Microstructure and Properties of Nanostructured Coating on Ti6Al4V
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Nanotubes
2.3. Microstructure and Surface Characterization
2.4. Corrosion Tests
3. Results and Discussion
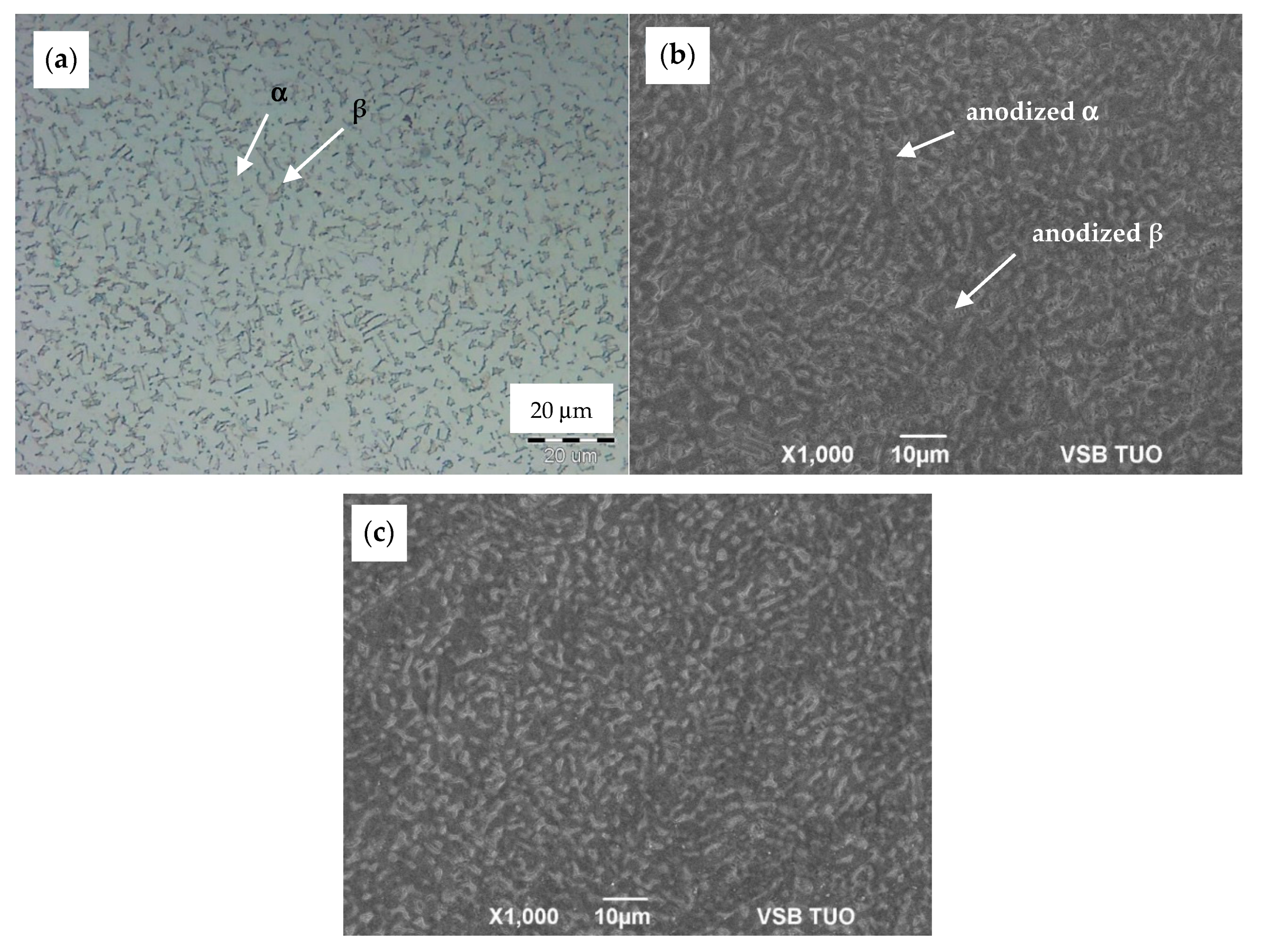
3.1. Microstructure Before and After Anodization
3.2. X-ray Diffraction Analysis
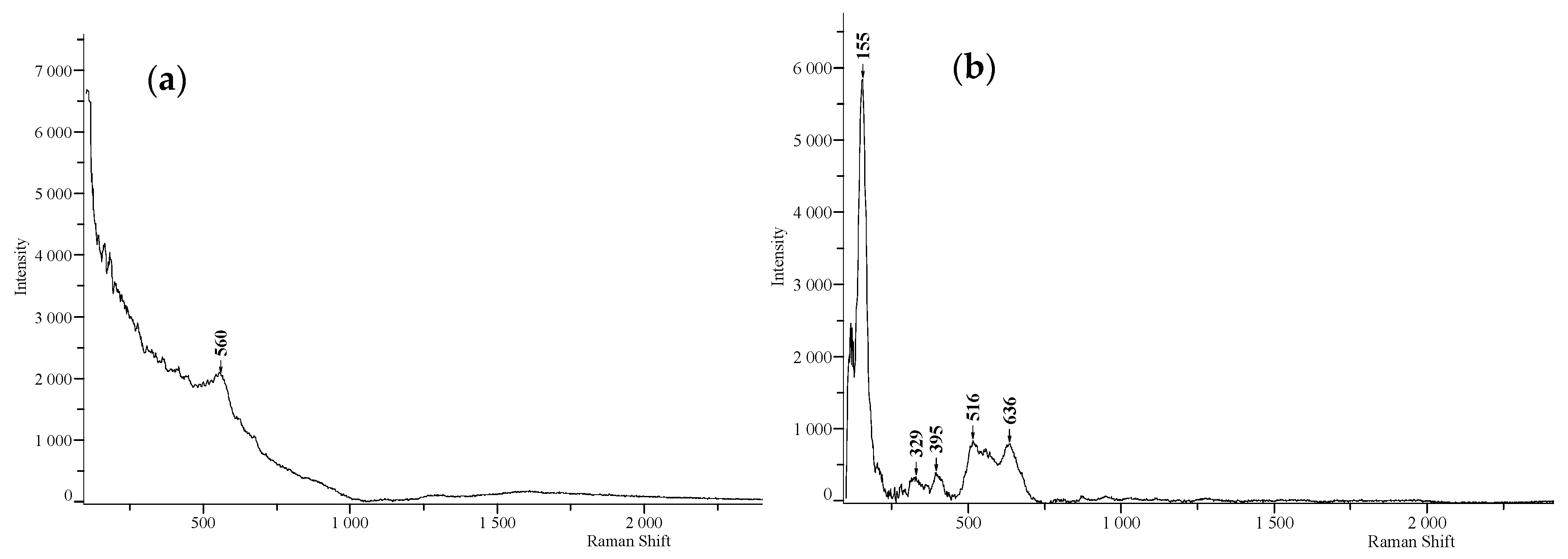
3.3. Raman Spectroscopy Analysis
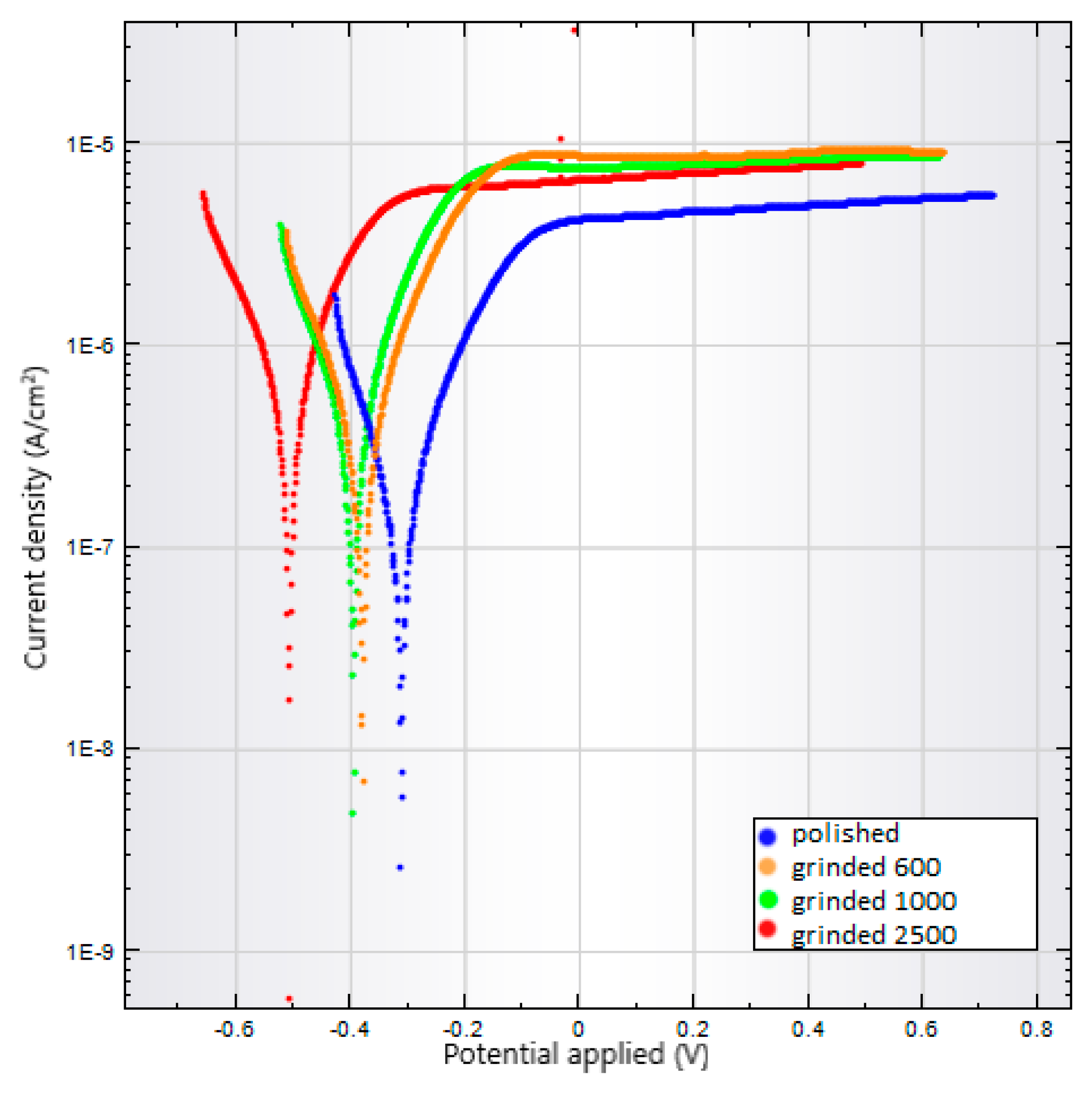
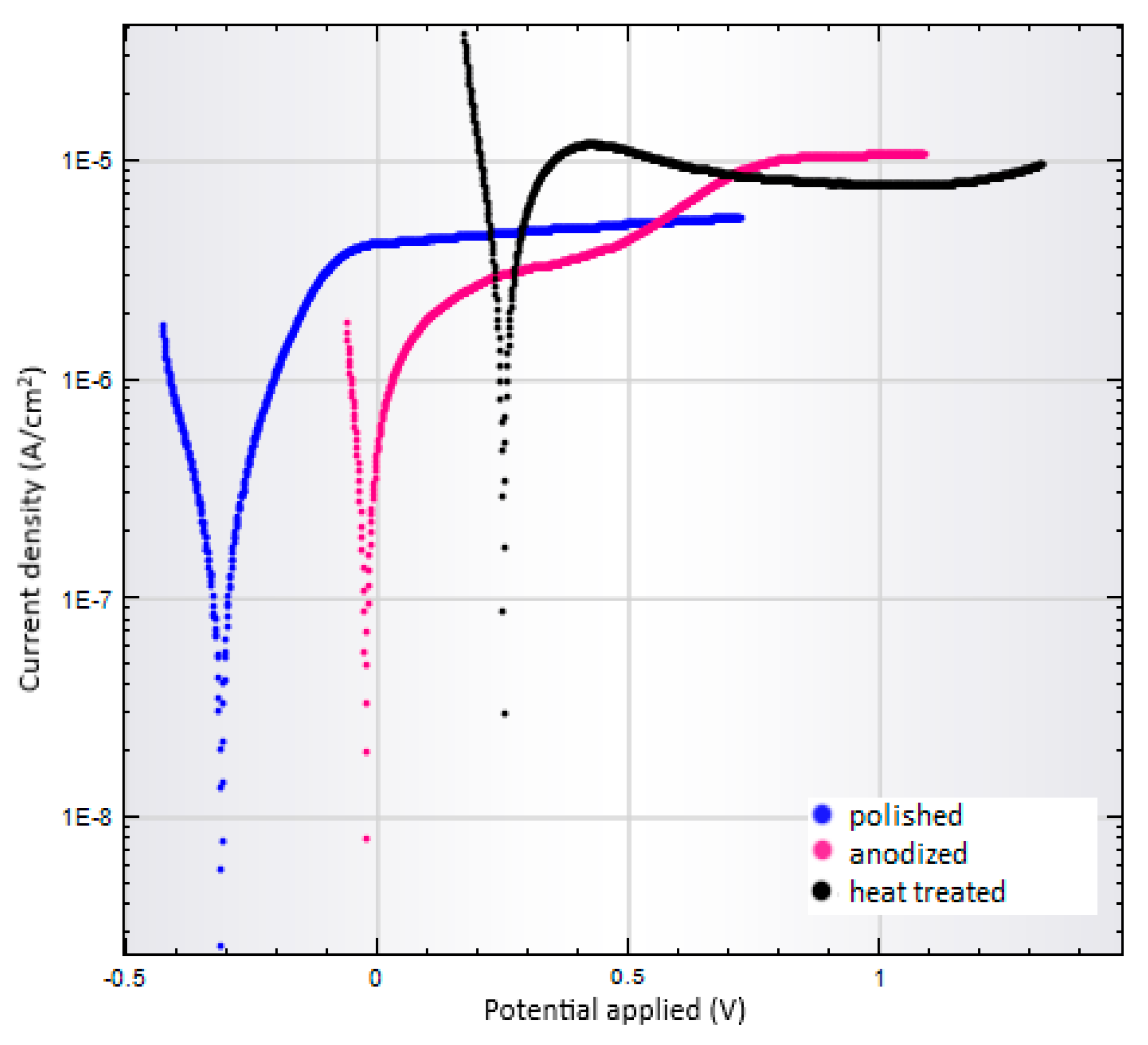
3.4. Corrosion Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nasirpouri, F.I.; Yousefi, I.; Moslehifard, E.; Kralil-Allafi, J. Tuning surface morphology and crystallinity of anodic TiO2 nanotubes and their response to biomimetic bone growth for implant applications. Surf. Coat. Technol. 2017, 315, 163–171. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Zhang, X.; Chang, X.; Zhang, X.; Li, Y.C.; Ye, T.; Han, R.; Han, S.; Gao, Y.; et al. Nanotube-formed Ti substrates coated with silicate/silver co-doped hydroxyapatite as prospective materials for bone implant. J. Alloys Compd. 2017, 697, 182–199. [Google Scholar] [CrossRef]
- Grimes, C.A.; Mor, G.K. TiO2 Nanotubes Arrays: Synthesis, Properties, and Applications; Springer: New York, NY, USA, 2009; p. 358. [Google Scholar]
- Gunputh, U.F.; Huirong, L.; Handy, R.D.; Tredwin, C. Anodised TiO2 NT as a scaffold for antibacterial silver nanoparticles on titanium implant. Mater. Sci. Eng. 2018, 91, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd, P.; Pham, X.N. Socioeconomic Renovation in Viet Nam: The Origin, Evolution, and Impact of doi moi; Institute of Southeast Asian Studies: Singapore, 2000; p. 174. [Google Scholar]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 NT. Mater. Sci. Eng. R 2013, 74, 377–406. [Google Scholar] [CrossRef] [Green Version]
- Khudhair, D.; Bhatti, A.; Li, H.Y.; Hamedani, A.; Garmestani, H.; Hodgson, P.; Nahavandi, S. Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations. Mater. Sci. Eng. C 2016, 59, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Hilaeio, F.; Roche, V.; Nogueira, R.P.; Jorge Junior, A.M. Influence of morphology and crystalline structure of TiO2 nanotubes on their electrochemical properties and apatite-forming ability. Electrochim. Acta. 2017, 245, 337–349. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Tavoosi, M.; Mozafarinia, R.; Ghasemi, A.; Doostmohammadi, A. Preparation and characterization of TiO2 nanotube arrays on Ti6Al4V surface for enhancement of cell treatment. Surf. Coat. Technol. 2017, 321, 409–415. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y.; Liu, J.; Yao, J. Influence of the Ti alloy substrate on the anodic oxidation in an environmentally-friendly electrolyte. Surf. Coat. Technol. 2018, 344, 680–688. [Google Scholar] [CrossRef]
- Petrášová, I.; Losertová, M. Electrochimical behavior of biocompatible alloys. Mater. Technol. 2015, 49, 207–211. [Google Scholar]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Losertová, M.; Štamborská, M.; Lapin, J.; Mareš, V. Comparison of deformation behavior of 316L Stainless Steel and Ti6Al4V alloy applied in traumatology. Metalurgija 2016, 55, 667–670. [Google Scholar]
- Elmer, J.W.; Palmer, T.A.; Babu, S.S.; Specht, E.D. In situ observations of lattice expansion and transformation rates of α and βphases in Ti–6Al–4V. Mater. Sci. Eng. A 2005, 391, 104–113. [Google Scholar] [CrossRef]
- Losertová, M.; Štefek, O.; Galajda, M.; Konečná, K.; Simha Martynková, G.; Čech Barabaszová, K. Microstructure and Electrochemical Behavior of TiO2 Nanotubes Coated on Titanium-based Substrate Before and After Thermal Treatment. J. Nanosci. Nanotechnol. 2019, 19, 2989–2996. [Google Scholar] [CrossRef] [PubMed]
- Atapour, M.; Pilchak, A.; Frankel, G.S.; Williams, J.C.; Fathi, M.H.; Shamanian, M. Corrosion Behavior of Ti-6Al-4V with Different Thermomechanical Treatments and Microstructures. Corrosion 2010, 66, 065004-9. [Google Scholar] [CrossRef]
- Novoselova, T.; Malinov, S.; Sha, W.; Zhecheva, A. High-temperature synchrotron X-ray diffraction study of phases in a gamma TiAl alloy. Mater. Sci. Eng. A 2004, 371, 103–112. [Google Scholar] [CrossRef]
- Verma, R.; Gangwar, J.; Srivastava, A.K. Multiphase TiO2 nanostructures: A review of efficient synthesis, growth mechanism, probing capabilities, and applications in bio-safety and health. RSC Adv. 2017, 7, 44199–44224. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, N.; Bouziani, A.; Park, J.; Micusik, M.; Kim, S.Y.; Majkova, E.; Omastova, M.; Ozturka, A. Synthesis and enhanced photocatalytic activity of nitrogen-doped triphasic TiO2 nanoparticles. J. Photoch. Photobio. A 2019, 377, 92–100. [Google Scholar] [CrossRef]


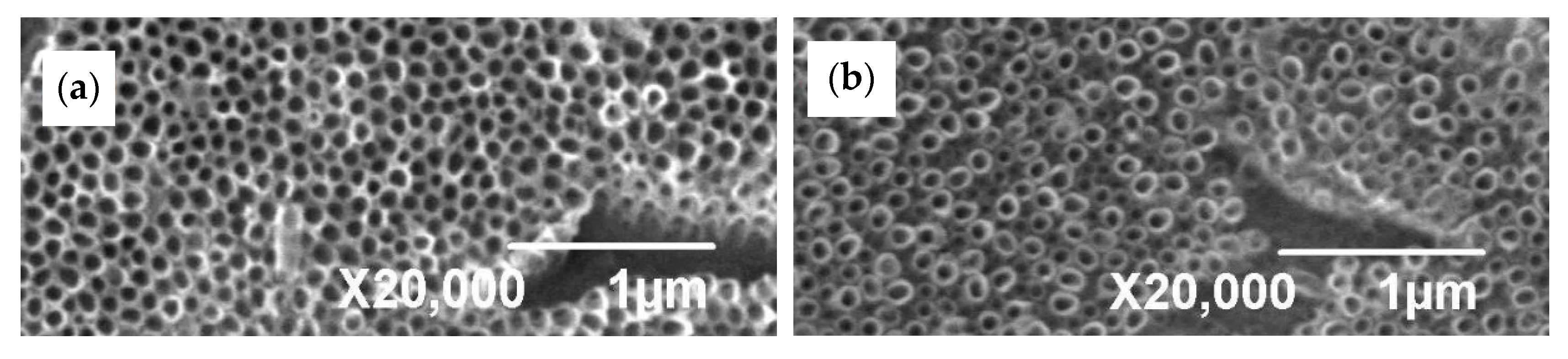
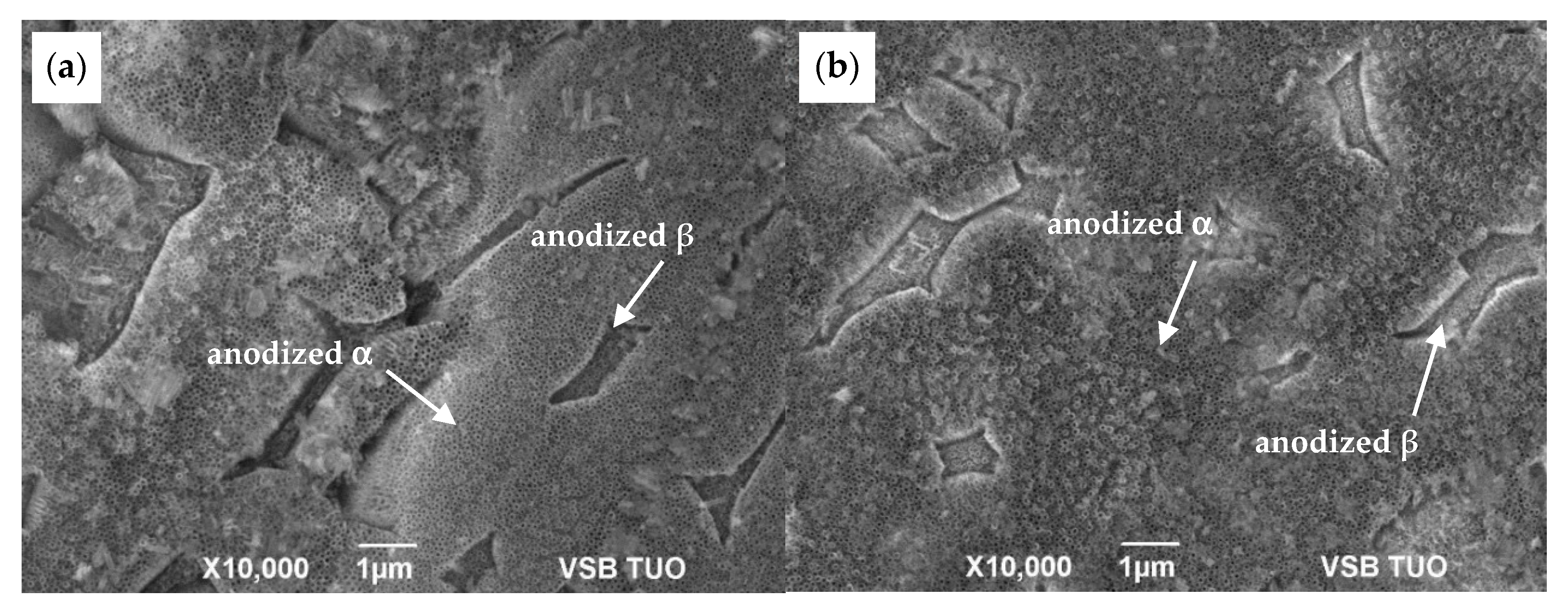


| NT Dimensions Sample | Outer Diameter [nm] | Inner Diameter [nm] | Wall Thickness [nm] |
|---|---|---|---|
| Ground AN 1 | 122 | 82 | 40 |
| Polished AN | 131 | 84 | 48 |
| Ground HT 2 | 98 | 62 | 36 |
| Polished HT | 106 | 56 | 50 |
| Sample of Ti6Al4V | Corrosion Rate (mm/year) | Potential Corrosion (mV) | Corrosion Current Density (nA/cm2) | Polarization Resistance (kΩ) |
|---|---|---|---|---|
| Ground 600 | 0.0116 | −379 | 337 | 72.6 |
| Ground 1000 | 0.0122 | −395 | 352 | 63.6 |
| Ground 2500 | 0.0219 | −507 | 635 | 48.5 |
| Polished | 0.0043 | −311 | 123 | 158.3 |
| As-anodized | 0.0182 | −24 | 527 | 42.9 |
| Anodized-heat treated | 0.0998 | 251 | 2894 | 6.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordanovová, V.; Losertová, M.; Štencek, M.; Lukášová, T.; Simha Martynková, G.; Peikertová, P. Microstructure and Properties of Nanostructured Coating on Ti6Al4V. Materials 2020, 13, 708. https://doi.org/10.3390/ma13030708
Jordanovová V, Losertová M, Štencek M, Lukášová T, Simha Martynková G, Peikertová P. Microstructure and Properties of Nanostructured Coating on Ti6Al4V. Materials. 2020; 13(3):708. https://doi.org/10.3390/ma13030708
Chicago/Turabian StyleJordanovová, Veronika, Monika Losertová, Michal Štencek, Tereza Lukášová, Gražyna Simha Martynková, and Pavlína Peikertová. 2020. "Microstructure and Properties of Nanostructured Coating on Ti6Al4V" Materials 13, no. 3: 708. https://doi.org/10.3390/ma13030708
APA StyleJordanovová, V., Losertová, M., Štencek, M., Lukášová, T., Simha Martynková, G., & Peikertová, P. (2020). Microstructure and Properties of Nanostructured Coating on Ti6Al4V. Materials, 13(3), 708. https://doi.org/10.3390/ma13030708






