The Significance and Utilisation of Biomimetic and Bioinspired Strategies in the Field of Biomedical Material Engineering: The Case of Calcium Phosphat—Protein Template Constructs
Abstract
:1. Introduction
2. Biomineralisation
- Electrostatic interaction,
- Capillary forces,
- Size-exclusion models.
3. The Biomimetic Strategy in Biomedical Material Engineering
3.1. Biomimetic Composites and Scaffolds for Bone Regeneration Purposes
3.1.1. Collagen and Gelatin/CaP Systems
- (1)
- (2)
- (1)
- (2)
- (3)
3.1.2. Other Protein/CaP Systems
Synthetic Peptide/CaP Systems
Natural Protein/CaP Systems
Utilisation of Waste Biomaterials
Synthesis of CaP Nanoparticles
3.2. Coatings
3.2.1. Metallic Substrates
3.2.2. Polymer and Ceramic Substrates
3.3. Reconstruction and Regeneration Strategies with Concern to Dental Applications
3.3.1. Systems Based on Amelogenin
3.3.2. Systems Based on Amino Acids and Peptides
3.3.3. Systems Based on Dendrimers
3.3.4. Other Systems
3.4. Smart Devices
3.4.1. Delivery Systems
3.4.2. Smart Constructs
4. Summary and Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Schmitt, O.H. Some interesting and useful biomimetic transforms. In Proceedings of the 3rd International Biophysics Congress of the International Union for Pure and Applied Biophysics at the Massachusetts Institute of Technology, Cambridge, MA, USA, 29 August–3 September 1969; Volume 297. [Google Scholar]
- Vincent, J.F.V.; Bogatyreva, O.A.; Bogatyrev, N.R.; Bowyer, A.; Pahl, A.K. Biomimetics: Its practice and theory. J. R. Soc. Interface 2006, 3, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarikaya, M.; Aksay, I.A. Nacre: Properties, crystallography, morphology, and formation. In Design and Processing of Materials by Biomimetics; Sarikaya, M., Aksay, I.A., Eds.; American Institute of Physics: Washington, DC, USA, 1993; pp. 35–86. [Google Scholar]
- Sarikaya, M. An introduction to biomimetics: A structural viewpoint. Microsc. Res. Tech. 1994, 27, 360–375. [Google Scholar] [CrossRef]
- Green, D.; Walsh, D.; Mann, S.; Oreffo, R.O. The potential of biomimesis in bone tissue engineering: Lessons from the design and synthesis of invertebrate skeletons. Bone 2000, 30, 810–815. [Google Scholar] [CrossRef]
- Raucci, M.G.; Guarino, V.; Ambrosio, L. Biomimetic strategies for bone repair and regeneration. J. Funct. Biomater. 2012, 3, 688–705. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, M.; Ikoma, T.; Itoh, S.; Matsumoto, H.N.; Koyama, Y.; Takakuda, K.; Shinomiya, K.; Tanaka, J. Biomimetic synthesis of bone-like nanocomposites using the self-organization mechanism of hydroxyapatite and collagen. Comp. Sci. Technol. 2004, 64, 819–825. [Google Scholar] [CrossRef]
- Du, C.; Cui, F.Z.; Zhu, X.D.; De Groot, K. Three-dimensional nano-HAp/collagen matrix loading with osteogenic cells in organ culture. J. Biomed. Mater. Res A 1999, 44, 407–415. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Fan, H.; Wen, X.; Lu, J.; Zhang, X. In Situ synthesis of bone-like apatite/collagen nano-composite at low temperature. Mater. Lett. 2004, 58, 3569–3572. [Google Scholar] [CrossRef]
- Allo, B.A.; Costa, D.O.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactive and biodegradable nanocomposites and hybrid biomaterials for bone regeneration. J. Funct. Biomater. 2012, 3, 432–463. [Google Scholar] [CrossRef] [Green Version]
- Sugawara-Narutaki, A. Bio-inspired synthesis of polymer–inorganic nanocomposite materials in mild aqueous systems. Polym. J. 2013, 45, 269–276. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical structures of bone and bioinspired bone tissue engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Alves, N.M.; Leonor, I.B.; Azevedo, H.S.; Reis, R.L.; Mano, J.F. Designing biomaterials based on biomineralization of bone. J. Mater. Chem. 2010, 20, 2911–2921. [Google Scholar] [CrossRef] [Green Version]
- Vriezema, D.M.; Comellas Aragone’s, M.; Elemans, J.A.A.W.; Cornelissen, J.J.L.M.; Rowan, A.E.; Nolte, R.J.M. Self-assembled nanoreactors. Chem. Rev. 2005, 105, 1445–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maria, S.M.; Prukner, C.; Sheikh, Z.; Müller, F.A.; Komarova, S.V.; Barralet, J.E. Characterization of biomimetic calcium phosphate labeled with fluorescent dextran for quantification of osteoclastic activity. Acta Biomater. 2015, 20, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Sommerdijk, N.A. Biomineralization as an inspiration for materials chemistry. Angew. Chem. Int. Ed. Engl. 2012, 51, 6582–6596. [Google Scholar] [CrossRef] [PubMed]
- Fabritius, H.O.; Ziegler, A.; Friák, M.; Nikolov, S.; Huber, J.; Seidl, B.H.M.; Ruangchai, S.; Alagboso, F.I.; Karsten, S.; Lu, J. Functional adaptation of crustacean exoskeletal elements through structural and compositional diversity: A combined experimental and theoretical study. Bioinspir. Biomim. 2016, 11, 055006. [Google Scholar] [CrossRef]
- Neues, F.; Epple, M. X-ray microcomputer tomography for the study of biomineralized endo-and exoskeletons of animals. Chem. Rev. 2008, 108, 4734–4741. [Google Scholar] [CrossRef]
- Jiang, S.D.; Yao, Q.Z.; Ma, Y.F.; Zhou, G.T.; Fu, S.Q. Phosphate-dependent morphological evolution of hydroxyapatite and implication for biomineralisation. Gondwana Res. 2015, 28, 858–868. [Google Scholar] [CrossRef]
- Qi, C.; Musetti, S.; Fu, L.H.; Zhu, Y.J.; Huang, L. Biomolecule-assisted green synthesis of nanostructured calcium phosphates and their biomedical applications. Chem. Soc. Rev. 2019, 48, 2698–2737. [Google Scholar] [CrossRef]
- Veis, A. Biomineralization. Rev. Mineral. Geochem. 2003, 54, 249–289. [Google Scholar] [CrossRef]
- Poole, R.A.; Kojima, T.; Tasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and structure of articular cartilage-A template for tissue repair. Clin. Orthop. Relat. Res. 2001, 391, S26–S33. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Connell, S.; Kirkham, J.; Shore, R.; Smith, A. Dental enamel—A biological ceramic: Regular substructures in enamel hydroxyapatite crystals revealed by atomic force microscopy. J. Mater. Chem. 2004, 14, 2242–2248. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Tan, J.; Nancollas, G.H. Constant composition dissolution kinetics studies of human dentin. J. Dent. Res. 1996, 75, 1019–1026. [Google Scholar] [CrossRef]
- Giachelli, C.M. Inducers and inhibitors of biomineralization: Lessons from pathological calcification. Orthod. Craniofac. Res. 2005, 8, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V. When 1 + 1 > 2: Nanostructured composites for hard tissue engineering applications. Mater. Sci. Eng. C 2015, 57, 434–451. [Google Scholar] [CrossRef] [Green Version]
- Hellmich, C.; Ulm, F.J.; Dormieux, L. Can the diverse elastic properties of trabecular and cortical bone be attributed to only a few tissue-independent phase properties and their interactions? Arguments from a multiscale approach. Biomech. Model Mechanobiol. 2004, 2, 219–238. [Google Scholar] [CrossRef]
- Campi, G.; Fratini, M.; Bukreeva, I.; Ciasca, G.; Burghammer, M.; Brun, F.; Tromba, G.; Mastrogiacomo, M.; Cedola, A. Imaging collagen packing dynamics during mineralization of engineered bone tissue. Acta Biomater. 2015, 23, 309–316. [Google Scholar] [CrossRef]
- Li, Y.; Aparicio, C. Discerning the subfibrillar structure of mineralized collagen fibrils: A model for the ultrastructure of bone. PLoS ONE 2013, 8, e76782. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, L.; Putnis, C.V. Atomic force microscopy imaging of classical and nonclassical surface growth dynamics of calcium orthophosphates. Cryst. Eng. Commun. 2018, 20, 2886–2896. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, S.; Pan, H.; Tang, R. Amorphous phase mediated crystallization: Fundamentals of biomineralization. Crystals 2018, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Bolean, M.; Simão, A.M.S.; Barioni, M.B.; Favarin, B.Z.; Sebinelli, H.G.; Veschi, E.A.; Janku, T.A.B.; Bottini, M.; Hoylaerts, M.F.; Itri, R.; et al. Biophysical aspects of biomineralization. Biophys. Rev. 2017, 9, 747–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Azaïs, T.; Robin, M.; Vallée, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.M.; Nassif, N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef]
- Quan, B.D.; Sone, E.D. The effect of polyaspartate chain length on mediating biomimetic remineralization of collagenous tissues. J. R. Soc. Interface 2018, 15, 20180269. [Google Scholar] [CrossRef] [Green Version]
- Tejero, R.; Bierbaum, S.; Douglas, T.; Reinstorf, A.; Worch, H.; Scharnweber, D. Glucuronic acid and phosphoserine act as mineralization mediators of collagen I based biomimetic substrates. J. Mater. Sci. Mater. Med. 2010, 21, 407–418. [Google Scholar] [CrossRef]
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [Green Version]
- Jie, Y.; Cai, Z.; Li, S.; Xie, Z.; Ma, M.; Huang, X. Hydroxyapatite nucleation and growth on collagen electrospun fibers controlled with different mineralization conditions and phosvitin. Macromol. Res. 2017, 25, 905–912. [Google Scholar] [CrossRef]
- Rodriguez, D.E.; Thula-Mata, T.; Toro, E.J.; Yeh, Y.W.; Holt, C.; Holliday, L.S.; Gower, L.B. Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta Biomater. 2014, 10, 494–507. [Google Scholar] [CrossRef] [Green Version]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Guo, Q.; Ma, G.; Shen, L.; Yu, H.; Lin, B.; Lu, N.; Huang, K. Application of recombinant collagen type I combined with polyaspartic acid in biomimetic biomineralization. Chin. Acad. Med. Sci. 2017, 39, 318–323. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Tsetsekou, A.; Kammenou, M.I.; Boukos, N. Biomimetic synthesis of ribbon-like hydroxyapatite employing poly(L-arginine). Mater. Sci. Eng. C 2016, 58, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a detailed understanding of magnesium ions on hydroxyapatite crystallization inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- Eiden-Aßmann, S.; Viertelhaus, M.; Heiß, A.; Hoetzer, K.A.; Felsche, J. The influence of amino acids on the biomineralization of hydroxyapatite in gelatin. J. Inorg. Biochem. 2002, 91, 481–486. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toroian, D.; Lim, J.E.; Price, P.A. The size exclusion characteristics of type I collagen: Implications for the role of noncollagenous bone constituents in mineralization. J. Biol. Chem. 2007, 282, 22437–22447. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.J.; Heinz, H. Accurate force field parameters and pH resolved surface models for hydroxyapatite to understand structure, mechanics, hydration, and biological interfaces. J. Phys. Chem. C 2016, 120, 4975–4992. [Google Scholar] [CrossRef] [Green Version]
- Bleek, K.; Taubert, A. New developments in polymer-controlled, bioinspired calcium phosphate mineralization from aqueous solution. Acta Biomater. 2013, 9, 6283–6321. [Google Scholar] [CrossRef]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic composite scaffolds containing bioceramics andcollagen/gelatin for bone tissue engineering—A mini review. Int. J. Biol. Macromol. 2016, 93, 1390–1401. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Kluijtmans, S.G.M.J. Biomineralization of recombinant peptide scaffolds: Interplay among chemistry, architecture, and mechanics. ACS Biomater. Sci. Eng. 2017, 3, 1100–1108. [Google Scholar] [CrossRef]
- Ding, S.; Tang, M.; Chen, J.; Li, L.; Li, H. Effects of collagen assembly form on biomimetic mineralization. Chin. J. Mater. Res. 2016, 30, 51–56. [Google Scholar] [CrossRef]
- Xia, Z.; Villa, M.M.; Wei, M. A biomimetic collagen–apatite scaffold with a multi-level lamellar structure for bone tissue engineering. J. Mater. Chem. B 2014, 2, 1998–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Chen, L.; Cai, X.; Tong, T.; Tong, H.; Hu, J. A novel method for the fabrication of homogeneous hydroxyapatite/collagen nanocomposite and nanocomposite scaffold with hierarchical porosity. J. Mater. Sci. Mater. Med. 2011, 22, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Preti, L.; Lambiase, B.; Campodoni, E.; Sandri, M.; Ruffini, A.; Pugno, N.; Tampieri, A.; Sprio, S. Nature-inspired processes and structures: New paradigms to develop highly bioactive devices for hard tissue regeneration. In Bio-Inspired Technology; IntechOpen: London, UK, 2019. [Google Scholar]
- Eppell, S.J.; Tong, W.; McMasters, J.; Soenjaya, Y.; Barbu, A.M.; Ko, A.; Baskin, J.Z. Minor Review: An overview of a synthetic nanophase bone substitute. Materials 2018, 11, 1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, C.; Heinemann, S.; Kruppke, B.; Worch, H.; Thomas, J.; Wiesmann, H.P.; Hanke, T. Electric field-assisted formation of organically modified hydroxyapatite (ormoHAP) spheres in carboxymethylated gelatin gels. Acta Biomater. 2016, 44, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Gelinsky, M.; Kawasaki, T.; Kohgo, T.; König, U.; Pompe, W.; Watari, F. Biomimetic porous scaffolds with high elasticity made from mineralized collagen—An animal study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Matsumoto, H.N.; Yamada, T.; Koyama, Y.; Takakuda, K.; Tanaka, J. Glutaraldehyde cross-linked hydroxyapatite/collagen self-organized nanocomposites. Biomaterials 2004, 25, 63–69. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C. Biomimetic collagen/hydroxyapatite composite scaffolds: Fabrication and characterizations. J. Bionic Eng. 2014, 11, 600–609. [Google Scholar] [CrossRef]
- Tomomatsu, O.; Tachibana, A.; Yamauchi, K.; Tanabe, T. A film of collagen/calcium phosphate composite prepared by enzymatic mineralization in an aqueous phase. J. Ceram. Soc. Jpn. 2008, 116, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Strange, D.G.T.; Oyen, M.L. Biomimetic bone-like composites fabricated through an automated alternate soaking proces. Acta Biomater. 2011, 7, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Kruppke, B.; Farack, J.; Wagner, A.S.; Beckmann, S.; Heinemann, C.; Glenske, K.; Rößler, S.; Wiesmann, H.P.; Wenisch, S.; Hanke, T. Gelatine modified monetite as a bone substitute material: An In Vitro assessment of bone biocompatibility. Acta Biomater. 2016, 32, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Song, J.H.; Kim, H.E. Nanofiber generation of gelatin-hydroxyapatite biomimetics for guided tissue regeneration. Adv. Funct. Mater. 2005, 15, 1988–1994. [Google Scholar] [CrossRef]
- Li, X.; Feng, Q.; Jiao, Y.; Cui, F. Collagen-based scaffolds reinforced by chitosan fibres for bone tissue engineering. Polym. Int. 2005, 54, 1034–1040. [Google Scholar] [CrossRef]
- Han, G.S.; Lee, S.; Kim, D.W.; Kim, D.H.; Noh, J.H.; Park, J.H.; Roy, S.; Ahn, T.K.; Jung, H.S. A simple method to control morphology of hydroxyapatite nano- and microcrystals by altering phase transition route. Cryst. Growth Des. 2013, 13, 3414–3418. [Google Scholar] [CrossRef]
- Lickorish, D.; Ramshaw, J.A.M.; Werkmeister, J.A.; Glattauer, V.; Howlett, C.R. Collagen—Hydroxyapatite composite prepared by biomimetic proces. J. Biomed. Mater. Res. Part A 2004, 68, 19–27. [Google Scholar] [CrossRef]
- Narasimha Raghavan, R.; Muthukumar, T.; Somanathan, N.; Sastry, T.P. Biomimetic mineralization of novel silane crosslinked collagen. Mater. Sci. Eng. C 2013, 33, 1983–1988. [Google Scholar] [CrossRef]
- Li, Y.; Thula, T.T.; Jee, S.; Perkins, S.L.; Aparicio, C.; Douglas, E.P.; Gower, L.B. Biomimetic mineralization of woven bone-like nanocomposites: Role of collagen cross-links. Biomacromolecules 2012, 13, 49–59. [Google Scholar] [CrossRef]
- Tarik Arafat, M.; Tronci, G.; Yin, J.; Wood, D.J.; Russell, S.J. Biomimetic wet-stable fibres via wet spinning and diacid-based crosslinking of collagen triple helices. Polymer 2015, 77, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Bigi, A.; Boanini, E.; Panzavolta, S.; Roveri, N. Biomimetic Growth of hydroxyapatite on gelatin films doped with sodium polyacrylate. Biomacromolecules 2000, 1, 752–756. [Google Scholar] [CrossRef]
- Kollmann, T.; Simon, P.; Carrillo-Cabrera, W.; Braunbarth, C.; Poth, T.; Rosseeva, E.V.; Kniep, R. Calcium phosphate-gelatin nanocomposites: Bulk preparation (shape-and phase-control), characterization, and application as dentine repair material. Chem. Mater. 2010, 22, 5137–5153. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Cojazzi, G.; Falini, G.; Panzavolta, S. Morphological and structural investigation of octacalcium phosphate hydrolysis in the presence of polyacrylic acids: Effect of relative molecular weights. Crys. Growth Des. 2001, 1, 239–244. [Google Scholar] [CrossRef]
- Chang, M.C.; Ko, C.C.; Douglas, W.H. Preparation of hydroxyapatite-gelatin nanocomposite. Biomaterials 2003, 24, 2853–2862. [Google Scholar] [CrossRef]
- Chang, M.C. Organic—Inorganic interaction between hydroxyapatite and gelatin with the aging of gelatin in aqueous phosphoric acid solution. J. Mater. Sci. Mater. Med. 2008, 19, 3411–3418. [Google Scholar] [CrossRef]
- Liu, X.; Lin, K.; Wu, C.; Wang, Y.; Zou, Z.; Chang, J. Multilevel hierarchically ordered artificial biomineral. Small 2014, 10, 152–159. [Google Scholar] [CrossRef]
- Ito, H.; Oaki, Y.; Imai, H. Selective synthesis of various nanoscale morphologies of hydroxyapatite via an intermediate phase. Cryst. Growth Des. 2008, 8, 1055–1059. [Google Scholar] [CrossRef]
- Furuichi, K.; Oaki, Y.; Imai, H. Preparation of nanotextured and nanofibrous hydroxyapatite through dicalcium phosphate with gelatin. Chem. Mater. 2006, 18, 229–234. [Google Scholar] [CrossRef]
- Chang, M.C.; Douglas, W.H.; Tanaka, J. Organic-inorganic interaction and the growth mechanism of hydroxyapatite crystals in gelatin matrices between 37 and 80 °C. J. Mater. Sci. Mater. Med. 2006, 17, 387–396. [Google Scholar] [CrossRef]
- Kim, Y.K.; Gu, L.S.; Bryan, T.E.; Kim, J.R.; Chen, L.; Liu, Y.; Yoon, J.C.; Breschi, L.; Pashley, D.H.; Tay, F.R. Mineralisation of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium, phosphate and hydroxyl ions. Biomaterials 2010, 31, 6618–6627. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Luo, X.; Ye, B.; Li, Z.; Wu, D.; Li, L.; Li, H.; Zhou, C. Biomimetic mineralization of collagen fibers regulated with “soft template”. J. Funct. Mater. 2016, 47, 03129–03135. [Google Scholar] [CrossRef]
- Yu, L.; Martin, I.J.; Kasi, R.M.; Wei, M. Enhanced intrafibrillar mineralization of collagen fibrils induced by brushlike polymers. ACS Appl. Mater. Interfaces 2018, 10, 28440–28449. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zilm, M.; Wei, M. Fabrication of intrafibrillar and extrafibrillar mineralized collagen/apatite scaffolds with a hierarchical structure. J. Biomed. Mater. Res. Part A 2016, 104, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Thula, T.T.; Rodriguez, D.E.; Lee, M.H.; Pendi, L.; Podschun, J.; Gower, L.B. In Vitro mineralization of dense collagen substrates: A biomimetic approach toward the development of bone-graft materials. Acta Biomater. 2011, 7, 3158–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antebi, B.; Cheng, X.; Harris, J.N.; Gower, L.B.; Chen, X.D.; Ling, J. Biomimetic collagen–hydroxyapatite composite fabricated via a novel perfusion-flow mineralization technique. Tissue Eng. C Methods 2013, 19, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingender, B.; Bradley, P.; Saxena, N.; Ruberti, J.W.; Gower, L.B. Biomimetic organization of collagen matrices to template bone-like microstructures. Matrix Biol. 2016, 52, 384–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, M.; Guo, P.; Keeney, M.; Yang, F.; Hsu, T.M.; Fuller, G.G.; Martin, C.R.; Zare, R.N. Preparation of mineralized nanofibers: Collagen fibrils containing calcium phosphate. Nano Lett. 2011, 11, 1383–1388. [Google Scholar] [CrossRef] [Green Version]
- Suchý, T.; Šupová, M.; Bartoš, M.; Sedláček, R.; Piola, M.; Soncini, M.; Fiore, G.B.; Sauerová, P.; Hubálek Kalbáčová, M. Dry versus hydrated collagen scaffolds: Are dry states representative of hydrated states? J. Mater. Sci. Mater. Med. 2018, 29, 20. [Google Scholar] [CrossRef]
- Chang, M.C.; Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef]
- Sader, M.S.; Martins, V.C.A.; Gomez, S.; LeGeros, R.Z.; Soares, G.A. Production and In Vitro characterization of 3D porous scaffolds made of magnesium carbonate apatite (MCA)/anionic collagen using a biomimetic approach. Mater. Sci. Eng. C 2013, 33, 4188–4196. [Google Scholar] [CrossRef]
- Chang, M.C.; Ko, C.C.; Douglas, W.H. Conformational change of hydroxyapatite/gelatin nanocomposite by Glutaraldehyde. Biomaterials 2003, 24, 3087–3094. [Google Scholar] [CrossRef]
- Chang, M.C.; Douglas, W.H. Cross-linkage of hydroxyapatite/gelatin nanocomposite using imide-based zero-length cross-linker. J. Mater. Sci. Mater. Med. 2007, 18, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tan, G.; Tan, Y.; Wang, H.; Liao, J.; Ning, C. Biomimetic mineralization of anionic gelatin hydrogels: Effect of degree of methacrylation. RSC Adv. 2014, 4, 21997–22008. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Liao, S.; Watari, F.; Uo, M.; Ohkawa, S.; Tamura, K.; Wang, W.; Cui, F. The preparation and characteristics of a carbonated hydroxyapatite/collagen composite at room temperature. J. Biomed. Mater. Res. B 2005, 74, 817–821. [Google Scholar] [CrossRef]
- Sachlos, E.; Gotora, D.; Czernuszka, J.T. Collagen scaffolds reinforced with biomimetic composite nano-sized carbonate-substituted hydroxyapatite crystals and shaped by rapid prototyping to contain internal microchannels. Tissue Eng. 2006, 12, 2479–2487. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, S.S.; Cui, F.Z. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem. Mater. 2003, 15, 3221–3226. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Feng, Q.; Guo, X. Incorporation of strontium into hydroxyapatite via biomineralization of collagen fibrils. Ceram. Inter. 2015, 41, 8773–8778. [Google Scholar] [CrossRef]
- Minardi, S.; Corradetti, B.; Taraballi, F.; Sandri, M.; Eps, J.V.; Cabrera, F.J.; Weiner, B.K.; Tampieri, A.; Tasciotti, E. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials 2015, 62, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Tampieri, A.; Sandri, M.; Landi, E.; Sprio, S.; Valentini, F.; Boskey, A. Synthetic biomineralisation yielding HA/collagen hybrid composite. Adv. Appl. Ceram. 2008, 107, 298–302. [Google Scholar] [CrossRef]
- Yu, S.H.; Chen, S.F. Recent advances in polymer directed crystal growth and mediated self-assembly of nanoparticles. Cur. Nanosci. 2006, 2, 81–92. [Google Scholar] [CrossRef]
- Tsetsekou, A.; Brasinika, D.; Vaou, V.; Chatzitheodoridis, E. On the synthesis of tailored biomimetic hydroxyapatite nanoplates through a bioinspired approach in the presence of collagen or chitosan and L-arginine. Mater. Sci. Eng. C 2014, 43, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Sang, L.; Wang, X.; Xu, S.; Li, X. Low temperature, pH-triggered synthesis of collagen–chitosan–hydroxyapatite nanocomposites as potential bone grafting substitutes. Mater. Lett. 2011, 65, 2395–2397. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Pan, P.; Fan, T.; Chen, M.; Zhang, Q. In Situ strategy for bone repair by facilitated endogenous tissue engineering. Colloids Surf. B Biointerfaces 2015, 135, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.H.; Liang, M.H.; Wang, P.; Luo, Y. Biomimetic composite microspheres of collagen/chitosan/nano-hydroxyapatite: In-situ synthesis and characterization. Mater. Sci. Eng. C 2016, 58, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, C.; Zhu, M.; Tian, J.; Rong, J. In Vitro and In Vivo characterization of homogeneous chitosan-based composite scaffolds. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2012, 27, 100–106. [Google Scholar] [CrossRef]
- Kanungo, B.P.; Gibson, L.J. Density–property relationships in mineralized collagen–glycosaminoglycan scaffolds. Acta Biomater. 2009, 5, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, Y.; Nan, K. Biologically inspired growth of hydroxyapatite crystals on bio-organics-defined scaffolds. Mater. Res. Bull. 2013, 48, 1128–1131. [Google Scholar] [CrossRef]
- Ren, X.; Tu, V.; Bischoff, D.; Weisgerber, D.W.; Lewis, M.S.; Yamaguchi, D.T.; Miller, T.A.; Harley, B.A.C.; Lee, J.C. Nanoparticulate mineralized collagen scaffolds induce In Vivo bone regeneration independent of progenitor cell loading or exogenous growth factor stimulation. Biomaterials 2016, 89, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Kanungo, B.P.; Silva, E.; Van Vliet, K.; Gibson, L.J. Characterization of mineralized collagen–glycosaminoglycan scaffolds for bone regeneration. Acta Biomater. 2008, 4, 490–503. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Kozlowska, J.; Osyczka, A.M. New composite materials prepared by calcium phosphateprecipitation in chitosan/collagen/hyaluronic acid spongecross-linked by EDC/NHS. Int. J. Biol. Macromol. 2018, 107, 247–253. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, W.; Hu, W.; Zhou, L.; Wang, S.; Zhang, S. Collagen/silk fibroin bi-template induced biomimetic bone-like substitutes. J. Biomed. Mater. Res. A 2011, 99, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, J.; Ran, J.; Shen, X.; Tong, H. Preparation and evaluation of collagen-silk fibroin/hydroxyapatite nanocomposites for bone tissue engineering. Int. J. Biolog. Macromol. 2014, 65, 1–7. [Google Scholar] [CrossRef]
- Cai, X.; Tong, H.; Zhao, Y.; Shen, X.; Hu, J. A novel approach of homogenous inorganic/organic composite through In Situ precipitation in gelatine/poly(acrylic acid) gel. In Key Engineering Materials; Trans Tech Publications: Stafa, Zurich, Switzerland, 2008; Volume 361, pp. 499–502. [Google Scholar] [CrossRef]
- Bera, T.; Ramachandrarao, P. Morphological changes in biomimetically synthesized hydroxyapatite and silver nanoparticles for medical applications. J. Mater. Sci. 2009, 44, 2264–2270. [Google Scholar] [CrossRef]
- Wang, Y.; Manh, N.V.; Wang, H.; Zhong, X.; Zhang, X.; Li, C. Synergistic intrafibrillar/extrafibrillar mineralization of collagen scaffolds based on a biomimetic strategy to promote the regeneration of bone defects. Int. J. Nanomed. 2016, 11, 2053–2067. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Cao, S.; Wang, H.; Li, Y.; Kishen, A.; Deng, X.; Yang, X.; Wang, Y.; Cong, C.; Wang, H.; et al. Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an In Vitro tooth model of deep caries. PLoS ONE 2015, 10, e0116553. [Google Scholar] [CrossRef]
- Huang, Z.; Feng, Q.; Yu, B.; Li, S. Biomimetic properties of an injectable chitosan/nano-hydroxyapatite/collagen composite. Mater. Sci. Eng. C 2011, 31, 683–687. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Y.; Feng, Q.L.; Zhao, W.; Yu, B.; Tian, J.; Li, S.J.; Lin, B.M. In Vivo bone regeneration with injectable chitosan/hydroxyapatite/collagen composites and mesenchymal stem cells. Front. Mater. Sci. 2011, 5, 301–310. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, B.; Feng, Q.; Li, S.; Chen, Y.; Luo, L. In Situ-forming chitosan/nano-hydroxyapatite/collagen gel for the delivery of bone marrow mesenchymal stem cells. Carbohydr. Pol. 2011, 85, 261–267. [Google Scholar] [CrossRef]
- Ehrlich, H.; Douglas, T.; Scharnweber, D.; Hanke, T.; Born, R.; Bierbaum, S.; Worch, H. Hydroxyapatite crystal growth on modified collagen I-templates in a model dual membrane diffusion system. Zeitschrift für Anorganische und Allgemeine Chemie 2005, 631, 1825–1830. [Google Scholar] [CrossRef]
- Ehrlich, H.; Hanke, T.; Simon, P.; Born, R.; Fischer, C.; Frolov, A.; Langrock, T.; Hoffmann, R.; Schwarzenbolz, U.; Henle, T.; et al. Carboxymethylation of the fibrillar collagen with respect to formation of hydroxyapatite. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 542–551. [Google Scholar] [CrossRef]
- George, A.; Ravindran, S. Protein templates in hard tissue engineering. Nano Today 2010, 5, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Mallapragada, S.K. Bioinspired synthesis of organic/inorganic nanocomposite materials mediated by biomolecules. In Book, on Biomimetics; Pramatarova, L.D., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Yusufoglu, Y.; Hu, Y.; Kanapathipillai, M.; Kramer, M.; Kalay, Y.E.; Thiyagarajan, P.; Akinc, M.; Schmidt-Rohr, K.; Mallapragada, S. Bioinspired synthesis of self-assembled calcium phosphate nanocomposites using block copolymer-peptide conjugates. J. Mater. Res. 2008, 23, 3196–3212. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.Y.; Yusufoglu, Y.; Kanapathipillai, M.; Yang, C.Y.; Wu, Y.Q.; Thiyagarajan, P.; Deming, T.; Akinc, M.; Schmidt-Rohr, K.; Mallapragada, S. Self-assembled calcium phosphate nanocomposites using block copolypeptide templates. Soft Matter 2009, 5, 4311–4320. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhou, Z.M.; Zhang, Z.B.; Du, B.; Zhang, Z.; Wang, Q.; Yuan, P.; Liu, L.R.; Zhang, Q.Q. Biomimetic synthesis of platelet-shaped hydroxyapatite mesocrystals in a collagen mimetic peptide–PEG hybrid hydrogel. Mater. Lett. 2015, 159, 150–153. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Iafisco, M.; Montesi, M.; Sandri, M.; Sprio, S.; Tampier, A. Biomimetic mineralization of recombinant collagen type I derived protein to obtain hybrid matrices for bone regeneration. J. Struct. Biol. 2016, 196, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Belén Ramírez-Rodríguez, B.; Montesi, M.; Panseri, S.; Sprio, S.; Tampieri, A.; Sandri, M. Biomineralized recombinant collagen-based scaffold mimicking native bone enhances mesenchymal stem cell interaction and differentiation. Tis. Eng. A 2017, 23, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Fok, A.; Rodriguez-Cabello, J.C.; Aparicio, C. Biomimetic mineralization of recombinamer-based hydrogels toward controlled morphologies and high mineral density. ACS Appl. Mater. Interfaces 2015, 7, 25784–25792. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Rodriguez-Cabello, J.C.; Aparicio, C. Intrafibrillar mineralization of self-assembled elastin-like recombinamer fibrils. ACS Appl. Mater. Interfaces 2017, 9, 5838–5846. [Google Scholar] [CrossRef]
- Prieto, S.; Shkilnyy, A.; Rumplasch, C.; Ribeiro, A.; Arias, F.J.; Rodríguez-Cabello, J.C.; Taubert, A. biomimetic calcium phosphate mineralization with multifunctional elastin-like recombinamers. Biomacromolecules 2011, 12, 1480–1486. [Google Scholar] [CrossRef]
- Chen, P.H.; Tseng, Y.H.; Mou, Y.; Tsai, Y.L.; Guo, S.M.; Huang, S.J.; Yu, S.S.F.; Chan, J.C.C. Adsorption of a statherin peptide fragment on the surface of nanocrystallites of hydroxyapatite. J. Am. Chem. Soc. 2008, 130, 2862–2868. [Google Scholar] [CrossRef]
- Cui, H.G.; Webber, M.J.; Stupp, S.I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Biopolymers 2010, 94, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Jochum, M.; Peter, C. Molecular dynamics simulations of peptides at the air−water interface: Influencing factors on peptide-templated mineralization. Langmuir 2014, 30, 15486–15495. [Google Scholar] [CrossRef] [PubMed]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Selfassembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newcomb, C.J.; Bitton, R.; Velichko, Y.S.; Snead, M.L.; Stupp, S.I. The role of nanoscale architecture in supramolecular templating of biomimetic hydroxyapatite mineralization. Small 2012, 8, 2195–2202. [Google Scholar] [CrossRef] [Green Version]
- Spoerke, E.D.; Anthony, S.G.; Stupp, S.I. Enzyme directed templating of artificial bone mineral. Adv. Mater. 2009, 21, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Mata, A.; Geng, Y.; Henrikson, K.J.; Aparicio, C.; Stock, S.R.; Satcher, R.L.; Stupp, S.I. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials 2010, 31, 6004–6012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gungormus, M.; Branco, M.; Fong, H.; Schneider, J.P.; Tamerler, C.; Sarikaya, M. Self assembled bi-functional peptide hydrogels with biomineralization-directing peptides. Biomaterials 2010, 31, 7266–7274. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Zhang, J.; Xie, L.; Jandt, K.D. Biomimetic growth of hydroxyapatite on super water-soluble carbon nanotube-protein hybrid nanofibers. Carbon 2011, 49, 2216–2226. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, J.; Chen, F.; Bai, D.; Shao, C.; Wang, J.; Xi, P.; Zeng, Z. Gelatin functionalized graphene oxide for mineralization of hydroxyapatite: Biomimetic and In Vitro evaluation. Nanoscale 2014, 6, 5315–5322. [Google Scholar] [CrossRef]
- Wang, J.; Ouyang, Z.; Ren, Z.; Li, J.; Zhang, P.; Wei, G.; Su, Z. Self-assembled peptide nanofibers on graphene oxide as a novel nanohybrid for biomimetic mineralization of hydroxyapatite. Carbon 2015, 89, 20–30. [Google Scholar] [CrossRef]
- Yoh, R.; Matsumoto, T.; Sasaki, J.I.; Sohmura, T. Biomimetic fabrication of fibrin/apatite composite material. J. Biomed. Mater. Res. A 2008, 87, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; He, W.; Wang, Y.; Zhang, X.; Li, Z.; Yan, S.; Zhou, W.; Wang, G. Biomineralization of large hydroxyapatite particles using ovalbumin as biosurfactant. Mater. Lett. 2008, 62, 3603–3605. [Google Scholar] [CrossRef]
- Neelakandeswari, N.; Sangami, G.; Dharmaraj, N. Preparation and characterization of nanostructured hydroxyapatite using a biomaterial. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2011, 41, 513–516. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ji, X.; Banks, C.E.; Song, J. Flower-like agglomerates of hydroxyapatite crystals formed on an egg-shell membrane. Coll. Surf. B 2011, 82, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Neoh, K.G.; Kishen, A. A biomimetic strategy to form calcium phosphate crystals on type I collagen substrate. Mater. Sci. Eng. C 2010, 30, 822–826. [Google Scholar] [CrossRef]
- Cui, J.; Ma, C.; Li, Z.; Wu, L.; Wei, W.; Chen, M.; Peng, B.; Deng, Z. Polydopamine-functionalized polymer particles as templates for mineralization of hydroxyapatite: Biomimetic and In Vitro bioactivity. RSC Adv. 2016, 6, 6747–6755. [Google Scholar] [CrossRef]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Liu, Z.; Qu, S.; Zheng, X.; Xiong, X.; Fu, R.; Tang, K.; Zhong, Z.; Weng, J. Effect of polydopamine on the biomimetic mineralization of mussel-inspired calcium phosphate cement In Vitro. Mater. Sci. Eng. C 2014, 44, 44–51. [Google Scholar] [CrossRef]
- Gao, X.; Song, J.; Ji, P.; Zhang, X.; Li, X.; Xu, X.; Wang, M.; Zhang, S.; Deng, Y.; Deng, F.; et al. Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 3499–3515. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.B. Mussel-inspired transformation of CaCO3 to bone minerals. Biomaterials 2010, 31, 6628–6634. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, Y.; Bai, Y.; Gao, X.; Zhang, H.; Xu, A.; Huang, E.; Deng, F.; Wei, S. In Vitro biocompability/osteogenesis and In Vivo bone formation evalution of peptide-decorated apatite nanocomposites assisted via polydopamine. J. Biomed. Nanotechnol. 2016, 12, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nemoto, R.; Senna, M. Microstructure and chemical states of hydroxyapatite/silk fibroin nanocomposites synthesized via a wet-mechanochemical route. J. Nanoparticle Res. 2002, 4, 535–540. [Google Scholar] [CrossRef]
- Wang, L.; Nemoto, R.; Senna, M. Changes in microstructure and physico-chemical properties of hydroxyapatite silk fibroin nanocomposite with varying silk fibroin content. J. Eur. Ceram. Soc. 2004, 24, 2707–2715. [Google Scholar] [CrossRef]
- Furuzono, T.; Taguchi, T.; Kishida, A.; Akashi, M.; Tamada, Y. Preparation and characterization of apatite deposited on silk fabric using an alternate soaking process. J. Biomed. Mater. Res. 2000, 50, 344–352. [Google Scholar] [CrossRef]
- Kino, R.; Ikoma, T.; Monkawa, A.; Yunoki, S.; Munekata, M.; Tanaka, J.; Asakura, T. Deposition of bone-like apatite on modified silk fibroin films from simulated body fluid. J. Appl. Polym. Sci. 2006, 99, 2822–2830. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Senna, M. High-affinity integration of hydroxyapatite nanoparticles with chemically modified silk fibroin. J. Nanopart. Res. 2007, 9, 919–929. [Google Scholar] [CrossRef]
- Yang, M.; He, W.; Shuai, Y.; Min, S.; Zhu, L. Nucleation of hydroxyapatite crystals by self-assembled Bombyx mori silk fibroin. J. Polym. Sci. B 2013, 51, 742–748. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, J.B.; Kim, H.H.; Ki, C.S.; Park, S.Y.; Kim, H.J.; Park, Y.H. Surface coating of hydroxyapatite on silk nanofibre through biomineralisation using ten times concentrated simulated body fluid and the evaluation for bone regeneration. Macromol. Res. 2014, 22, 710–716. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, U.J.; Kim, H.S.; Li, C.; Wada, M.; Leisk, G.G.; Kaplan, D.L. Bone tissue engineering with premineralised silk scaffolds. Bone 2008, 42, 1226–1234. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Li, Y.; Jin, J.; Cai, Y.; Wei, K.; Yao, J. Deposition behavior and properties of silk fibroin scaffolds soaked in simulated body fluid. Mater. Chem. Phys. 2008, 111, 92–97. [Google Scholar] [CrossRef]
- Kong, X.D.; Cui, F.Z.; Wang, X.M.; Zhang, M.; Zhang, W. Silk fibroin regulated mineralisation of hydroxyapatite nanocrystals. J. Cryst. Growth 2004, 270, 197–202. [Google Scholar] [CrossRef]
- Huang, X.; Bai, S.; Lu, Q.; Liu, X.; Liu, S.; Zhu, H. Osteoinductive-nanoscaled silk/HA composite scaffolds for bone tissue engineering application. Biomed. Mater. Res. B 2015, 103, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, X.; Liu, S.; Zhang, A.; Lu, Q.; Kaplan, D.L.; Zhu, H. Biomineralization regulation by nano-sized features in silk fibroin proteins: Synthesis of water-dispersible nano-hydroxyapatite. J. Biomed. Mater. Res. B 2014, 102, 1720–1729. [Google Scholar] [CrossRef]
- Midha, S.; Tripathi, R.; Geng, H.; Lee, P.D.; Ghosh, S. Elucidation of differential mineralisation on native and regenerated silk matrices. Mater. Sci. Eng. C 2016, 68, 663–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Jin, H.J.; Botsaris, G.D.; Kaplan, D.L. Silk apatite composites from electrospun fibers. J. Mater. Res. 2005, 20, 3374–3384. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Sang, F.; Ding, B.; Chen, L.; Cui, S.; Li, K.; Han, Q.; Tan, W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite–tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2016, 58, 342–351. [Google Scholar] [CrossRef]
- Jin, Y.; Kundu, B.; Cai, Y.; Kundu, S.C.; Yao, J. Bio-inspired mineralization of hydroxyapatite in 3D silk fibroin hydrogel for bone tissue engineering. Colloids Surf. B 2015, 134, 339–345. [Google Scholar] [CrossRef]
- Ming, J.; Jiang, Z.; Wang, P.; Bie, S.; Zuo, B. Silk fibroin/sodium alginate fibrous hydrogels regulated hydroxyapatite crystal growth. Mater. Sci. Eng. C 2015, 51, 287–293. [Google Scholar] [CrossRef]
- Ming, J.F.; Bie, S.Y.; Jiang, Z.J.; Wang, P.; Zuo, B.Q. Novel hydroxyapatite nanorods crystal grow in silk fibroin/sodium alginate nanofiber hydrogel. Mater. Lett. 2014, 126, 169–173. [Google Scholar] [CrossRef]
- Utku, F.S.; Basaran, K.; Sunar, Y.; Celebioglu, H.; Kapici, I. Generation of silk fibroin-Ca-P composite biomimetic bone replacement material using electrochemical deposition. J. Elect. Electron. Eng. 2017, 17, 3439–3443. [Google Scholar]
- Panda, N.; Bissoyi, A.; Pramanik, K.; Biswas, A. Development of novel electrospun nanofibrous scaffold from P. ricini and a. mylitta silk fibroin blend with improved surface and biological properties. Mater. Sci. Eng. C 2015, 48, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.X.; Cai, X.; Mo, S.B.; Chen, L.; Shen, X.Y.; Tong, H. Fabrication and characterization of chitosan-silk fibroin/hydroxyapatite composites via In Situ precipitation for bone tissue engineering. Chin. J. Polym. Sci. 2015, 33, 1661–1671. [Google Scholar] [CrossRef]
- Polak, R.; Rodas, A.C.D.; Chicoma, D.L.; Giudici, R.; Beppu, M.M.; Higa, O.Z.; Pitombo, R.N.M. Inhibition of calcification of bovine pericardium after treatment with biopolymers, E-beam irradiation and In Vitro endothelization. Mater. Sci. Eng. C 2013, 33, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.M.; Rodas, A.C.D.; Weska, R.F.; Aimoli, C.G.; Higa, O.Z.; Maizato, M.; Leiner, A.A.; Pitombo, R.N.M.; Polakiewicz, B.; Beppu, M.M. Bovine pericardium coated with biopolymeric films as an alternative to prevent calcification: In Vitro calcification and cytotoxicity results. Mater. Sci. Eng. C 2010, 30, 575–582. [Google Scholar] [CrossRef]
- Cao, B.; Mao, C. Oriented nucleation of hydroxylapatite crystals on spider dragline silks. Langmuir 2007, 23, 10701–10705. [Google Scholar] [CrossRef] [PubMed]
- Ajeesh, M.; Francis, B.F.; Annie, J.; Harikrishna Varma, P.R. Nano iron oxide hydroxyapatite composite ceramics with enhanced radiopacity. J. Mater. Sci. Mater. Med. 2010, 21, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Bahrololoom, M.E.; Vashaee, D.; Tayebi, L. In Situ preparation of iron oxide nanoparticles in natural hydroxyapatite/chitosan matrix for bone tissue engineering application. Ceram. Int. 2015, 41, 3094–3100. [Google Scholar] [CrossRef]
- Cojocaru, F.D.; Balan, V.; Popa, M.I.; Lobiuc, A.; Antoniac, A.; Antoniac, I.V.; Verestiuc, L. Biopolymers—Calcium phosphates composites with inclusions of magnetic nanoparticles for bone tissue engineering. Inter. J. Biol. Macromol. 2019, 125, 612–620. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M. Regenerated cellulose/wool blend enhanced biomimetic hydroxyapatite mineralization. Inter. J. Biol. Macromol. 2016, 92, 920–925. [Google Scholar] [CrossRef]
- Salama, A.; Shukry, N.; El-Gendy, A.; El-Sakhawy, M. Bioactive cellulose grafted soy protein isolate towards biomimetic calcium phosphate mineralization. Ind. Crops Prod. 2017, 95, 170–174. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhang, W.; Yao, H.B.; Zhu, H.Z.; Mao, L.B.; Yu, S.H. Bioinspired crystallization of continuous calcium phosphate films on a Langmuir monolayer of zein protein: Their mechanical performance, hydrophilicity, and biocompatibility. Cryst. Growth Des. 2013, 13, 3505–3513. [Google Scholar] [CrossRef]
- Shahlori, R.; Waterhouse, G.I.N.; Nelson, A.R.J.; McGillivray, D.J. Morphological, chemical and kinetic characterisation of zein protein-induced biomimetic calcium phosphate films. J. Mater. Chem. B 2015, 3, 6213–6223. [Google Scholar] [CrossRef]
- Selvakumar, R.; Seethalakshmi, N.; Thavamani, P.; Naidu, R.; Megharaj, M. Recent advances in the synthesis of inorganic nano/microstructures using microbial biotemplates and their applications. RSC Adv. 2014, 4, 52156–52169. [Google Scholar] [CrossRef] [Green Version]
- Kajander, E.O.; Ciftçioglu, N. Nanobacteria: An alternative mechanism for pathogenic intra-and extracellular calcification and stone formation. Proc. Natl. Acad. Sci. USA 1998, 95, 8274–8279. [Google Scholar] [CrossRef] [Green Version]
- Kajander, E.O. Nanobacteria—Propagating calcifying nanoparticles. Lett. Appl. Microbiol. 2006, 42, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Kumara, M.T.; Muralidharan, S.; Tripp, B.C. Generation and characterization of inorganic and organic nanotubes on bioengineered Flagella of Mesophilic bacteria. J. Nanosci. Nanotechnol. 2007, 7, 2260–2272. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, E.R.; Torres, M.G.; Muñoz, S.V.; Rosas, E.R.; Vázquez, C.; Rodríguez Talavera, R. Growth of hydroxyapatite on the cellular membrane of the bacterium Bacillus thuringiensis for the preparation of hybrid biomaterials. Mater. Sci. Eng. C 2016, 58, 614–621. [Google Scholar] [CrossRef]
- Li, D.; Newton, S.M.C.; Klebba, P.E.; Mao, C. Flagellar display of bone-protein-derived peptides for studying peptide-mediated biomineralization. Langmuir 2012, 28, 16338–16346. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Cao, B.; Mao, C. Bacteriophage bundles with prealigned Ca2+ initiate the oriented nucleation and growth of hydroxylapatite. Chem. Mater. 2010, 22, 3630–3636. [Google Scholar] [CrossRef] [Green Version]
- Šupová, M. Isolation and preparation of nanoscale bioapatites from natural sources: A Review. J. Nanosci. Nanotechnol. 2014, 14, 546–563. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Banerjee, P.; Madhu, S.; Chandra Babu, N.K.; Shanthi, C. Bio-mimetic mineralization potential of collagen hydrolysate obtained from chromium tanned leather waste. Mater. Sci. Eng. C 2015, 49, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S.; Guha, A. Waste utilization for the controlled synthesis of nanosized hydroxyapatite. Mater. Sci. Eng. C 2009, 29, 1326–1329. [Google Scholar] [CrossRef]
- Lin, K.; Wu, C.; Chang, J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 2014, 10, 4071–4102. [Google Scholar] [CrossRef]
- Meldrum, F.C.; Cölfen, H.; Cölfen, H. Controlling mineral morphologies and structures in biological and synthetic systems. Chem. Rev. 2008, 108, 4332–4432. [Google Scholar] [CrossRef]
- Teng, S.; Shi, J.; Chen, L. Formation of calcium phosphates in gelatin with a novel diffusion system. Coll. Surf. B 2006, 49, 87–92. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Zhang, L.; Li, Y.; Wei, S. In Situ synthesis and In Vitro biocompatibility of needle-like nano-hydroxyapatite in agar–gelatin-co-hydrogel. Mater. Lett. 2013, 104, 8–12. [Google Scholar] [CrossRef]
- Han, Y.; Li, S.; Wang, X. A novel thermolysis method of colloidal protein precursors to prepare hydroxyapatite nanocrystals. Cryst. Res. Technol. 2009, 44, 336–340. [Google Scholar] [CrossRef]
- Han, Y.; Li, S.; Wang, X.; Jia, L.; He, J. Preparation of hydroxyapatite rod-like crystals by protein precursor method. Mater. Res. Bull. 2007, 42, 1169–1177. [Google Scholar] [CrossRef]
- Zhao, H.; He, W.; Wang, Y.; Zhang, X.; Li, Z.; Yan, S.; Zhou, W. Biomimetic synthesis and characterization of hydroxyapatite crystal with low phase transformation temperature. J. Chem. Eng. Data 2008, 53, 2735–2738. [Google Scholar] [CrossRef]
- Gang, Z.H.; Qingshan, Z. Glutamic acid-mediated synthesis of ultralong hydroxyapatite nanoribbons under hydrothermal conditions. Chem. Lett. 2005, 34, 788–789. [Google Scholar] [CrossRef] [Green Version]
- Gopi, D.; Indira, J.; Collins Arun Prakash, V.; Kavitha, L. Spectroscopic characterization of porous nanohydroxyapatite synthesized by a novel amino acid soft solution freezing method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Feng, Q.; Liu, H.; Yang, X. Preparation of biomimetic hydroxyapatite by biomineralization and calcination using poly(L-lactide)/gelatin composite fibrous mat as template. Mater. Lett. 2013, 91, 275–278. [Google Scholar] [CrossRef]
- Mohandes, F.; Salavati-Niasari, M.; Fereshteh, Z.; Fathi, M. Novel preparation of hydroxyapatite nanoparticles and nanorods with the aid of complexing agents. Ceram. Int. 2014, 40, 12227–12233. [Google Scholar] [CrossRef]
- Sudo, S.Z.; Schotzko, N.K.; Folke, L.E.A. Use of hydroxyapatite coated glass beads for preclinical testing of potential antiplaque agents. Appl. Environ. Microbiol. 1976, 32, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Calcium orthophosphate coatings, films and layers. Prog. Biomater. 2012, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Heimann, R.B. The challenge and promise of low-temperature bioceramic coatings: An editorial. Surf. Coat. Technol. 2016, 301, 1–5. [Google Scholar] [CrossRef]
- Koju, N.; Sikder, P.; Ren, Y.; Zhou, H.; Bhaduri, S.B. Biomimetic coating technology for orthopedic implants. Cur. Opin. Chem. Eng. 2017, 15, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Lemaître, J.; Bowen, P. A comparative study of simulated body fluids in the presence of proteins. Acta Biomater. 2017, 53, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Tas, A.C. The use of physiological solutions or media in calcium phosphate synthesis and processing. Acta Biomater. 2014, 10, 1771–1792. [Google Scholar] [CrossRef]
- Chakraborty, J.; Sinha, M.K.; Basu, D. Biomolecular template-induced biomimetic coating of hydroxyapatite on an SS 316 L substrate. J. Am. Ceram. Soc. 2007, 90, 1258–1261. [Google Scholar] [CrossRef]
- Ao, H.; Xie, Y.; Tan, H.; Wu, X.; Liu, G.; Qin, A.; Zheng, X.; Tang, T. Improved hMSC functions on titanium coatings by type I collagen immobilization. J. Biomed. Mater. Res. A 2014, 102, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Tapsir, Z.; Saidin, S. Synthesis and characterization of collagen–hydroxyapatite immobilized on polydopamine grafted stainless steel. Surf. Coat. Technol. 2016, 285, 11–16. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-alloys and surface modifications to improve the mechanical properties and the biological response to orthopedic and dental implants: A review. Biomed. Res. Int. 2016, 2908570. [Google Scholar] [CrossRef] [Green Version]
- Iafisco, M.; Foltran, I.; Sabbatini, S.; Tosi, G.; Roveri, N. Electrospun nanostructured fibers of collagen-biomimetic apatite on titanium alloy. Bioinorg. Chem. Appl. 2012, 123953. [Google Scholar] [CrossRef]
- Sukhodub, L.F. Materials and coatings based on biopolymerapatite nanocomposites: Obtaining, structural characterization and In Vivo tests. Mater. und Werkst. Entwickl. Fert. Prüfung Eig. und Anwend. Tech. Werkst. 2009, 40, 318–325. [Google Scholar] [CrossRef]
- Ciobanu, G.; Ciobanu, O. Investigation on the effect of collagen and vitamins on biomimetic hydroxyapatite coating formation on titanium surfaces. Mater. Sci. Eng. C 2013, 33, 1683–1688. [Google Scholar] [CrossRef]
- Tan, G.; Zhou, L.; Ning, C.; Tan, C.; Ni, G.; Liao, J.; Yu, P.; Chen, X. Biomimetically-mineralized composite coatings on titanium functionalized with gelatin methacrylate hydrogels. Appl. Surf. Sci. 2013, 279, 293–299. [Google Scholar] [CrossRef]
- Pan, M.; Kong, X.; Cai, Y.; Yao, J. Hydroxyapatite coating on the titanium substrate modulated by a recombinant collagen-like protein. Mater. Chem. Phys. 2011, 126, 811–817. [Google Scholar] [CrossRef]
- Ceylan, H.; Kocabey, S.; Gulsuner, H.U.; Balcik, O.S.; Guler, M.O.; Tekinay, A.B. Bone-like mineral nucleating peptide nanofibers induce differentiation of human mesenchymal stem cells into mature osteoblasts. Biomacromolecules 2014, 15, 2407–2418. [Google Scholar] [CrossRef] [Green Version]
- Sargeant, T.D.; Guler, M.O.; Oppenheimer, S.M.; Mata, A.; Satcher, L.R.; Dunand, D.C.; Stupp, S.I. Hybrid bone implants: Self-assembly of peptide amphiphile nanofiberswithin porous titanium. Biomaterials 2008, 29, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C.; Freche, M. In vitro crystallization of octacalcium phosphate on type I collagen: Influence of serum albumin. J. Mater. Sci. Mater. Med. 1999, 10, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Stenport, V.; Kjellin, P.; Andersson, M.; Currie, F.; Sul, Y.T.; Wennerberg, A.; Arvidsson, A. Precipitation of calcium phosphate in the presence of albumin on titanium implants with four different possibly bioactive surface preparations. An In Vitro study. J. Mater. Sci. Mater. Med. 2008, 19, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Neshatian, M.; Holcroft, J.; Ganss, B. The enamel protein ODAM promotes mineralization in a collagen matrix. Con. Tis. Res. 2018, 59, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Schickle, K.; Spitz, J.; Neuss, S.; Telle, R. Biomimetic In Situ nucleation of calcium phosphates by protein immobilization on high strength ceramic materials. J. Europ. Ceram. Soc. 2018, 38, 271–277. [Google Scholar] [CrossRef]
- Petrakova, N.V.; Kuvshinova, E.A.; Ashmarin, A.A.; Konovalov, A.A.; Nikitina, Y.O.; Egorov, A.A.; Sviridova, I.K.; Barinov, S.M.; Komlev, V.S. Calcium phosphate ceramic surface coating via precipitation approach. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Barcelona, Spain, 2019; Volume 525, p. 012101. [Google Scholar]
- Andersson, M.; Currie, F.; Kjellin, P.; Sul, Y.T.; Stenport, V. Nucleation and growth of calcium phosphates in the presence of fibrinogen on titanium implants with four potentially bioactive surface preparations. An In Vitro study. J. Mater. Sci. Mater. Med. 2009, 20, 1869–1879. [Google Scholar] [CrossRef]
- Areva, S.; Paldan, H.; Peltola, T.; Närhi, T.; Jokinen, M.; Lindén, M. Use of sol–gel-derived titania coating for direct soft tissue attachment. J. Biomed. Mater. Res A 2004, 70, 169–178. [Google Scholar] [CrossRef]
- Shou, G.; Dong, L.; Liu, Z.; Cheng, K.; Weng, W. Facet-specific mineralization behavior of nano-CaP on anatase polyhedral microcrystals. ACS Biomater. Sci. Eng. 2017, 3, 875–881. [Google Scholar] [CrossRef]
- Chakraborty, J.; Chatterjee, S.; Sinha, M.K.; Basu, D. Effect of albumin on the growth characteristics of hydroxyapatite coatings on alumina substrates. J. Am. Ceram. Soc. 2007, 90, 3360–3363. [Google Scholar] [CrossRef]
- Kokubo, T. Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials; ISO: Geneva, Switzerland, 2014; p. STD-917469. [Google Scholar]
- Zhuang, J.; Lin, J.; Li, J.; Wang, H.; Cheng, K.; Weng, W. Electrochemical deposition of mineralized BSA/collagen coating. Mater. Sci. Eng. C 2016, 66, 66–76. [Google Scholar] [CrossRef]
- D´Elia, N.L.; Gravina, N.; Ruso, J.M.; Marco-Brown, J.L.; Sieben, J.M.; Messina, P.V. Albumin-mediated deposition of bone-like apatite onto nano-sized surfaces: Effect of surface reactivity and interfacial hydration. J. Coll. Inter. Sci. 2017, 494, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial surface treatment for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Salmeron-Sanchez, M.; Dalby, M.J. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue Eng. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Sun, T.; Wang, M. Electrochemical deposition of apatite/collagen composite coating on NiTi shape memory alloy and coating properties. In Proceedings of the Materials Research Society Symposium, MRS Fall Meeting, Boston, MA, USA, 1–4 December 2009; Volume 1239, pp. 141–146. [Google Scholar] [CrossRef]
- Graf, H.L.; Stoeva, S.; Armbruster, F.P.; Neuhaus, J.; Hilbig, H. Effect of bone sialoprotein and collagen coating on cell attachment to TICER and pure titanium implant surfaces. Int. J. Oral Maxillofac. Surg. 2008, 37, 634–640. [Google Scholar] [CrossRef]
- Sano, K.I.; Shiba, K. A hexapeptide motif that electrostatically binds to the surface of titanium. J. Am. Chem. Soc. 2003, 125, 14234–14235. [Google Scholar] [CrossRef]
- Kelly, M.; Williams, R.; Aojula, A.; O’Neill, J.; Trzińscka, Z.; Grover, L.; Scott, R.A.H.; Peacock, A.F.A.; Logan, A.; Stamboulis, A.; et al. Peptide aptamers: Novel coatings for orthopaedic implants. Mater. Sci. Eng. C 2015, 54, 84–93. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Elahinia, M. Biodegradable magnesium alloys. In Metals for Biomedical Devices, 2nd ed.; Woodhead Publishing Series in Biomaterials: Cambridge, UK; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–289. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Lin, B.; Zhong, M.; Zheng, C.; Cao, L.; Wang, D.; Wang, L.; Liang, J.; Cao, B. Preparation and characterization of dopamine-induced biomimetic hydroxyapatite coatings on the AZ31 magnesium alloy. Surf. Coat. Technol. 2015, 281, 82–88. [Google Scholar] [CrossRef]
- Cui, W.; Beniash, E.; Gawalt, E.; Xu, Z.; Sfeir, C. Biomimetic coating of magnesium alloy for enhanced corrosionresistance and calcium phosphate deposition. Acta Biomater. 2013, 9, 8650–8659. [Google Scholar] [CrossRef]
- Abdelkebir, K.; Morin-Grognet, S.; Gaudière, F.; Coquerel, G.; Labat, B.; Atmani, H.; Ladam, G. Biomimetic layer-by-layer templates for calcium phosphate biomineralization. Acta Biomater. 2012, 8, 3419–3428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Y.; Altankov, G.; Grabiec, U.; Bennett, M.; Salmeron-Sanchez, M.; Dehghani, F.; Groth, T. Molecular composition of GAG-collagen I multilayers affects remodeling of terminal layers and osteogenic differentiation of adipose-derived stem cells. Acta Biomater. 2016, 41, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Y.; Li, L.H.; Zhou, C.R.; Heyroth, F.; Fuhrmann, B.; Maeder, K.; Groth, T. Improved stability and cell response by intrinsic cross-linking of multilayers from collagen I and oxidized glycosaminoglycans. Biomacromolecules 2014, 15, 4272–4280. [Google Scholar] [CrossRef]
- Kong, J.; Wei, B.; Groth, T.; Chen, Z.; Li, L.; He, D.; Huang, R.; Chu, J.; Zhao, M. Biomineralization improves mechanical and osteogenic properties of multilayer-modified PLGA porous scaffolds. J. Biomed. Mater. Res. A 2018, 106, 2714–2725. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, J.; Yuan, X.; Xia, Y. Coating Electrospun Poly(ε-caprolactone) Fibers with gelatin and calcium phosphate and their use as biomimetic scaffolds for bone tissue engineering. Langmuir 2008, 24, 14145–14150. [Google Scholar] [CrossRef] [PubMed]
- Tarik Arafat, M.; Lam, C.X.F.; Ekaputra, A.K.; Wong, S.Y.; Li, X.; Gibson, I. Biomimetic composite coating on rapid prototyped scaffolds for bone tissue engineering. Acta Biomater. 2011, 7, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Sakai, A.; Sakamoto, Y.; Matsuno, H.; Serizawa, T. Facile surface functionalization of polystyrene substrates with biomimetic apatite by utilizing serum proteins. Chem. Lett. 2010, 39, 220–222. [Google Scholar] [CrossRef]
- Iijima, K.; Sakai, A.; Komori, A.; Sakamoto, Y.; Matsuno, H.; Serizawa, T.; Hashizume, M. Control of biomimetic hydroxyapatite deposition on polymer substrates using different protein adsorption abilities. Col. Surf. B 2015, 130, 77–83. [Google Scholar] [CrossRef]
- Iijima, K.; Suzuki, R.; Iizuka, A.; Ueno-Yokohata, H.; Kiyokawa, N.; Hashizume, M. Surface functionalization of tissue culture polystyrene plates with hydroxyapatite under body fluid conditions and its effect on differentiation behaviors of mesenchymal stem cells. Colloids Surf. B 2016, 147, 351–359. [Google Scholar] [CrossRef]
- Iijima, K.; Iizuka, A.; Suzuki, R.; Ueno-Yokohata, H.; Kiyokawa, N.; Hashizume, M. Effect of protein adsorption layers and solution treatments on hydroxyapatite deposition on polystyrene plate surfaces in simulated body fluids. J. Mater. Sci. Mater. Med. 2017, 28, 193. [Google Scholar] [CrossRef]
- Iijima, K.; Iizuka, A.; Suzuki, R.; Ueno-Yokohata, H.; Kiyokawa, N.; Hashizume, M. Preparation of 3D porous hydroxyapatite cell scaffolds using polystyrene templates under biomimetic conditions. J. Ceram. Soc. Jpn. 2018, 126, 956–958. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, L.; Wang, J.; Jiang, L.; Zhao, H.; Yishake, M.; Fan, Z. Bioinspired modification of poly(L-lactic acid)/nano-sized β-tricalcium phosphate composites with gelatin/hydroxyapatite coating for enhanced osteointegration and osteogenesis. J. Biomed. Nanotechnol. 2018, 14, 884–899. [Google Scholar] [CrossRef] [PubMed]
- Elkassas, D.; Arafa, A. The innovative applications of therapeutic nanostructures in dentistry. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1543–1562. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Iafisco, M.; Delgado-López, J.M.; Sarda, S.; Drouet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Green, D.W.; Goto, T.K.; Kim, K.S.; Jung, H.S. Calcifying tissue regeneration via biomimetic materials chemistry. J. R. Soc. Interface 2014, 11, 20140537. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Luz, G.M.; Mano, J.F. Mineralized structures in nature: Examples and inspirations for the design of new composite materials and biomaterials. Compos. Sci. Technol. 2010, 70, 1777–1788. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Zou, B.; Narayanan, K.; George, A. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone 2004, 34, 921–932. [Google Scholar] [CrossRef]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1. [Google Scholar] [CrossRef]
- Sauro, S.; Pashley, D.H. Strategies to stabilise dentine-bonded interfaces through remineralising operative approaches – State of The Art. In. J. Adhes. Adhes. 2016, 69, 39–57. [Google Scholar] [CrossRef]
- Bonchev, A.; Simeonov, M.; Vassileva, R. Review: Biomimetic approach for remineralization of human enamel. Int. J. Sci. Res. 2018, 7, 1416–1420. [Google Scholar] [CrossRef]
- Cao, C.Y.; Mei, M.L.; Li, Q.; Lo, E.C.M.; Chu, C.H. Methods for biomimetic mineralisation of human enamel: A systematic review. Materials 2015, 8, 2873–2886. [Google Scholar] [CrossRef] [Green Version]
- Firth, A.; Aggeli, A.; Burke, J.; Yang, X.; Kirkham, J. Biomimetic selfassembling peptides as injectable scaffolds for hard tissue engineering. Nanomedicine. 2006, 1, 189–199. [Google Scholar] [CrossRef]
- Fan, Y.; Nelson, J.R.; Alvarez, J.R.; Hagan, J.; Berrier, A.; Xu, X. Amelogenin assisted Ex Vivo remineralization of human enamel: Effects of supersaturation degree and fluoride concentration. Acta Biomater. 2011, 7, 2293–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wang, L.; Quin, Y.; Sun, Z.; Henneman, Z.; Moradian-Oldak, J.; Nancollas, G.H. How amelogenin orchestrates the organization of hierarchical elongated microstructures of apatite. J. Phys. Chem. B 2010, 114, 2293–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Avila, O.; Wu, S.; Kim, S.J.; Cheng, Y.; Khan, F.; Samudrala, R.; Sali, A.; Horst, J.A.; Habelitz, S. Self-assembly of filamentous amelogenin requires calcium and phosphate: From dimers via nanoribbons to fibrils. Biomacromolecules 2012, 13, 3494–3502. [Google Scholar] [CrossRef] [Green Version]
- Friddle, R.W.; Battle, K.; Trubetskoy, V.; Tao, J.; Salter, E.A.; Moradian-Oldak, J.; De Yoreo, J.J.; Wierzbicki, A. Single-molecule determination of the face-specific adsorption of amelogenin’s C-terminus on hydroxyapatite. Angew. Chem. Int. Ed. Engl. 2011, 50, 7541–7545. [Google Scholar] [CrossRef]
- Wen, H.B.; Moradian-Oldak, J.; Fincham, A.G. Dose-dependent modulation of octacalcium phosphate crystal habit by amelogenins. J. Dent. Res. 2000, 79, 1902–1906. [Google Scholar] [CrossRef]
- Iijima, M.; Moriwaki, Y.; Takagi, T.; Moradian-Oldak, J. Effects of bovine amelogenins on the crystal morphology of octacalcium phosphate in a model system of tooth enamel formation. J. Cryst. Growth 2001, 222, 615–626. [Google Scholar] [CrossRef]
- Ruan, Q.; Moradian-Oldak, J. Amelogenin and enamel biomimetics. J. Mater. Chem. B Mater. Biol. Med. 2015, 3, 3112–3129. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Litman, A.; Margolis, H.C.; Yamakoshi, Y.; Simmer, J.P. Biomimetic enamel regeneration mediated by leucine-rich amelogenin peptide. J. Dent. Res. 2017, 96, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Ning, T.Y.; Cao, Y.; Zhang, W.B.; Mei, M.L.; Chu, C.H. A novel self-assembled oligopeptide amphiphile for biomimetic mineralization of enamel. BMC Biotechnol. 2014, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uskoković, V.; Li, W.; Habelitz, S. Amelogenin as a promoter of nucleation and crystal growth of apatite. J. Cryst. Growth 2011, 316, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.Y.; Green, S.; Wiedemann-Bidlack, F.B.; Beniash, E.; Yamakoshi, Y.; Simmer, J.P.; Margolis, H.C. Regulation of calcium phosphate formation by amelogenins under physiological conditions. Eur. J. Oral Sci. 2011, 119, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogan, S.; Fong, H.; Yucesoy, D.T.; Cousin, T.; Gresswell, C.; Dag, S.; Huang, G.; Sarikaya, M. Biomimetic tooth repair: Amelogenin-derived peptide enables In Vitro remineralization of human enamel. ACS Biomater. Sci. Eng. 2018, 4, 1788–1796. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Z.; Yang, J.; Lu, D.; Kishen, A.; Li, Y.; Chen, Z.; Que, K.; Zhang, Q.; Deng, X.; et al. Oriented and ordered biomimetic remineralization of the surface of demineralized dental enamel using HAP@ACP nanoparticles guided by glycine. Sci. Rep. 2017, 7, 40701. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Tao, J.; Xu, X.; Tang, R. Adsorption processes of Gly and Glu amino acids on hydroxyapatite surfaces at the atomic level. Langmuir 2007, 23, 8972–8981. [Google Scholar] [CrossRef]
- Gopi, D.; Indira, J.; Kavitha, L.; Sekar, M.; Kamachi Mudali, U. Synthesis of hydroxyapatite nanoparticles by a novel ultrasonic assisted with mixed hollow sphere template method. Spectrochim. Acta A 2012, 93, 131–134. [Google Scholar] [CrossRef]
- Gopi, D.; Nithiya, S.; Shinyjoy, E.; Kavitha, L. Spectroscopic investigation on formation and growth of mineralized nanohydroxyapatite for bone tissue engineering applications. Spectrochim. Acta A 2012, 92, 194–200. [Google Scholar] [CrossRef]
- Tao, J.; Pan, H.; Zeng, Y.; Xu, X.; Tang, R. Roles of amorphous calcium phosphate and biological additives in the assembly of hydroxyapatite nanoparticles. J. Phys. Chem. B 2007, 111, 13410–13418. [Google Scholar] [CrossRef]
- Li, L.; Mao, C.; Wang, J.; Xu, X.; Pan, H.; Deng, Y.; Gu, X.; Tang, R. Bio-inspired enamel repair via Glu-directed assembly of apatite nanoparticles: An approach to biomaterials with optimal characteristics. Adv. Mater. 2011, 23, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Z.; Cao, Y.; Liu, W.; Chu, C.H.; Li, Q.L. Polydopamine-induced tooth remineralization. ACS Appl. Mater. Interfaces 2012, 4, 6901–6910. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Jin, J.; Mei, D.; Xia, N.; Yao, J. Effect of silk sericin on assembly of hydroxyapatite nanocrystals into enamel prism-like structure. J. Mater. Chem. 2009, 19, 5751–5758. [Google Scholar] [CrossRef]
- Cai, Y.; Mei, D.; Jiang, T.; Yao, J. Synthesis of oriented hydroxyapatite crystals: Effect of reaction conditions in the presence or absence of silk sericin. Mater. Lett. 2010, 64, 2676–2678. [Google Scholar] [CrossRef]
- Reynolds, E.C.; Black, C.L.; Cai, F.; Cross, K.J.; Eakins, D.; Huq, N.L. Advances in enamel remineralisation: Casein phosphopeptide-amorphous calcium phosphate. J. Clin. Dent. 1999, 10, 86–88. [Google Scholar]
- Rahiotis, C.; Vougiouklakis, G. Effect of a CPP-ACP agent on the demineralization andremineralization of dentine In Vitro. J. Dent. 2007, 35, 695–698. [Google Scholar] [CrossRef]
- Cao, Y.; Mei, M.L.; Xu, J.; Lo, E.C.M.; Li, Q.; Chu, C.H. Biomimetic mineralisation of phosphorylated dentine by CPP-ACP. J. Dent. 2013, 41, 818–825. [Google Scholar] [CrossRef]
- Rad-Malekshahi, M.; Lempsink, L.; Amidi, M.; Hennink, W.E.; Mastrobattista, E. Biomedical applications of self-assembling peptides. Bioconjugate Chem. 2016, 27, 3–18. [Google Scholar] [CrossRef]
- Hosseinkhani, H.; Hong, P.D.; Yu, D.S. Self-assembled proteins and peptides for regenerative medicine. Chem. Rev. 2013, 113, 4837–4861. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Y.; Wei, W.; Mao, J. GEPIs-HA hybrid: A novel biomaterial for tooth repair. Med. Hypotheses 2008, 71, 591–593. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Gao, L.; Wu, C.; Chang, J. Synthesis of artificial dental enamel by an elastin-like polypeptide assisted biomimetic approach. J. Mater. Chem. B 2018, 5, 844–853. [Google Scholar] [CrossRef]
- Mao, J.; Shi, X.; Wu, Y.B.; Gong, S.Q. Identification of specific hydroxyapatite {001} binding heptapeptide by phage display and its nucleation effect. Materials 2016, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.M.; Tian, L.L.; Cheng, Z.J.; Cui, F.Z. In Situ remineralizaiton of partially demineralized human dentine mediated by a biomimetic non-collagen peptide. Soft Matter 2011, 20, 9673–9680. [Google Scholar] [CrossRef]
- He, G.; Gajjeraman, S.; Schultz, D.; Cookson, D.; Qin, C.; Butler, W.T.; Hao, J.; George, A. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry 2005, 44, 16140–16148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beniash, E.; Deshpande, A.S.; Fang, P.A.; Lieb, N.S.; Zhang, X.; Sfeir, C.S. Possible role of DMP1 in dentin mineralization. J. Struc. Biol. 2011, 174, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.Y.; Li, C.C.; Hsu, C.C. Characterization of the effects of 3DSS peptide on remineralized enamel in artificial saliva. J. Mech. Behav. Biomed. Mater. 2012, 6, 74–79. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chung, H.Y.; Yang, J.M.; Shi, W.; Wu, B. Influence of 8DSS peptide on nano-mechanical behavior of human enamel. J. Dent. Res. 2011, 90, 88–92. [Google Scholar] [CrossRef]
- Chung, H.Y.; Li, C.C. Asparagine-serine-serine peptide regulates enamel remineralization In Vitro. J. Mater. Res. 2013, 28, 2890–2896. [Google Scholar] [CrossRef]
- Chung, H.Y.; Li, C.C. Microstructure and nanomechanical properties of enamel remineralized with asparagine–serine–serine peptide. Mater. Sci. Eng. C 2013, 33, 969–973. [Google Scholar] [CrossRef]
- Melcher, M.; Facey, S.J.; Henkes, T.M.; Subkowski, T.; Hauer, B. Accelerated nucleation of hydroxyapatite using an engineered hydrophobin fusion protein. Biomacromolecules 2016, 17, 1716–1726. [Google Scholar] [CrossRef]
- Ma, B.; Gong, S.; Zhou, B.; Mao, J. Self-assembled nanofiber networks formed from biomineralization-directing genetically engineered peptides. J. Biomater. Tissue Eng. 2017, 7, 355–362. [Google Scholar] [CrossRef]
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Müller, B.; Pieles, U. Biomimetic remineralization of carious lesions by self-assembling peptide. J. Dent. Res. 2017, 96, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.C.; Brookes, S.J.; Aggeli, A. A Self-assembling peptide scaffolds promote enamel remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Wierichs, R.J.; Kogel, J.; Lausch, J.; Esteves-Oliveira, M.; Meyer-Lueckel, H. Effects of self-assembling peptide P11-4, fluorides, and caries infiltration on artificial enamel caries lesions In Vitro. Caries Res. 2017, 51, 451–459. [Google Scholar] [CrossRef]
- Huang, Z.; Newcomb, C.J.; Bringas, C., Jr.; Stupp, S.I.; Snead, M.L. Biological synthesis of tooth enamel instructed by an artificial matrix. Biomaterials 2010, 31, 9202–9211. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.H.; Zhou, P.L.; Yang, S.P.; Yu, X.B.; Yang, L.Z. Controllable synthesis of hydroxyapatite nanocrystals via a dendrimer-assisted hydrothermal process. Mater. Res. Bull. 2007, 42, 1611–1618. [Google Scholar] [CrossRef]
- Yan, S.; Zhou, Z.H.; Zhang, F.; Yang, S.P.; Yang, L.; Yu, X.B. Effect of anionic PAMAM with amido groups starburst dendrimers on the crystallization of Ca10(PO4)6(OH)2 by hydrothermal method. Mater. Chem. Phys. 2006, 99, 164–169. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Z.; Yang, S.P.; Mao, L.; Chen, H.; Yu, X. Hydrothermal synthesis of hydroxyapatite nanorods in the presence of anionic starburst dendrimer. Mater. Lett. 2005, 59, 1422–1425. [Google Scholar] [CrossRef]
- Pramanik, N.; Imae, T. Fabrication and characterization of dendrimer functionalized mesoporous hydroxyapatite. Langmuir 2012, 28, 14018–14027. [Google Scholar] [CrossRef]
- Chen, H.; Holl, M.M.B.; Orr, B.G.; Majoros, I.; Clarkson, B.H. Interaction of dendrimers (artificial proteins) with biological hydroxyapatite crystals. J. Dent. Res. 2003, 82, 443–448. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Orr, B.G.; Holl, M.M.B.; Majoros, I.; Clarkson, B.H. Nanoscale probing of the enamel nanorod surface using polyamidoamine dendrimers. Langmuir 2004, 20, 4168–4171. [Google Scholar] [CrossRef]
- Chen, L.; Liang, K.; Li, J.; Wu, D.; Zhou, X.; Li, J. Regeneration of biomimetic hydroxyapatite on etched human enamel by anionic PAMAM template In Vitro. Arch. Oral Biol. 2013, 58, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, H.; Tang, B.; Liang, K.; Li, J. Biomimetic remineralization of human enamel in the presence of polyamidoamine dendrimers In Vitro. Caries Res. 2015, 49, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, J.; Li, J.; Chen, L.; Liang, K.; Wu, W.; Chen, X.; Li, J. Bioinspired intrafibrillar mineralization of human dentinen by PAMAM dendrimer. Biomaterials 2013, 34, 6738–6747. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; He, H.; Wang, L.; Jia, X.; Feng, H. Oriented crystallization of hydroxyapatite by the biomimetic amelogenin nanospheres from self-assemblies of amphiphilic dendrons. Chem. Commun. 2011, 47, 10100–10102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Chen, Y.; Li, L.; Sun, J.; Gu, X.H.; Xu, X.R.; Pan, H.H.; Tang, R.K. Remineralization of dentin collagen by meta-stabilized amorphous calcium phosphate. Cryst. Eng. Commun. 2013, 15, 6151–6158. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Sun, J.; Mao, C.; Wang, W.; Pan, H.; Tang, R.; Gu, X. Hierarchical structure and mechanical properties of remineralized dentin. J. Mech. Behav. Biomed. Mater. 2014, 40, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Arola, D.D.; Gu, L.; Kim, Y.K.; Mai, S.; Liu, Y.; Pashley, D.H.; Tay, F.R. Functional biomimetic analogs help remineralize apatite-depleteddemineralized resin-infiltrated dentin via a bottom–up approach. Acta Biomater. 2010, 6, 2740–2750. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Mai, S.; Li, N.; Yiu, C.K.Y.; Mao, J.; Pashley, D.H.; Tay, F.R. Differences between top-down and bottom-up approaches in mineralizing thick, partially demineralized collagen scaffolds. Acta Biomater. 2011, 7, 1742–1751. [Google Scholar] [CrossRef] [Green Version]
- Abuna, G.; Feitosa, V.P.; Correr, A.B.; Cama, G.; Giannini, M.; Sinhoreti, M.A.; Pashley, D.H.; Sauro, S. Bonding performance of experimental bioactive/biomimetic self-etch adhesives doped with calcium-phosphate fillers and biomimetic analogs of phosphoproteins. J. Dent. 2016, 52, 79–86. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Biomimetic remineralization of resin-bonded acid-etched dentin. J. Dent. Res. 2009, 88, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.; Qi, Y.; Niu, L.N.; Elshafiy, S.; Mao, J.; Breschi, L.; Pashley, D.H.; Tay, F.R. The use of sodium trimetaphosphate as a biomimetic analog of matrix phosphoproteins for remineralization of artificial caries-like dentin. Dent. Mater. 2011, 27, 465–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Gu, L.; Huang, Z.; Sun, Q.; Chen, H.; Ling, J.; Mai, S. Intrafibrillar mineralization of polyacrylic acid-bound collagen fibrils using a two-dimensional collagen model and Portland cement-based resins. Eur. J. Oral Sci. 2017, 125, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, S. Regeneration of human tooth enamel. Angew. Chem. Int. Edit. 2004, 43, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Golomb, G.; Schoen, F.J.; Smith, M.S.; Linden, J.; Dixion, M.; Levy, R.J. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardum used in cardiac-valve bioprostheses. Am. J. Pathol. 1987, 127, 122–130. [Google Scholar]
- Levy, R.J.; Schoen, F.J.; Sherman, F.S.; Nichols, J.; Hawley, M.A.; Lund, S.A. Calcification of subcutaneously implanted type I collagen sponges. Effects of formaldehyde and glutaraldehyde pretreatments. Am. J. Pathol. 1986, 122, 71–82. [Google Scholar]
- Cilli, R.; Prakki, A.; de Araujo, P.A.; Pereira, J.C. Influence of glutaraldehyde priming on bond strength of an experimental adhesive system applied to wet and dry dentine. J. Dent. 2009, 37, 212–218. [Google Scholar] [CrossRef]
- Bedran-Russo, A.K.; Pereira, P.N.; Duarte, W.R.; Drummond, J.L.; Yamauchi, M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 268–272. [Google Scholar] [CrossRef]
- Al-Ammar, A.; Drummond, J.L.; Bedran-Russo, A.K. The use of collagen cross-linking agents to enhance dentin bond strength. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Mao, C.; Sun, J.; Chen, Y.; Wang, W.; Pan, H.; Tang, R.; Gu, X. Glutaraldehyde-induced remineralization improves the mechanical properties and biostability of dentin collagen. Mater. Sci. Eng. C 2016, 67, 657–665. [Google Scholar] [CrossRef]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic mineralization of biomaterials using simulated body fluids for bone tissue engineering and regenerative medicine. Tissue Eng. A 2017, 23, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Von der Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell. Tissue Res. 2010, 339, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. Natural and genetically engineered proteins for tissue engineering. Prog. Polym. Sci. 2012, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, H.; Zhang, X.; Ning, C.; Wang, Y. In Vitro study on the osteogenesis enhancement effect of BMP-2 incorporated biomimetic apatite coating on titanium surfaces. Dent. Mater. J. 2017, 36, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Hunziker, E.B.; Enggist, L.; Kuffer, A.; Buser, D.; Liu, Y. Osseointegration: The slow delivery of BMP-2 enhances osteoinductivity. Bone 2012, 51, 98–106. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, G.; Liu, Y.; Wismeijer, D.; Liu, Y. A novel BMP2-coprecipitated, layer-by-layer assembled biomimetic calcium phosphate particle: A biodegradable and highly-efficient osteoinducer. Clin. Implant Dent. Relat. Res. 2014, 16, 643–654. [Google Scholar] [CrossRef]
- Wang, D.; Tabassum, A.; Wu, G.; Deng, L.; Wismeijer, D.; Liu, Y. Bone regeneration in critical-sized bone defect enhanced by introducing osteoinductivity to biphasic calcium phosphate granules. Clin. Oral Implant. Res. 2017, 28, 251–260. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, Y.; Wu, G.; Wismeijer, D.; Pathak, J.L.; Liu, Y. BMP2-coprecipitated calcium phosphate granules enhance osteoinductivity of deproteinized bovine bone, and bone formation during critical-sized bone defect healing. Sci. Rep. 2017, 7, 41800. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Tomkins, M.; Fajardo, R.; Meinel, L.; Snyder, B.; Wade, K.; Chen, J.; Vunjak-Novakovic, G.; Kaplan, D.L. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 In Vitro and In Vivo. J. Biomed. Mater. Res. A 2006, 78, 324–334. [Google Scholar] [CrossRef]
- La, W.G.; Kang, S.W.; Yang, H.S.; Bhang, S.H.; Lee, S.H.; Park, J.H.; Kim, B.S. The efficacy of bone morphogenetic protein-2 depends on its mode of delivery. Artif. Organs 2010, 34, 1150–1153. [Google Scholar] [CrossRef]
- Murphy, C.M.; Schindeler, A.; Gleeson, J.P.; Yu, N.Y.C.; Cantrill, L.C.; Mikulec, K.; Peacock, L.; O’Brien, F.J.; Little, D.G. A collagen–hydroxyapatite scaffold allows for binding and co-delivery of recombinant bone morphogenetic proteins and bisphosphonates. Acta Biomater. 2014, 10, 2250–2258. [Google Scholar] [CrossRef]
- Yang, H.S.; La, W.G.; Bhang, S.H.; Lee, T.J.; Lee, M.; Kim, B.S. Apatite-coated collagen scaffold for bone morphogenetic protein-2 delivery. Tissue Eng. A 2011, 17, 2153–2164. [Google Scholar] [CrossRef] [PubMed]
- Sotome, S.; Uemura, T.; Kikuchi, M.; Chen, J.; Itoh, S.; Tanaka, J.; Tateishi, T.; Shinomiya, K. Synthesis and In Vivo evaluation of a novel hydroxyapatite/collagen–alginate as a bone filler and a drug delivery carrier of bone morphogenetic protein. Mater. Sci. Eng. C 2004, 24, 341–347. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.C.G.; Jo, J.; Wang, H.; Yamamoto, M.; Jansen, J.A.; Tabata, Y. Mineralization, biodegradation, and drug release behavior of gelatin/apatite composite microspheres for bone regeneration. Biomacromolecules 2010, 11, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Shinkai, M.; Takezawa, T.; Ohba, S.; Chung, U.; Nagamune, T. Bone regeneration using collagen type I vitrigel with bone morphogenetic protein-2. J. Biosci. Bioeng. 2009, 107, 318–323. [Google Scholar] [CrossRef]
- Jacobs, E.E.; Gronowicz, G.; Hurley, M.M.; Kuhn, L.T. Biomimetic calcium phosphate/polyelectrolyte multilayer coatings for sequential delivery of multiple biological factors. J. Biomed. Mater. Res. A 2017, 105, 1500–1509. [Google Scholar] [CrossRef] [Green Version]
- Charles, L.F.; Woodman, J.L.; Ueno, D.; Gronowicz, G.; Hurley, M.M.; Kuhn, L.T. Effects of low dose FGF-2 and BMP-2 on healing of calvarial defects in old mice. Exp. Gerontol. 2015, 64, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Gronowicz, G.; Jacobs, E.; Peng, T.; Zhu, L.; Hurley, M.; Kuhn, L.T. Calvarial bone regeneration is enhanced by sequential delivery of FGF-2 and BMP-2 from layer-by-layer coatings with a biomimetic calcium phosphate barrier layer. Tissue Eng. A 2017, 23, 1490–1501. [Google Scholar] [CrossRef]
- Alhamdi, J.; Jacobs, E.; Gronowicz, G.; Benkirane-Jessel, N.; Hurley, M.; Kuhn, L. Cell Type influences local delivery of biomolecules from a bioinspired apatite drug delivery system. Materials 2018, 11, 1703. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lin, X.; Liu, T.; Deng, L.; Huang, Y.; Liu, Y. Osteogenic enhancement between icariin and bone morphogenetic protein 2: A potential osteogenic compound for bone tissue engineering. Front. Pharm. 2019, 10, 201. [Google Scholar] [CrossRef]
- Suchý, T.; Šupová, M.; Klapková, E.; Horný, L.; Rýglová, Š.; Žaloudková, M.; Braun, M.; Sucharda, Z.; Ballay, R.; Veselý, J.; et al. The sustainable release of vancomycin and its degradation products from nanostructured collagen/hydroxyapatite composite layers. J. Pharm. Sci. 2016, 105, 1288–1294. [Google Scholar] [CrossRef] [Green Version]
- Suchý, T.; Šupová, M.; Klapková, E.; Adámková, V.; Závora, J.; Žaloudková, M.; Rýglová, Š.; Ballay, R.; Denk, F.; Pokorný, M.; et al. The release kinetics, antimicrobial activity and cytocompatibility of differently prepared collagen/hydroxyapatite/vancomycin layers: Microstructure vs. Nanostructure. Europ. J. Pharm. Sci. 2017, 100, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Suchý, T.; Šupová, M.; Sauerová, P.; Hubálek-Kalbáčová, M.; Klapková, E.; Pokorný, M.; Horný, L.; Závora, J.; Ballay, R.; Denk, F.; et al. Evaluation of collagen/hydroxyapatite electrospun layers loaded with vancomycin, gentamicin and their combination: Comparison of release kinetics, antimicrobial activity and cytocompatibility. Europ. J. Pharm. Biopharm. 2019, 140, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Pon-On, W.; Charoenphandhu, N.; Teerapornpuntakit, J.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. In Vitro study of vancomycin release and osteoblast-like cell growth on structured calcium phosphate-collagen. Mater. Sci. Eng. C 2013, 33, 1423–1431. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, H.; Zhang, S. Biomimetic coprecipitation of silk fibrin and calcium phosphate: Influence of selenite ions. Biol. Trace Elem. Res. 2017, 178, 338–347. [Google Scholar] [CrossRef]
- Kang, T.; Huang, Y.; Zhu, Q.; Cheng, H.; Pei, Y.; Feng, J.; Xu, M.; Jiang, G.; Song, Q.; Jiang, T.; et al. Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials 2018, 164, 80–97. [Google Scholar] [CrossRef]
- Lee, S.U.; Min, K.H.; Jeong, S.Y.; Bae, H.; Lee, S.C. Calcium phosphate-reinforced photosensitizer-loaded polymer nanoparticles for photodynamic therapy. Chem. Asian J. 2013, 8, 3222–3229. [Google Scholar] [CrossRef]
- Bock, N.; Riminucci, A.; Dionigi, C.; Russo, A.; Tampieri, A.; Landi, E.; Goranov, V.A.; Marcacci, M.; Dediu, V. A novel route in bone tissue engineering: Magnetic biomimetic scaffolds. Acta Biomater. 2010, 6, 786–796. [Google Scholar] [CrossRef]
- Chung, R.J. Study of hydroxyapatite nano composites with photoluminescence properties. Biomed. Eng. Appl. Basis Commun. 2011, 23, 107–112. [Google Scholar] [CrossRef]
- Xu, Y.M.; Meng, Z.Y.; Zhai, J. pH and calcium cooperative regulation nanofluidic gating device. Acta Chim. Sin. 2016, 74, 538–544. [Google Scholar] [CrossRef]
- Qu, T.; Tomar, V. An analysis of the effects of temperature and structural arrangements on the thermal conductivity and thermal diffusivity of tropocollagen–hydroxyapatite interfaces. Mater. Sci. Eng. C 2014, 38, 28–38. [Google Scholar] [CrossRef] [PubMed]
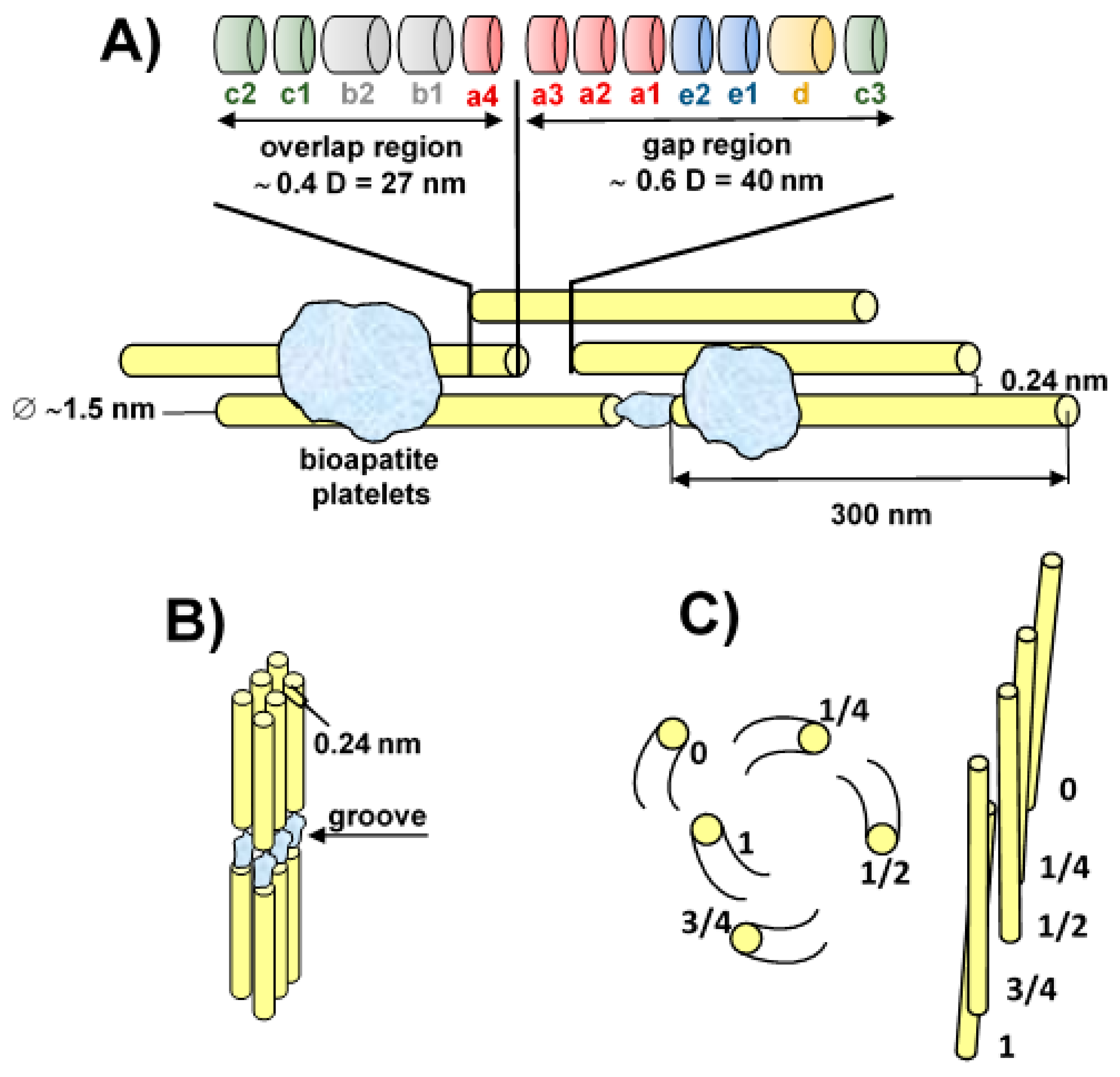

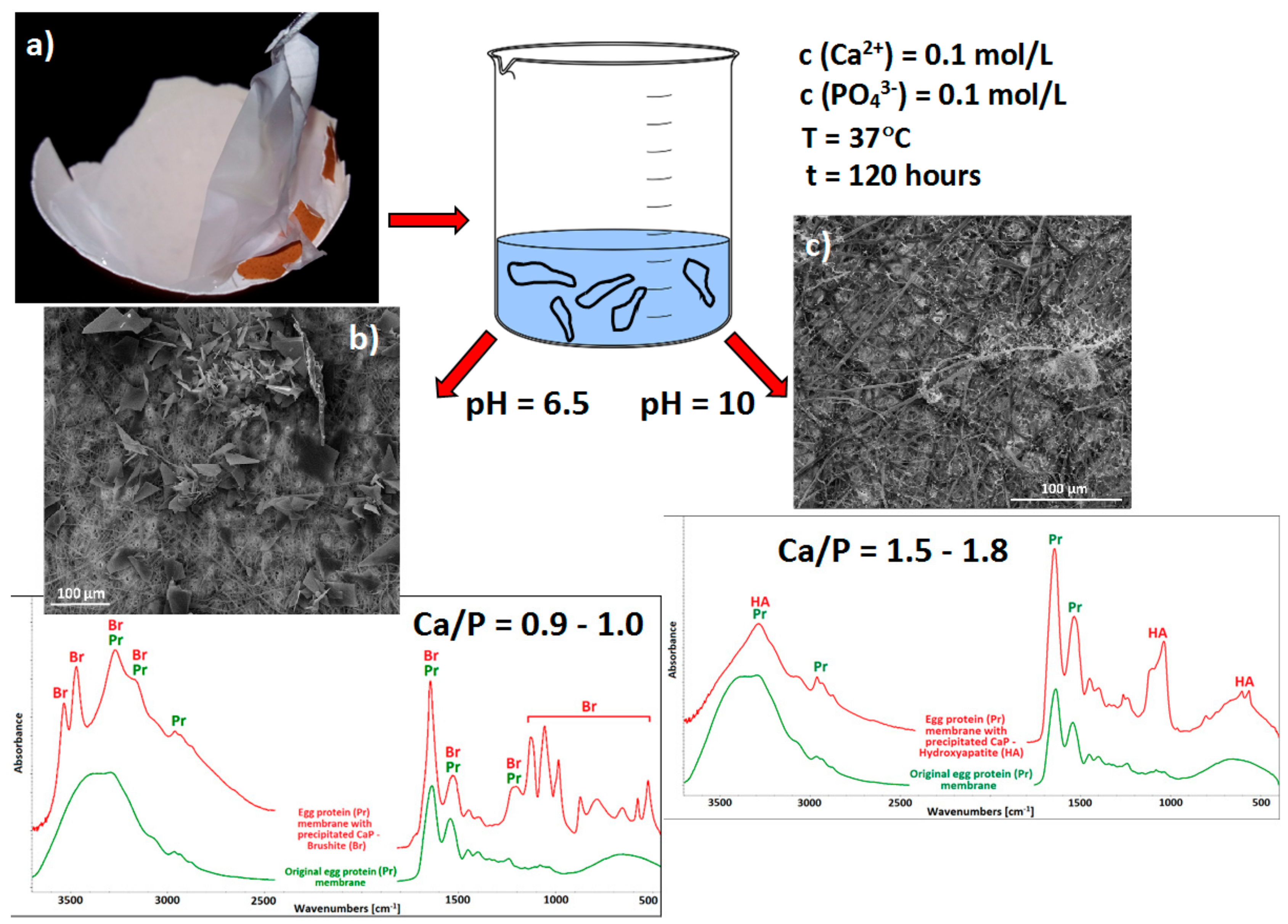

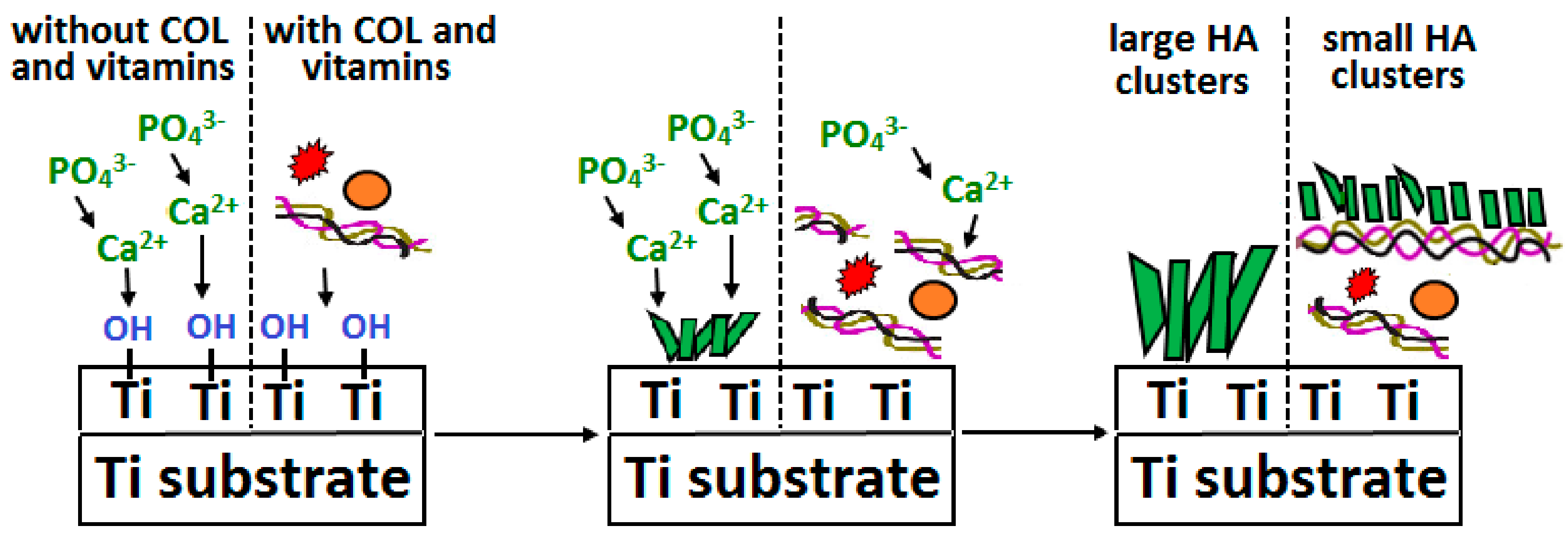


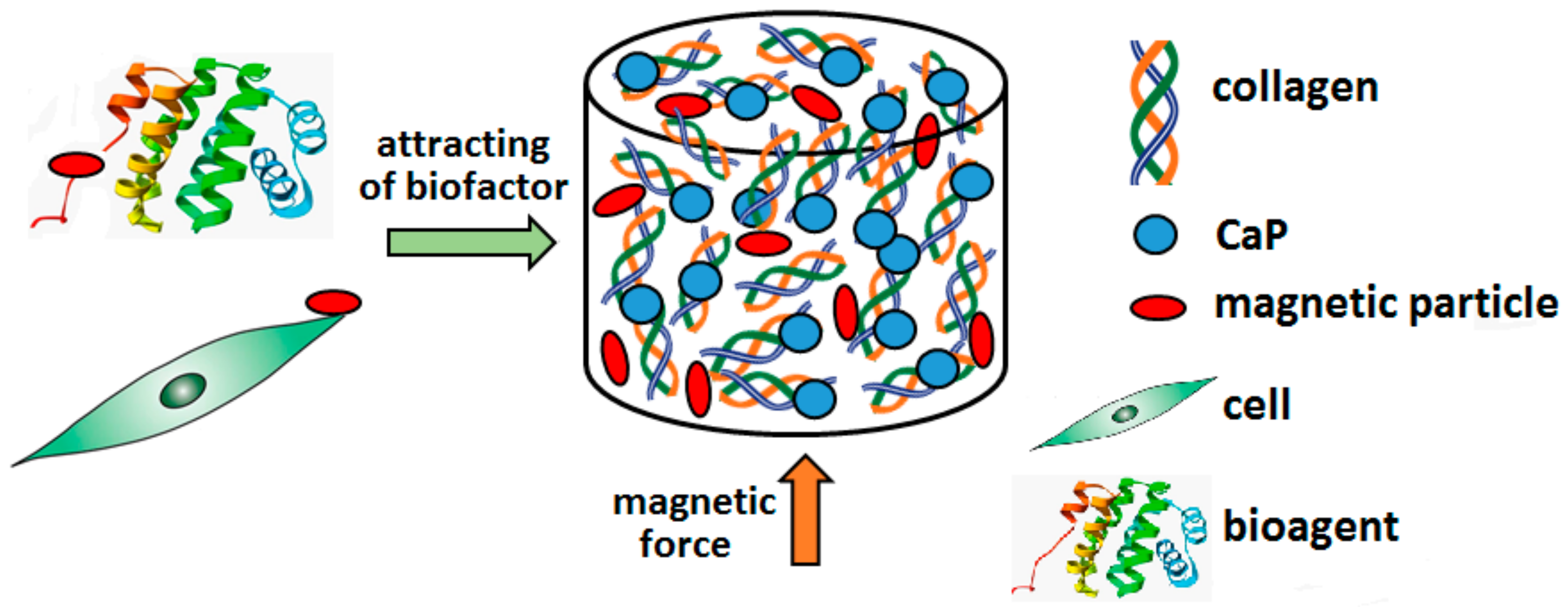

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šupová, M. The Significance and Utilisation of Biomimetic and Bioinspired Strategies in the Field of Biomedical Material Engineering: The Case of Calcium Phosphat—Protein Template Constructs. Materials 2020, 13, 327. https://doi.org/10.3390/ma13020327
Šupová M. The Significance and Utilisation of Biomimetic and Bioinspired Strategies in the Field of Biomedical Material Engineering: The Case of Calcium Phosphat—Protein Template Constructs. Materials. 2020; 13(2):327. https://doi.org/10.3390/ma13020327
Chicago/Turabian StyleŠupová, Monika. 2020. "The Significance and Utilisation of Biomimetic and Bioinspired Strategies in the Field of Biomedical Material Engineering: The Case of Calcium Phosphat—Protein Template Constructs" Materials 13, no. 2: 327. https://doi.org/10.3390/ma13020327
APA StyleŠupová, M. (2020). The Significance and Utilisation of Biomimetic and Bioinspired Strategies in the Field of Biomedical Material Engineering: The Case of Calcium Phosphat—Protein Template Constructs. Materials, 13(2), 327. https://doi.org/10.3390/ma13020327



