Preparation and Characterization of the Flame Retardant Decorated Plywood Based on the Intumescent Flame Retardant Adhesive
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
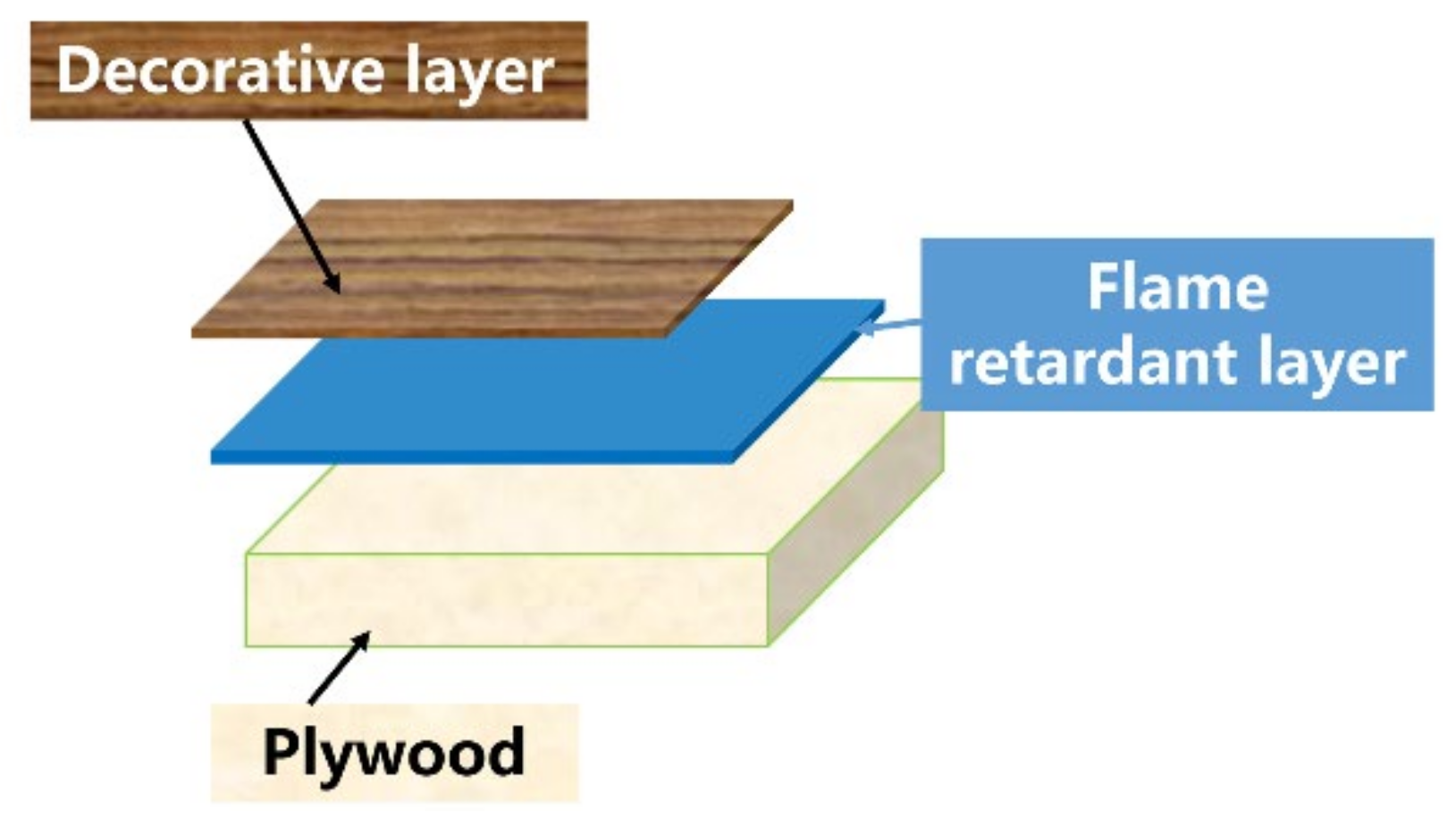
2.2. Sample Preparation
2.3. Measurements and Characterization
3. Results and Discussion
3.1. Shear Strength Test
3.2. Thermal Degradation Behavior of the Adhesive
3.3. Intumescent Behaviors of Adhesives
3.4. FTIR of Adhesives
3.5. Cone Calorimeter Measurements
3.6. Single Burning Item Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, L.; Chen, Z.L.; Fu, F.; Fan, M.Z. Investigation of Factory Fire Retardant Treatment of Eucalyptus Plywood. For. Prod. J. 2015, 65, 320–326. [Google Scholar]
- Wang, Y.C.; Zhao, J.P. Preliminary study on decanoic/palmitic eutectic mixture modified silica fume geopolymer-based coating for flame retardant plywood. Constr. Build. Mater. 2018, 189, 1–7. [Google Scholar] [CrossRef]
- Chuang, C.S.; Yang, T.H.; Tsai, K.C.; Tseng, T.Y.; Wang, M.K. Fire retardancy and CO/CO2 emission of intumescent coatings on thin plywood panel with waterborne vinyl acetate-acrylic resin. Wood Sci. Technol. 2013, 47, 353–367. [Google Scholar] [CrossRef]
- Cheng, R.X.; Wang, Q.W. The Influence of FRW-1 Fire Retardant Treatment on the Bonding of Plywood. J. Adhes. Sci. Technol. 2011, 25, 1715–1724. [Google Scholar] [CrossRef]
- Su, W.Y.; Subyakto, S.; Hata, T.; Nishimiya, K.; Imamura, Y.; Ishihara, S. Improvement of fire retardancy of plywood by incorporating boron or phosphate compounds in the glue. J. Wood Sci. 1998, 44, 131–136. [Google Scholar] [CrossRef]
- Wang, W.; Zammarano, M.; Shields, J.R.; Knowlton, E.D.; Kim, I.; Gales, J.A.; Hoehler, M.S.; Li, J.Z. A novel application of silicone-based flame-retardant adhesive in plywood. Constr. Build. Mater. 2018, 189, 448–459. [Google Scholar] [CrossRef]
- Maciulaitis, R.; Grigonis, M.; Malaiskiene, J. The impact of the aging of intumescent fire protective coatings on fire resistance. Fire Saf. J. 2018, 98, 15–23. [Google Scholar] [CrossRef]
- Stefansson, P.; Burud, I.; Thiis, T.; Gobakken, L.; Larnøy, E. Estimation of phosphorus-based flame retardant in wood by hyperspectral imaging—A new method. J. Spectr. Imaging 2018, 7, a3. [Google Scholar] [CrossRef]
- Demır, A.; Aydın, I.; Salca, E.A. Some technological properties of plywood after fire retardant treatment in different concentrations. Pro Ligno 2017, 13, 40–45. [Google Scholar]
- Grexa, O.; Horváthová, E.; Bešinová, O.G.; Lehocký, P. Flame retardant treated plywood. Polym. Degrad. Stab. 1999, 64, 529–533. [Google Scholar] [CrossRef]
- Kandelbauer, A.; Petek, P.; Medved, S.; Pizzi, A.; Teischinger, A. On the performance of a melamine–urea–formaldehyde resin for decorative paper coatings. Eur. J. Wood Wood Prod. 2010, 68, 63–75. [Google Scholar] [CrossRef]
- China Forestry Statistical Yearbook; China Forestry Publishing House: Beijing, China, 2018.
- Wang, Y.; Hou, M.; Zhang, Q.; Wu, X.; Zhao, H.; Xie, Q.; Chen, J. Organophosphorus Flame Retardants and Plasticizers in Building and Decoration Materials and Their Potential Burdens in Newly Decorated Houses in China. Environ. Sci. Technol. 2017, 51, 10991–10999. [Google Scholar] [CrossRef] [PubMed]
- Kulhavy, P.; Martinec, T.; Novak, O.; Petru, M.; Srb, P. Testing fireproof materials in a combustion chamber. EPJ Web Conf. 2017, 143, 02058. [Google Scholar] [CrossRef]
- Weil, E.D. Fire-Protective and Flame-Retardant Coatings-A State-of-the-Art Review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Gu, J.W.; Zhang, G.C.; Dong, S.L.; Zhang, Q.Y.; Kong, J. Study on preparation and fire-retardant mechanism analysis of intumescent flame-retardant coatings. Surf. Coat. Technol. 2007, 201, 7835–7841. [Google Scholar] [CrossRef]
- Duquesne, S.; Magnet, S.; Jama, C.; Delobel, R. Thermoplastic resins for thin film intumescent coatings-towards a better understanding of their effect on intumescence efficiency. Polym. Degrad. Stab. 2005, 88, 63–69. [Google Scholar] [CrossRef]
- Jimenez, M.; Bellayer, S.; Revel, B.; Duquesne, S.; Bourbigot, S. Comprehensive Study of the Influence of Different Aging Scenarios on the Fire Protective Behavior of an Epoxy Based Intumescent Coating. Ind. Eng. Chem. Res. 2013, 52, 729–743. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, S.; Zhang, Z.; Mai, C.; Xie, Y.; Wang, Q. Fire retardancy of an aqueous, intumescent, and translucent wood varnish based on guanylurea phosphate and melamine-urea-formaldehyde resin. Prog. Org. Coat. 2018, 121, 64–72. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Z.; Hu, Y. Microencapsulated ammonium polyphosphate with urea–melamine–formaldehyde shell: Preparation, characterization, and its flame retardance in polypropylene. Polym. Adv. Technol. 2008, 19, 1118–1125. [Google Scholar] [CrossRef]
- Ma, X.-X.; Wu, Y.-Z.; Zhu, H.-L. The fire-retardant properties of the melamine-modified urea–formaldehyde resins mixed with ammonium polyphosphate. J. Wood Sci. 2013, 59, 419–425. [Google Scholar] [CrossRef]
- ISO 5660-1: Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method); Internationan Organization for Standardization: Geneva, Switzerland, 2002.
- Kawalerczyk, J.; Dziurka, D.; Mirski, R.; Trocinski, A. Flour Fillers with Urea-Formaldehyde Resin in Plywood. Bioresources 2019, 14, 6727–6735. [Google Scholar]
- Demir, A.; Aydin, I.; Colak, S. Effect of various fire retardant chemicals in different concentrations on formaldehyde emission of plywood. Kastamonu Univ. Fac. For. J. 2017, 17, 509–516. [Google Scholar] [CrossRef][Green Version]
- Wang, L.L.; Wang, Y.C.; Yuan, J.F.; Li, G.Q. Thermal conductivity of intumescent coating char after accelerated aging. Fire Mater. 2013, 37, 440–456. [Google Scholar] [CrossRef]
- Li, G.X.; Yang, J.F.; He, T.S.; Wu, Y.H.; Liang, G.Z. An Investigation of the thermal degradation of the intumescent coating containing MoO3 and Fe2O3. Surf. Coat. Technol. 2008, 202, 3121–3128. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A.S. Intumescent coatings: A review on recent progress. J. Coat. Technol. Res. 2017, 14, 1–20. [Google Scholar] [CrossRef]
- Gravit, M.; Gumenyuk, V.; Sychov, M.; Nedryshkin, O. Estimation of the Pores Dimensions of Intumescent Coatings for Increase the Fire Resistance of Building Structures. Procedia Eng. 2015, 117, 119–125. [Google Scholar] [CrossRef]
- Wang, T.S.; Liu, T.; Ma, T.T.; Li, L.P.; Wang, Q.W.; Guo, C.G. Study on degradation of phosphorus and nitrogen composite UV-cured flame retardant coating on wood surface. Prog. Org. Coat. 2018, 124, 240–248. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.F.; Tai, Q.L.; Huang, H.; Hu, Y. Modification of lignin and its application as char agent in intumescent flame-retardant poly(lactic acid). Polym. Eng. Sci. 2012, 52, 2620–2626. [Google Scholar] [CrossRef]
- Lei, Z.; Cao, Y.; Xie, F.; Ren, H. Study on surface modification and flame retardants properties of ammonium polyphosphate for polypropylene. J. Appl. Polym. Sci. 2012, 124, 781–788. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Wang, X.M.; Zhang, S.F.; Gao, Q.; Li, J.Z. Effects of Melamine Addition Stage on the Performance and Curing Behavior of Melamine-Urea-Formaldehyde (MUF) Resin. Bioresources 2013, 8, 5500–5514. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, W.; Zhou, J.; Li, H.; Wang, X.; Chen, X.; Zhang, Z. Effects of microencapsulated APP-II on the microstructure and flame retardancy of PP/APP–II/PER composites. Polym. Degrad. Stab. 2014, 105, 150–159. [Google Scholar] [CrossRef]
- Vakhitova, L.N.; Taran, N.A.; Lapushkin, M.P.; Drizhd, V.L.; Lakhtarenko, N.V.; Popov, A.F. Solid-phase aminolysis in the ammonium polyphosphate-pentaerythritol-amine system. Theor. Exp. Chem. 2012, 48, 176–181. [Google Scholar] [CrossRef]
- Redfern, J.P. Rate of Heat Release Measurement Using the Cone Calorimeter. J. Therm. Anal. 1989, 35, 1861–1877. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, X.; Liu, G. Synthesis of an integrated intumescent flame retardant and its flame retardancy properties for polypropylene. Polym. Degrad. Stab. 2017, 138, 106–114. [Google Scholar] [CrossRef]
- Zhang, J.P.; Delichatsios, M.A.; McKee, M.; Ukleja, S. Experimental and numerical study of burning behaviors of flaxboard with intumescent coating and nanoparticles in the cone calorimeter and single burning item tests. Fire Mater. 2012, 36, 554–564. [Google Scholar] [CrossRef]







| Samples | MUF (g) | APP (g) | PER (g) |
|---|---|---|---|
| A1P3 | 100 | 10 | 30 |
| A1P2 | 100 | 13.3 | 26.7 |
| A1P1 | 100 | 20 | 20 |
| A2P1 | 100 | 26.7 | 13.3 |
| A3P1 | 100 | 30 | 10 |
| MAPP | 100 | 40 | 0 |
| MPER | 100 | 0 | 40 |
| MUF | 100 | 0 | 0 |
| Class | Criteria for Compliance | |
|---|---|---|
| A2 | FIGRA0.2MJ < 120 W/s; | |
| LFS < edge of specimen; | ||
| THR600s < 7.5 MJ | ||
| B1 | B | FIGRA0.2MJ < 120 W/s; |
| LFS < edge of specimen; | ||
| THR600s < 7.5 MJ | ||
| C | FIGRA0.4MJ < 250 W/s; | |
| LFS < edge of specimen; | ||
| THR600s < 7.5 MJ | ||
| B2 | D | FIGRA0.4MJ < 750 W/s |
| MUF:IFR (m/m) | 100:0 | 100:40 | 100:60 | 100:80 |
|---|---|---|---|---|
| Shear strength (MPa) | 1.47 ± 0.02 | 1.35 ± 0.03 | 0.97 ± 0.07 | 0.62 ± 0.10 |
| Sample ID | T-5% (°C) | Residue at 800 °C (wt%) |
|---|---|---|
| MAPP | 177.8 | 29.6% |
| A3P1 | 183.4 | 34.4% |
| A2P1 | 182.7 | 32.5% |
| A1P1 | 182.9 | 32.0% |
| A1P2 | 169.5 | 22.9% |
| A1P3 | 169.5 | 21.2% |
| MPER | 127.3 | 18.7% |
| Sample ID | MAPP | A3P1 | A2P1 | A1P1 | A1P2 | A1P3 | MPER |
|---|---|---|---|---|---|---|---|
| ex-V (mL) | 36 ± 2 | 133 ± 4 | 89 ± 4 | 87 ± 3 | 76 ± 2 | 64 ± 2 | 17 ± 1 |
| Sample ID | IT (s) | PHRR (kW/m2) | TTPa (s) | AHHR (kW/m2) | THR (MJ/m2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | 180 s | 300 s | 180 s | 300 s | ||
| DP | 26 ± 3 | 183 ± 6 | 174 ± 9 | 374 ± 10 | 40 ± 5 | 60 ± 10 | 330 ± 13 | 121 ±5 | 150 ± 10 | 19 ± 2 | 35 ± 3 |
| MAPP | 22 ± 2 | 183 ± 5 | 70 ± 5 | 289 ± 7 | 35 ± 4 | 294 ± 6 | 445 ± 9 | 18 ± 3 | 25 ± 3 | 3 ± 1 | 5 ± 1 |
| A3P1 | 21 ± 2 | 158 ± 3 | 57 ± 3 | 281 ± 5 | 33 ± 4 | 325 ± 6 | 530 ± 8 | 19 ± 2 | 16 ± 2 | 3 ± 1 | 4 ± 1 |
| A2P1 | 17 ± 2 | 151 ± 8 | 81 ± 11 | 297 ± 15 | 30 ± 8 | 278 ± 12 | 475 ± 16 | 18 ± 4 | 28 ± 5 | 3 ± 1 | 7±1 |
| A1P1 | 18 ± 2 | 153 ± 5 | 59 ± 7 | 306 ± 8 | 33 ± 3 | 156 ± 6 | 430 ± 11 | 35 ± 3 | 57 ± 4 | 5 ± 1 | 15 ± 2 |
| A1P2 | 17 ± 2 | 185 ± 4 | 114 ± 6 | 377 ± 5 | 30 ± 3 | 181 ± 5 | 405 ± 9 | 42 ± 3 | 69 ± 6 | 5 ± 2 | 18 ± 3 |
| Parameters of SBI | DP | FDP |
|---|---|---|
| FIGRA0.2MJ | 250.2 | 44.8 |
| FIGRA0.4MJ | 250.2 | 32.0 |
| LFS < edge of specimen | Yes | Yes |
| THR600s | 14.9 | 4.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Song, W.; Wu, Y.; Qu, W. Preparation and Characterization of the Flame Retardant Decorated Plywood Based on the Intumescent Flame Retardant Adhesive. Materials 2020, 13, 676. https://doi.org/10.3390/ma13030676
Wu M, Song W, Wu Y, Qu W. Preparation and Characterization of the Flame Retardant Decorated Plywood Based on the Intumescent Flame Retardant Adhesive. Materials. 2020; 13(3):676. https://doi.org/10.3390/ma13030676
Chicago/Turabian StyleWu, Muting, Wei Song, Yuzhang Wu, and Wei Qu. 2020. "Preparation and Characterization of the Flame Retardant Decorated Plywood Based on the Intumescent Flame Retardant Adhesive" Materials 13, no. 3: 676. https://doi.org/10.3390/ma13030676
APA StyleWu, M., Song, W., Wu, Y., & Qu, W. (2020). Preparation and Characterization of the Flame Retardant Decorated Plywood Based on the Intumescent Flame Retardant Adhesive. Materials, 13(3), 676. https://doi.org/10.3390/ma13030676






