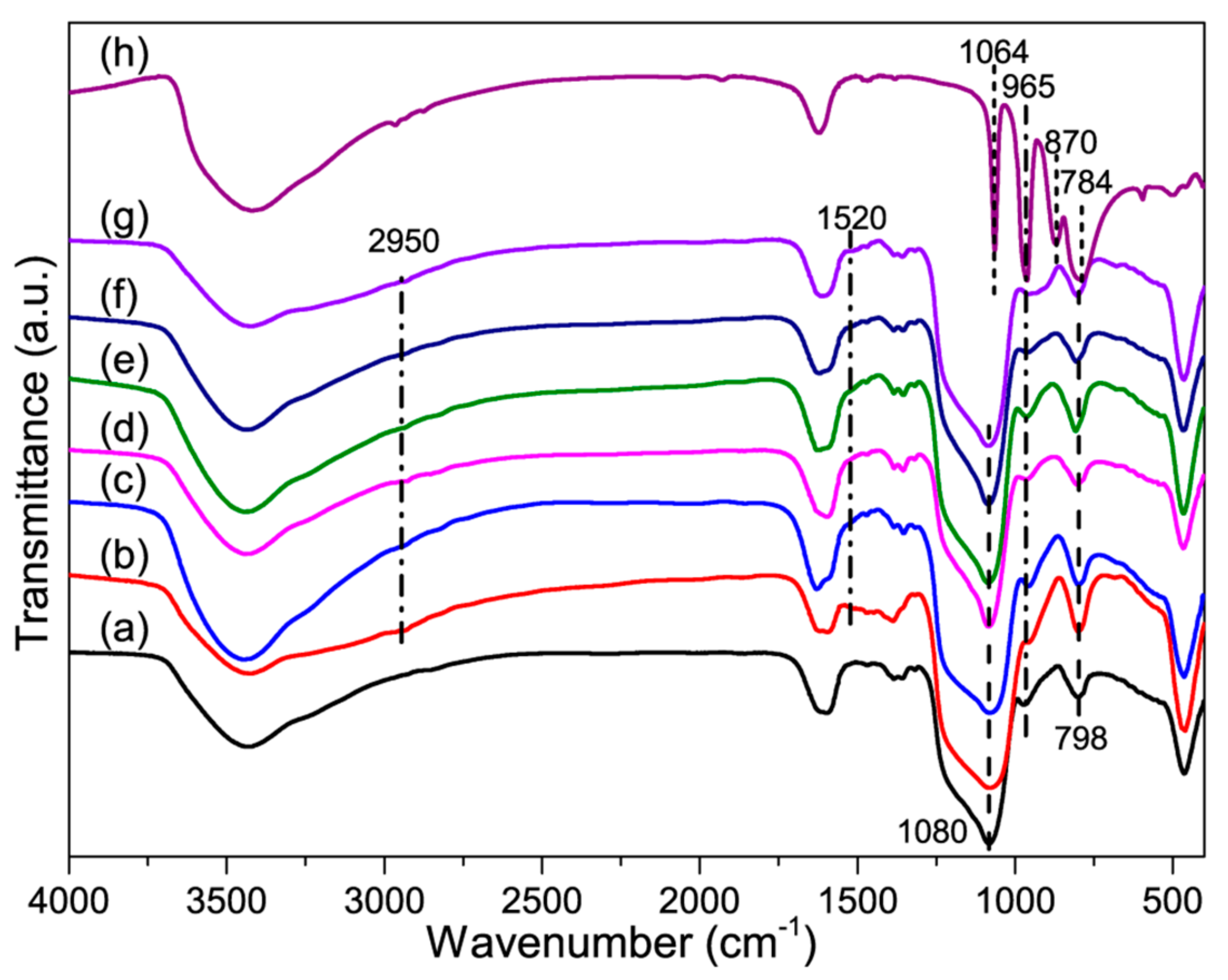
3.1.1. FT-IR Spectroscopy
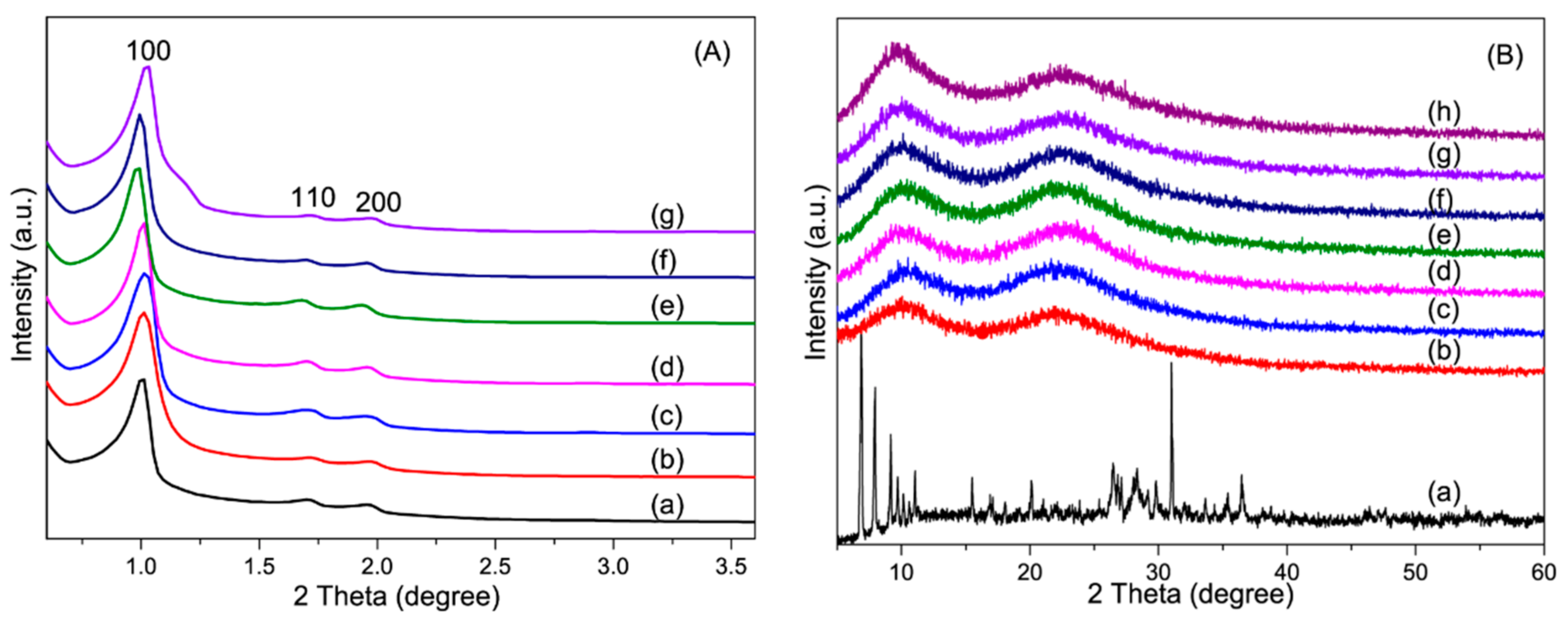
3.1.2. XRD
3.1.3. Nitrogen Adsorption–Desorption
3.1.4. 31P MAS NMR Spectroscopy
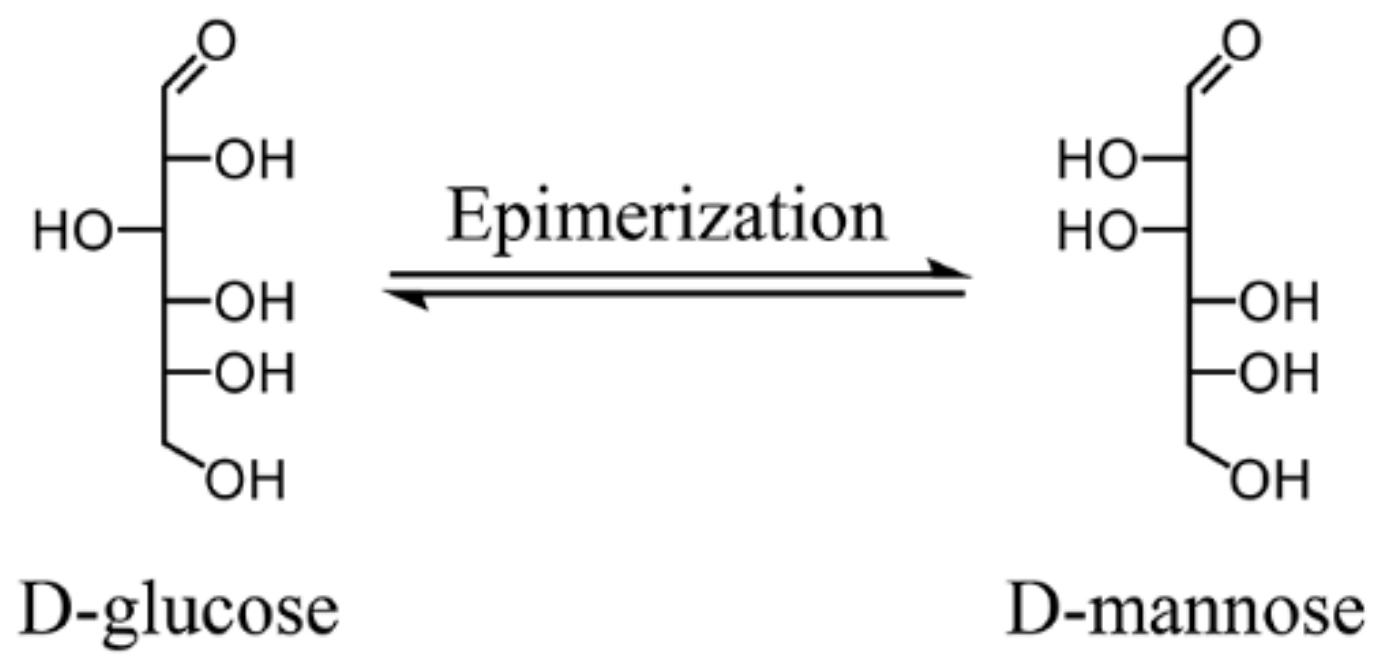
3.2. Catalytic Epimerization of Aldoses
3.2.1. Catalytic Activity of xPMo/NH2-SBA-15 for Glucose Epimerization
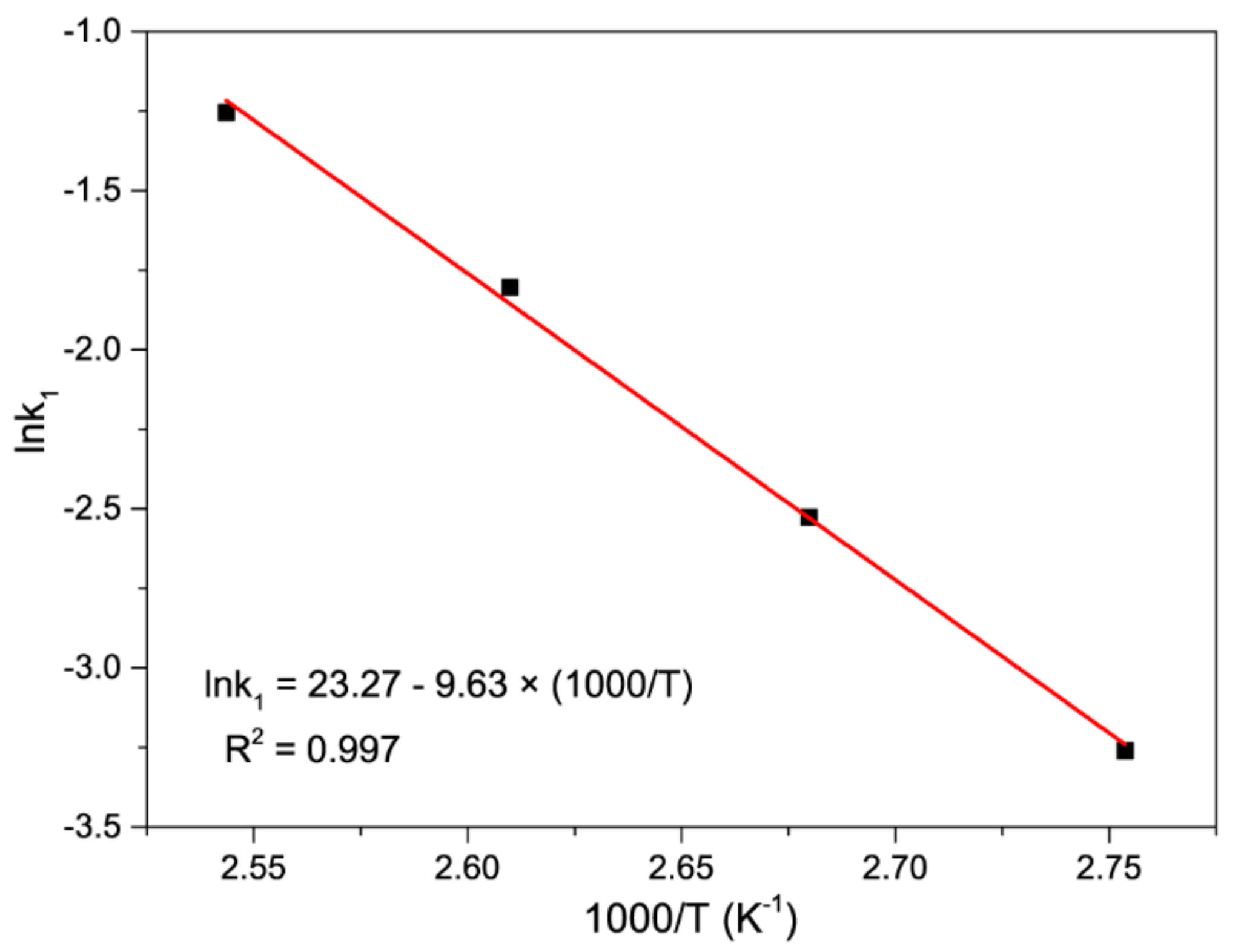
3.2.2. Calculation of the Activation Energy
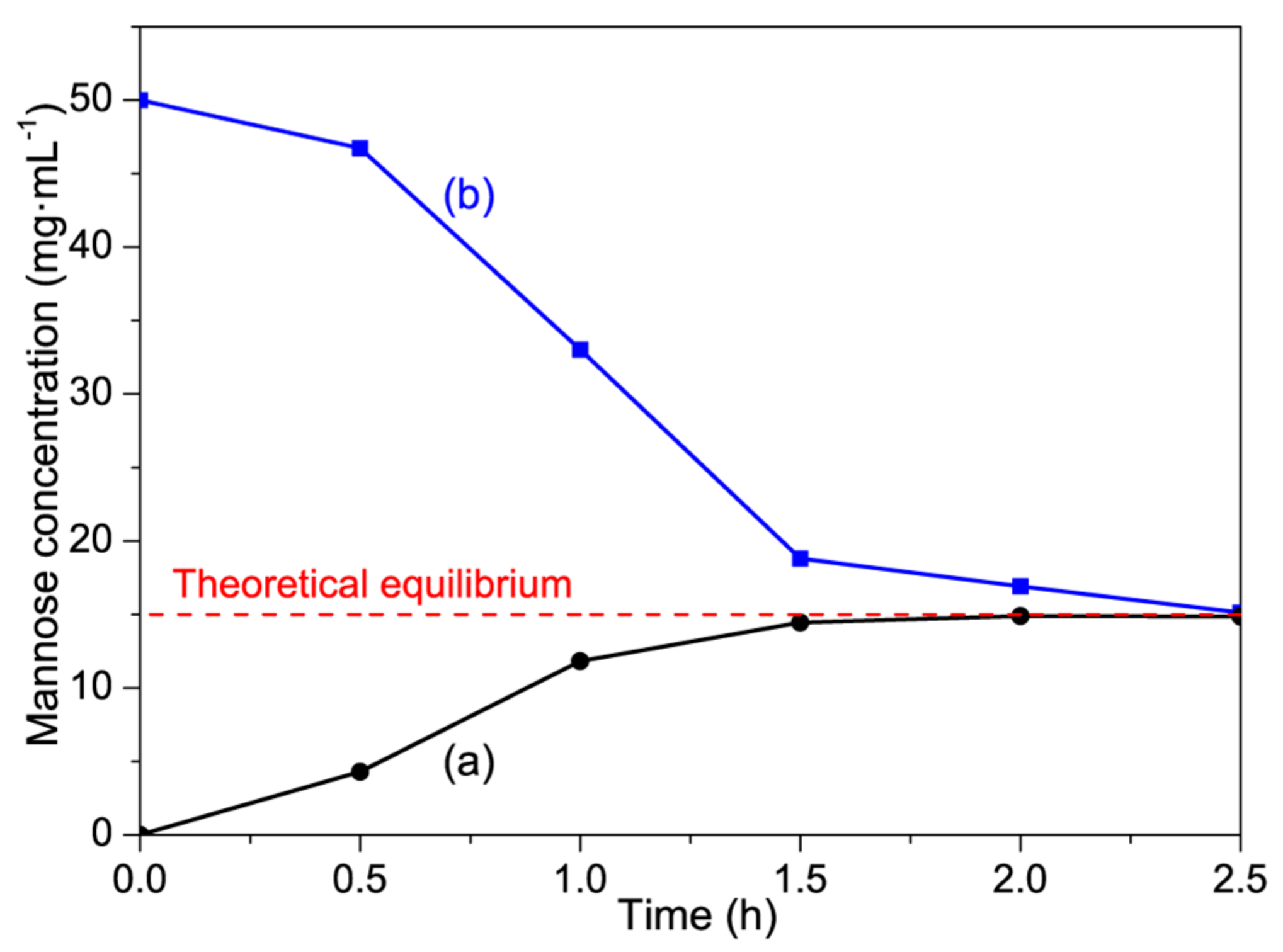
3.2.3. Catalytic Activity of 13.3PMo/NH2-SBA-15 for the Reverse Reaction
3.2.4. Reusability of the Catalyst for Glucose Epimerization
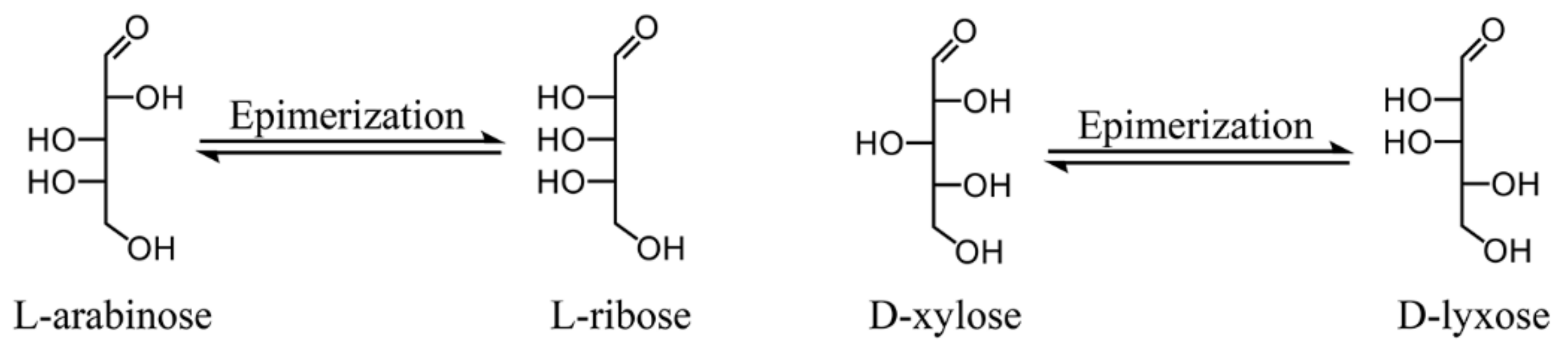
3.2.5. Catalytic Activity of 13.3PMo/NH2-SBA-15 for Other Aldoses Epimerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.L.; Pan, J.M.; Shen, Y.T.; Shi, W.D.; Liu, C.B.; Yu, L.B. Bronsted acidic polymer nanotubes with tunable wettability toward efficient conversion of one-pot cellulose to 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2015, 3, 871–879. [Google Scholar] [CrossRef]
- Yi, X.H.; Delidovich, I.; Sun, Z.; Wang, S.T.; Wang, X.H.; Palkovits, R. A heteropoly acid ionic crystal containing Cr as an active catalyst for dehydration of monosaccharides to produce 5-HMF in water. Catal. Sci. Technol. 2015, 5, 2496–2502. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Morales, I.; Moreno-Recio, M.; Santamaria-Gonzalez, J.; Maireles-Torres, P.; Jimenez-Lopez, A. Production of 5-hydroxymethylfurfural from glucose using aluminium doped MCM-41 silica as acid catalyst. Appl. Catal. B Environ. 2015, 164, 70–76. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic isomerization of biomass-derived aldoses: A review. ChemSusChem 2016, 9, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Marianou, A.A.; Michailof, C.M.; Ipsakis, D.K.; Karakoulia, S.A.; Kalogiannis, K.G.; Yiannoulakis, H.; Triantafyllidis, K.S.; Lappas, A.A. Isomerization of glucose into fructose over natural and synthetic MgO catalysts. ACS Sustain. Chem. Eng. 2018, 6, 16459–16470. [Google Scholar] [CrossRef]
- Ohara, M.; Takagaki, A.; Nishimura, S.; Ebitani, K. Syntheses of 5-hydroxymethylfurfural and levoglucosan by selective dehydration of glucose using solid acid and base catalysts. Appl. Catal. A Gen. 2010, 383, 149–155. [Google Scholar] [CrossRef]
- Jiao, H.F.; Zhao, X.L.; Lv, C.X.; Wang, Y.J.; Yang, D.J.; Li, Z.H.; Yao, X.D. Nb2O5-γ-Al2O3 nanofibers as heterogeneous catalysts for efficient conversion of glucose to 5-hydroxymethylfurfural. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.C.; Li, G.N.; Pidko, E.A.; Wiesfeld, J.J.; Rigutto, M.; Hensen, E.J.M. Dehydration of glucose to 5-hydroxymethylfurfural using Nb-doped tungstite. ChemSusChem 2016, 9, 2421–2429. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.W.; Pai, S.B.; Zhu, Y.L.; Lin, J.S.; Shanmuganathan, K.; Du, J.F.; Wang, C.G.; Kim, H.; Newton, M.G.; Cheng, Y.C.; et al. Structure-activity relationships of 1-(2-deoxy-2-fluoro-beta-L-arabinofuranosyl)pyrimidine nucleosides as anti-hepatitis B virus agents. J. Med. Chem. 1996, 39, 2835–2843. [Google Scholar] [CrossRef]
- Takagi, Y.; Nakai, K.; Tsuchiya, T.; Takeuchi, T. A 5’-(trifluoromethyl)anthracycline glycoside: Synthesis of antitumor-active 7-O-(2,6-dideoxy-6,6,6-trifluoro-alpha-L-lyxo-hexopyranosyl)adriamycinone. J. Med. Chem. 1996, 39, 1582–1588. [Google Scholar] [CrossRef]
- Cleland, W.W. What limits the rate of an enzyme-catalyzed reaction? Acc. Chem. Res. 1975, 8, 145–151. [Google Scholar] [CrossRef]
- Samuel, J.; Tanner, M.E. Mechanistic aspects of enzymatic carbohydrate epimerization. Nat. Prod. Rep. 2002, 19, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Angyal, S.J. A short note on the epimerization of aldoses. Carbohydr. Res. 1997, 300, 279–281. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Saravanamurugan, S.; Riisager, A. Glucose isomerization by enzymes and chemo-catalysts: Status and current advances. ACS Catal. 2017, 7, 3010–3029. [Google Scholar] [CrossRef]
- Dewit, G.; Kieboom, A.P.G.; Vanbekkum, H. Enolization and isomerization of monosaccharides in aqueous, alkaline-solution. Carbohydr. Res. 1979, 74, 157–175. [Google Scholar] [CrossRef]
- Moliner, M.; Roman-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef] [Green Version]
- Gunther, W.R.; Wang, Y.R.; Ji, Y.W.; Michaelis, V.K.; Hunt, S.T.; Griffin, R.G.; Roman-Leshkov, Y. Sn-Beta zeolites with borate salts catalyse the epimerization of carbohydrates via an intramolecular carbon shift. Nat. Commun. 2012, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bermejo-Deval, R.; Orazov, M.; Gounder, R.; Hwang, S.J.; Davis, M.E. Active sites in Sn-Beta for glucose isomerization to fructose and epimerization to mannose. ACS Catal. 2014, 4, 2288–2297. [Google Scholar] [CrossRef] [Green Version]
- Bilik, V. Reactions of saccharides catalyzed by molybdate ions. 2. Epimerization of D-glucose and D-mannose. Chem. Zvesti 1972, 26, 183–186. [Google Scholar]
- Bilik, V. Reactions of saccharides catalyzed by molybdate ions. 4. Epimerization of aldopentose. Chem. Zvesti 1972, 26, 372–375. [Google Scholar]
- Bilik, V.; Caplovic, J. Reactions of saccharides catalyzed by molybdate ions. 7. Preparation of L-ribose, D- and L-lyxose. Chem. Zvesti 1973, 27, 547–550. [Google Scholar]
- Bilik, V.; Matulova, M. Reactions of saccharides catalyzed by molybdate ions. 42. Epimerization and the molybdate complex of the aldoses. Chem. Pap. 1990, 44, 257–265. [Google Scholar]
- Petrus, L.; Petrusova, M.; Hricoviniova, Z. The Bilik reaction. Glycoscience 2001, 215, 15–41. [Google Scholar] [CrossRef]
- Chethana, B.K.; Lee, D.; Mushrif, S.H. First principles investigation into the metal catalysed 1,2 carbon shift reaction for the epimerization of sugars. J. Mol. Catal. A Chem. 2015, 410, 66–73. [Google Scholar] [CrossRef]
- Clark, E.L.; Hayes, M.L.; Barker, R. Paramolybdate anion-exchange resin, an improved catalyst for the C-1-C-2 rearangement and 2-epimerization of aldoses. Carbohydr. Res. 1986, 153, 263–270. [Google Scholar] [CrossRef]
- Stockman, R.; Dekoninck, J.; Sels, B.F.; Jacobs, P.A. The catalytic epimerization of sugars over immobilized heptamolybdate: Comparison of resins, layered double hydroxides and mesoporous silica as support. Stud. Surf. Sci. Catal. 2005, 156, 843–850. [Google Scholar] [CrossRef]
- Kockritz, A.; Kant, M.; Walter, M.; Martin, A. Rearrangement of glucose to mannose catalysed by polymer-supported Mo catalysts in the liquid phase. Appl. Catal. A Gen. 2008, 334, 112–118. [Google Scholar] [CrossRef]
- Takagaki, A.; Furusato, S.; Kikuchi, R.; Oyama, S.T. Efficient epimerization of aldoses using layered niobium molybdates. ChemSusChem 2015, 8, 3769–3772. [Google Scholar] [CrossRef]
- Ju, F.; VanderVelde, D.; Nikolla, E. Molybdenum-based polyoxometalates as highly active and selective catalysts for the epimerization of aldoses. ACS Catal. 2014, 4, 1358–1364. [Google Scholar] [CrossRef] [Green Version]
- Lari, G.M.; Groninger, O.G.; Mondelli, C.; Perez-Ramirez, J.; Li, Q.; Lopez, N. Catalyst and process design for the continuous manufacture of rare sugar alcohols by epimerization-hydrogenation of aldoses. ChemSusChem 2016, 9, 3373. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, E.; Eavani, S. Heterogenization of heteropoly compounds: A review of their structure and synthesis. RSC Adv. 2016, 6, 46433–46466. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Sutra, P.; Brunel, D. Preparation of MCM-41 type silica-bound manganese(III) Schiff-base complexes. Chem. Commun. 1996, 2485–2486. [Google Scholar] [CrossRef]
- Kim, H.; Jung, J.C.; Yeom, S.H.; Lee, K.Y.; Yi, J.; Song, I.K. Immobilization of a heteropolyacid catalyst on the aminopropyl-functionalized mesostructured cellular foam (MCF) silica. Mater. Res. Bull. 2007, 42, 2132–2142. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, H.; Ren, Y.H.; Wang, C.; Weng, Z.W.; Yue, B.; He, H.Y. Construction of g-C3N4-mNb2O5 composites with enhanced visible light photocatalytic activity. Nanomaterials 2018, 8, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.X.; Jin, Q.Z.; Shan, L.; Liu, Y.F.; Wang, X.G.; Huang, J.H. H3PW12O40 immobilized on silylated palygorskite and catalytic activity in esterification reactions. Appl. Clay. Sci. 2010, 47, 229–234. [Google Scholar] [CrossRef]
- Chen, C.; Xu, J.; Zhang, Q.H.; Ma, H.; Miao, H.; Zhou, L.P. Direct synthesis of bifunctionalized hexagonal mesoporous silicas and its catalytic performance for aerobic oxidation of cyclohexane. J. Phys. Chem. C 2009, 113, 2855–2860. [Google Scholar] [CrossRef]
- Villanneau, R.; Marzouk, A.; Wang, Y.; Ben Djamaa, A.; Laugel, G.; Proust, A.; Launay, F. Covalent grafting of organic-inorganic polyoxometalates hybrids onto mesoporous SBA-15: A key step for new anchored homogeneous catalysts. Inorg. Chem. 2013, 52, 2958–2965. [Google Scholar] [CrossRef]
- Kharat, A.N.; Moosavikia, S.; Jahromi, B.T.; Badiei, A. Liquid phase hydroxylation of benzene to phenol over vanadium substituted Keggin anion supported on amine functionalized SBA-15. J. Mol. Catal. A Chem. 2011, 348, 14–19. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari-Siahkali, A.; Philippou, A.; Dwyer, J.; Anderson, M.W. The acidity and catalytic activity of heteropoly acid on MCM-41 investigated by MAS NMR, FTIR and catalytic tests. Appl. Catal. A Gen. 2000, 192, 57–69. [Google Scholar] [CrossRef]
- Raj, N.K.K.; Deshpande, S.S.; Ingle, R.H.; Raja, T.; Manikandan, P. Heterogenized molybdovanadophosphoric acid on amine-functionalized SBA-15 for selective oxidation of alkenes. Catal. Lett. 2004, 98, 217–223. [Google Scholar] [CrossRef]
- Carraher, J.M.; Fleitman, C.N.; Tessonnier, J.P. Kinetic and Mechanistic Study of Glucose Isomerization Using Homogeneous Organic Bronsted Base Catalysts in Water. ACS Catal. 2015, 5, 3162–3173. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Carraher, J.M.; Swedberg, J.L.; Herndon, C.R.; Fleitman, C.N.; Tessonnier, J.P. Selective base-catalyzed isomerization of glucose to fructose. ACS Catal. 2014, 4, 4295–4298. [Google Scholar] [CrossRef]
- Kamada, M.; Kominami, H.; Kera, Y. Deposition and interaction of phosphododecatungstate on a silica gel surface modified with a silane coupling agent having anilino groups. J. Colloid. Interface Sci. 1996, 182, 297–300. [Google Scholar] [CrossRef]
- Angyal, S.J. The composition and conformation of sugars in solution. Angew. Chem. Int. Ed. Engl. 1969, 8, 157–166. [Google Scholar] [CrossRef]
- Delidovich, I.; Hoffmann, A.; Willms, A.; Rose, M. Porous tin-organic frameworks as selective epimerization catalysts in aqueous solutions. ACS Catal. 2017, 7, 3792–3798. [Google Scholar] [CrossRef]
- Rellan-Pineiro, M.; Garcia-Rates, M.; Lopez, N. A mechanism for the selective epimerization of the glucose mannose pair by Mo-based compounds: Towards catalyst optimization. Green Chem. 2017, 19, 5932–5939. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.L.; Pennings, N.J.; Serianni, A.S.; Barker, R. Epimerization of aldoses by molybdate involving a novel rearrangement of the carbon skeleton. J. Am. Chem. Soc. 1982, 104, 6764–6769. [Google Scholar] [CrossRef]


| Samples | Nitrogen Content a (wt%) | Molybdenum Content b (wt%) | SBET (m2·g−1) | Dpore (nm) | Vpore (cm3·g−1) |
|---|---|---|---|---|---|
| SBA-15 | - | - | 599 | 5.5 | 0.80 |
| NH2-SBA-15 | 1.6 | - | 343 | 5.1 | 0.54 |
| 3.3PMo/NH2-SBA-15 | 1.1 | 0.6 | 337 | 5.1 | 0.53 |
| 6.7PMo/NH2-SBA-15 | 1.1 | 1.4 | 326 | 5.1 | 0.53 |
| 10PMo/NH2-SBA-15 | 1.1 | 3.0 | 304 | 5.1 | 0.51 |
| 13.3PMo/NH2-SBA-15 | 1.2 | 5.4 | 286 | 5.1 | 0.50 |
| 16.7PMo/NH2-SBA-15 | 1.1 | 6.3 | 267 | 5.1 | 0.46 |
| Entry | Catalyst | Glucose Conversion (%) | Mannose Yield b (%) | Mannose Selectivity (%) | Fructose Yield (%) | Fructose Selectivity (%) |
|---|---|---|---|---|---|---|
| 1 | −c | 0 | 0 | 0 | 0 | 0 |
| 2 | SBA-15 | 0 | 0 | 0 | 0 | 0 |
| 3 | NH2-SBA-15 | 8.9 ± 0.1 | 0.5 ± 0.1 | 5.6 | 3.4 ± 0.1 | 38.2 |
| 4 | 3.3PMo/NH2-SBA-15 | 6.8 ± 0.2 | 2.0 ± 0.1 | 29.4 | 0 | 0 |
| 5 | 6.7PMo/NH2-SBA-15 | 7.4 ± 0.1 | 3.8 ± 0.1 | 51.4 | 0 | 0 |
| 6 | 10PMo/NH2-SBA-15 | 27.2 ± 0.2 | 22.9 ± 0.3 | 84.2 | 0 | 0 |
| 7 | 13.3PMo/NH2-SBA-15 | 34.8 ± 0.2 | 29.8 ± 0.1 | 85.6 | 0 | 0 |
| 8 | 16.7PMo/NH2-SBA-15 | 35.3 ± 0.1 | 29.7 ± 0.1 | 84.1 | 0 | 0 |
| Entry | Sugar | Conversion (%) | Epimer Yield (%) | Epimer Selectivity (%) |
|---|---|---|---|---|
| 1 | Glucose | 34.8 ± 0.2 | 29.8 ± 0.1 | 85.6 |
| 2 | Mannose | 66.2 ± 0.1 | 60.5 ± 0.1 | 91.4 |
| 3 | Arabinose | 27.7 ± 0.2 | 15.9 ± 0.3 | 57.4 |
| 4 | Xylose | 44.6 ± 0.1 | 33.3 ± 0.2 | 74.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, M.; Shang, J.; Ren, Y.; Yue, B.; He, H. H3PMo12O40 Immobilized on Amine Functionalized SBA-15 as a Catalyst for Aldose Epimerization. Materials 2020, 13, 507. https://doi.org/10.3390/ma13030507
Wang H, Wang M, Shang J, Ren Y, Yue B, He H. H3PMo12O40 Immobilized on Amine Functionalized SBA-15 as a Catalyst for Aldose Epimerization. Materials. 2020; 13(3):507. https://doi.org/10.3390/ma13030507
Chicago/Turabian StyleWang, Hui, Meiyin Wang, Jining Shang, Yuanhang Ren, Bin Yue, and Heyong He. 2020. "H3PMo12O40 Immobilized on Amine Functionalized SBA-15 as a Catalyst for Aldose Epimerization" Materials 13, no. 3: 507. https://doi.org/10.3390/ma13030507