Synthesis and Adsorption Performance of a Hierarchical Micro-Mesoporous Carbon for Toluene Removal under Ambient Conditions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.2.1. Synthesis of OMCs
2.2.2. Synthesis of KOMCs
2.3. Sample Characterization
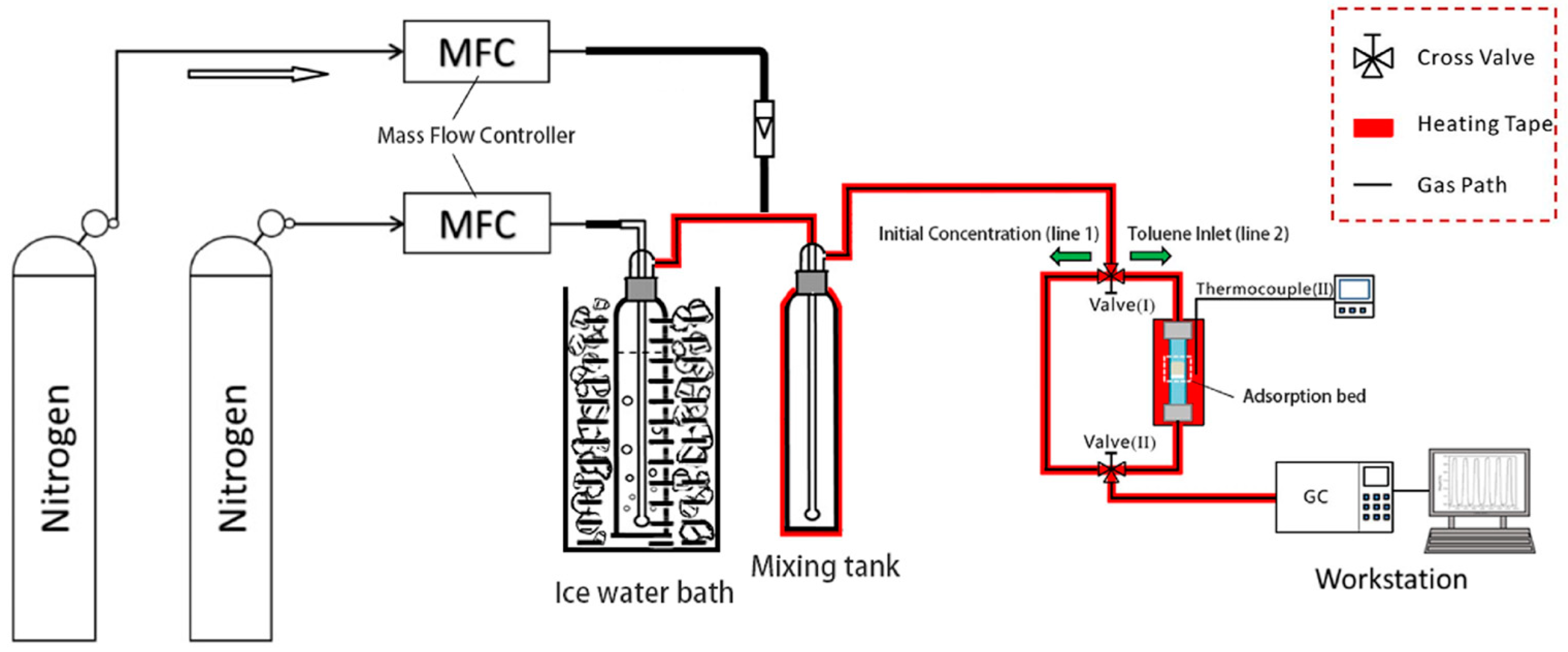
2.4. Dynamic Adsorption Measurements
3. Results and Discussion
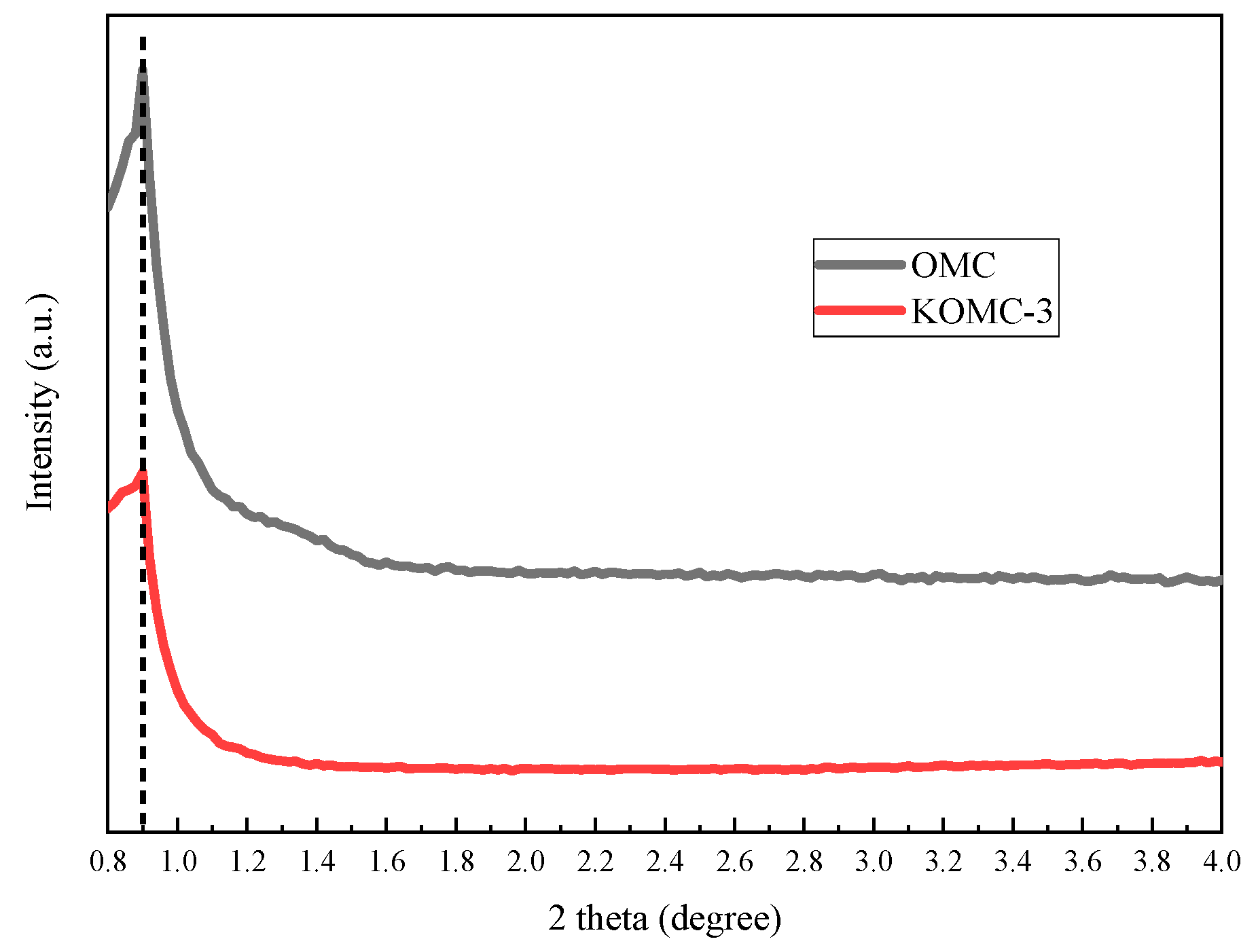
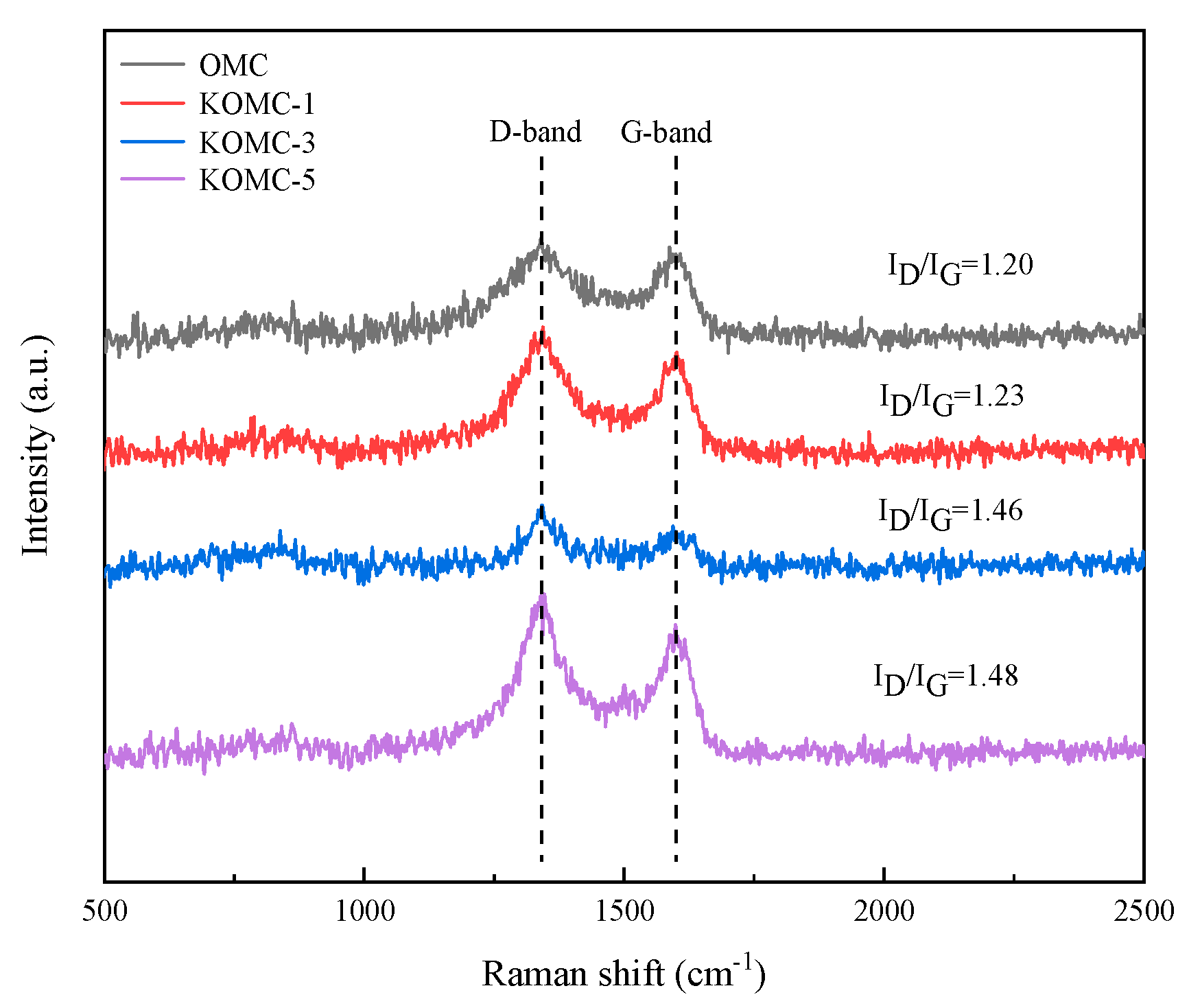
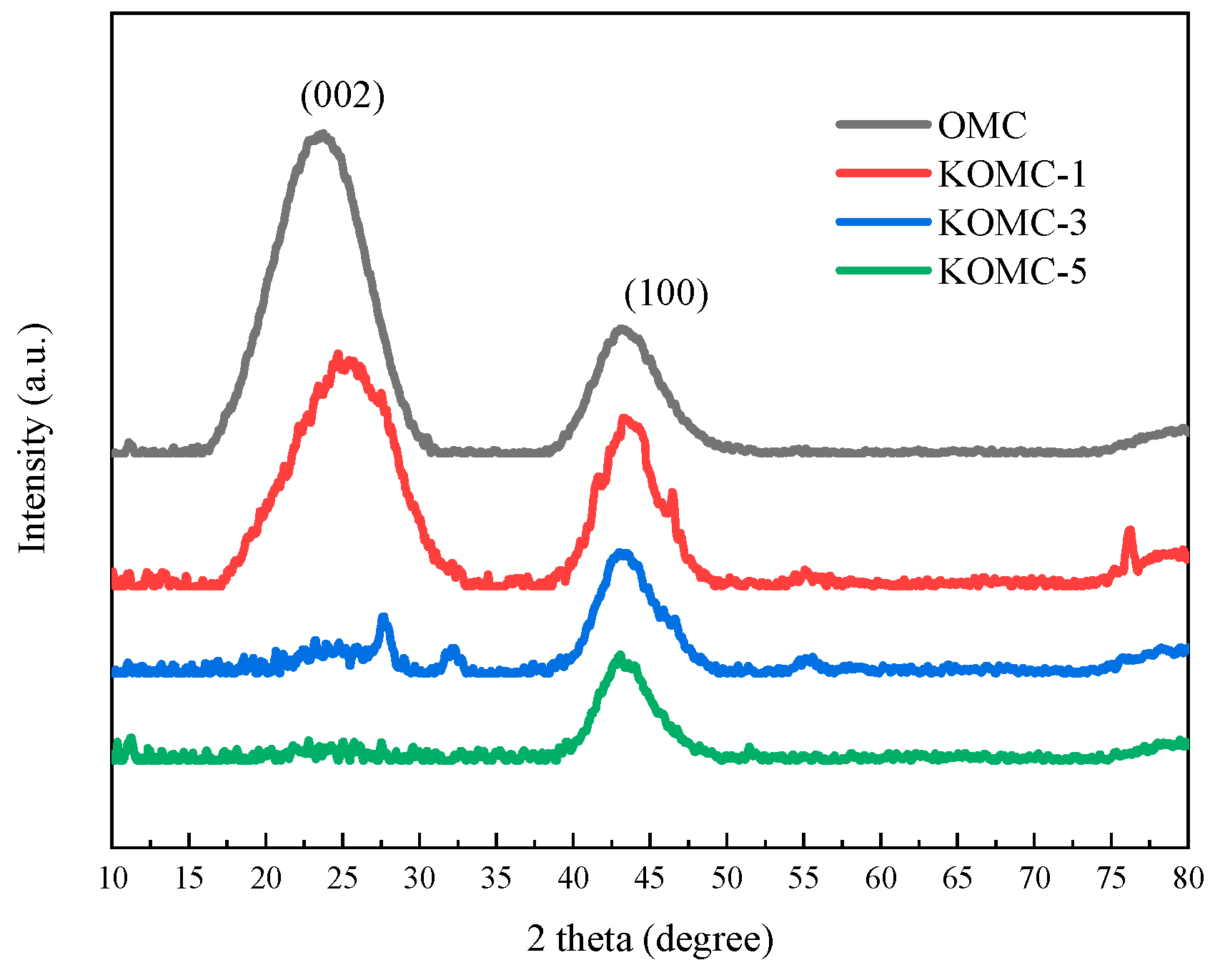
3.1. Characteristics of KOMCs
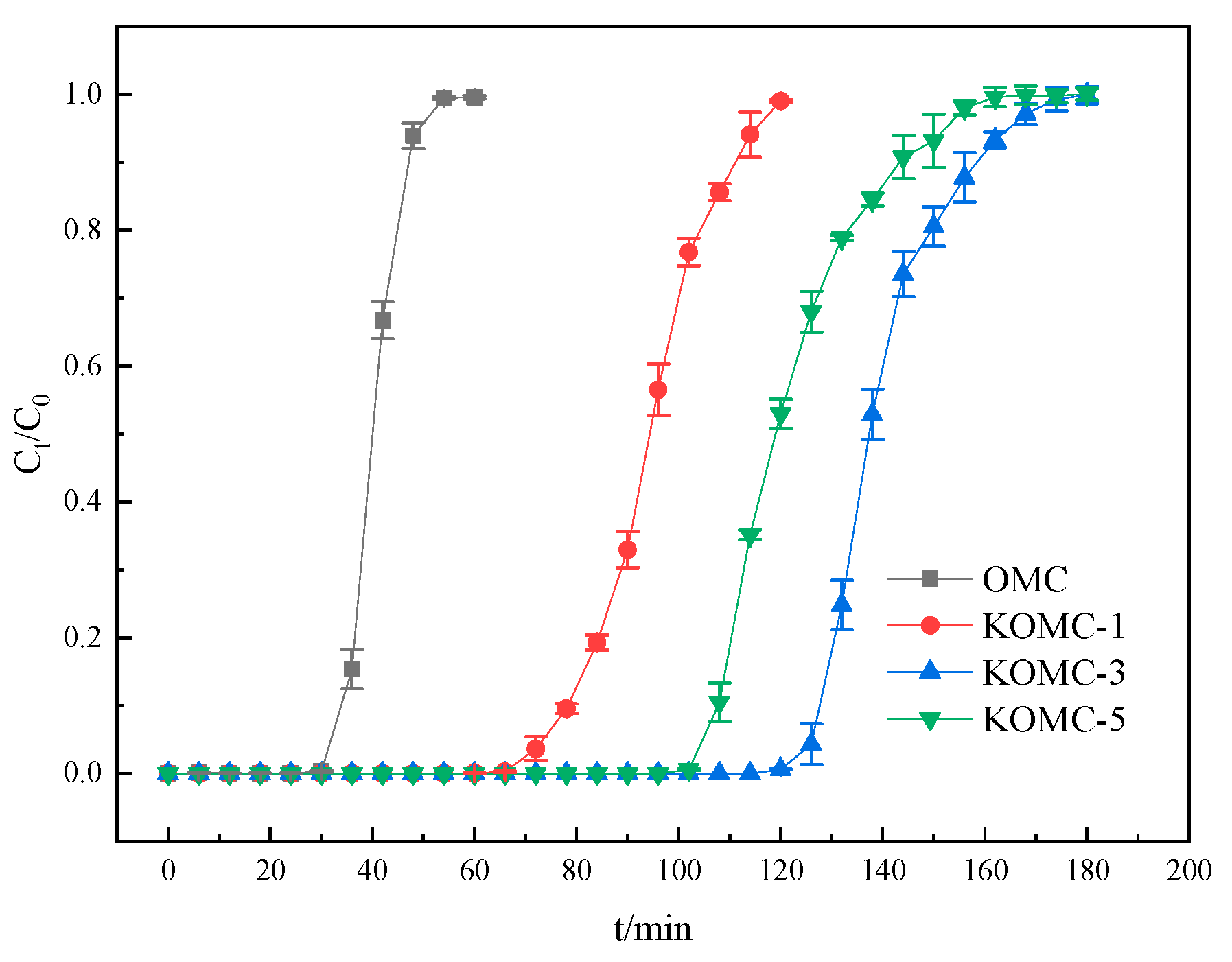
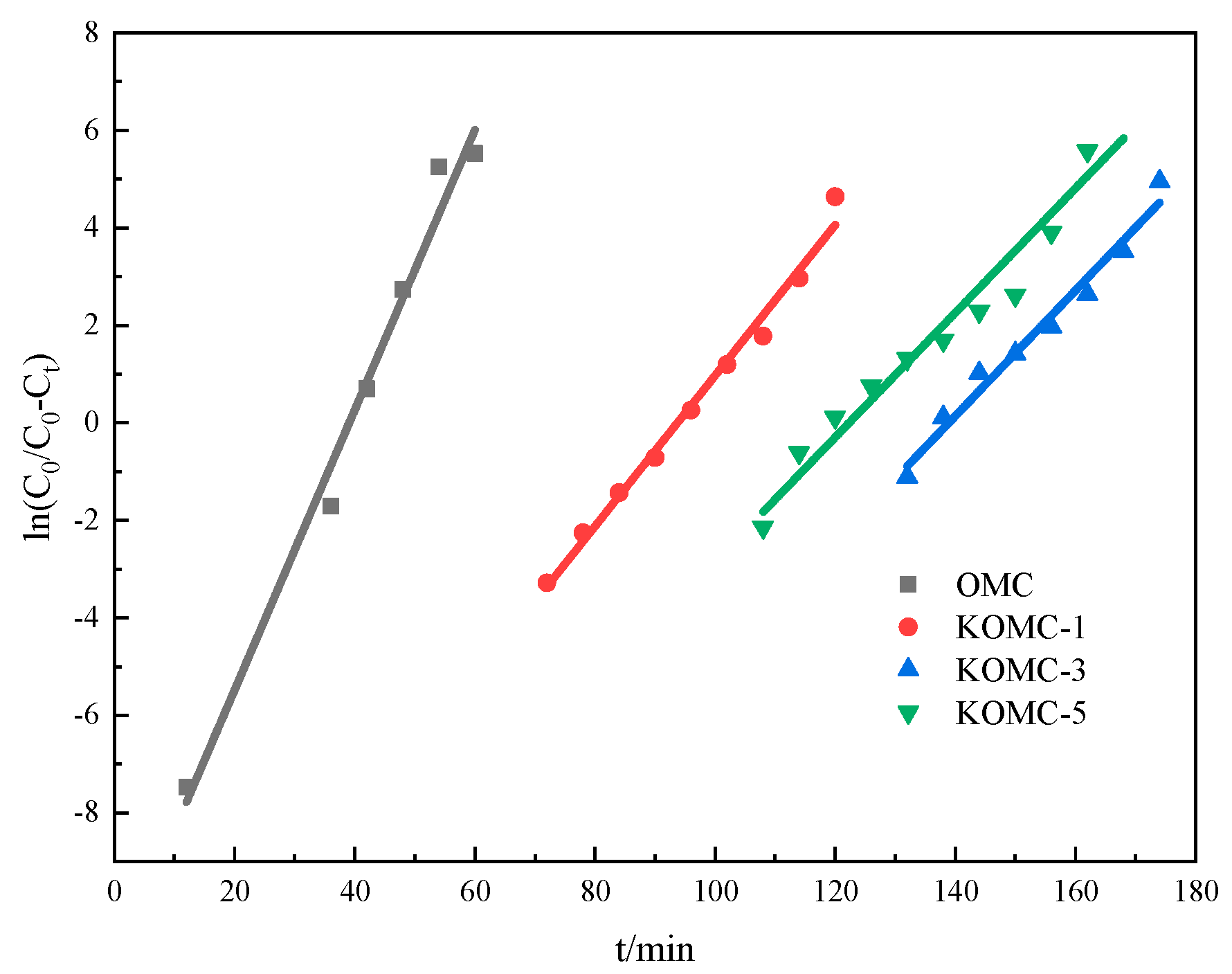
3.2. Dynamic Adsorption Performance of Toluene
3.3. Recyclability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Alivand, M.S.; Rashidi, A.; Samimi, A.; Mohebbi-Kalhori, D. Synthesis and adsorption performance of a modified micro-mesoporous MIL-101(Cr) for VOCs removal at ambient conditions. Chem. Eng. J. 2018, 341, 164–174. [Google Scholar] [CrossRef]
- Ouzzine, M.; Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical activated carbons for the adsorption of a real multicomponent VOC mixture. Carbon 2019, 148, 214–223. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Alabi, W.O.; Tope, B.B.; Jermy, R.B.; Aitani, A.M.; Hattori, H.; Al-Khattaf, S.S. Modification of Cs-X for styrene production by side-chain alkylation of toluene with methanol. Catal. Today 2014, 226, 117–123. [Google Scholar] [CrossRef]
- Hattori, H.; Alabi, W.O.; Jermy, B.R.; Aitani, A.M.; Al-Khattaf, S.S. Pathway to Ethylbenzene Formation in Side-Chain Alkylation of Toluene with Methanol Over Cesium Ion-Exchanged Zeolite X. Catal. Lett. 2013, 143, 1025–1029. [Google Scholar] [CrossRef]
- Ouzzine, M.; Lillo-Ródenas, M.A.; Linares-Solano, A. Photocatalytic oxidation of propene in gas phase at low concentration by optimized TiO2 nanoparticles. Appl. Catal. B Environ. 2013, 134–135, 333–343. [Google Scholar] [CrossRef]
- Cheng, Z.; Lu, L.; Kennes, C.; Yu, J.; Chen, J. Treatment of gaseous toluene in three biofilters inoculated with fungi/bacteria: Microbial analysis, performance and starvation response. J. Hazard. Mater. 2016, 303, 83–93. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Lu, B.; Jiang, A.; Wan, C. Study on the removal of indoor VOCs using biotechnology. J. Hazard. Mater. 2010, 182, 204–209. [Google Scholar] [CrossRef]
- Ozturk, B.; Kuru, C.; Aykac, H.; Kaya, S. VOC separation using immobilized liquid membranes impregnated with oils. Sep. Purif. Technol. 2015, 153, 1–6. [Google Scholar] [CrossRef]
- Yi, F.-Y.; Lin, X.-D.; Chen, S.-X.; Wei, X.-Q. Adsorption of VOC on modified activated carbon fiber. J. Porous Mater. 2008, 16, 521–526. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, S.; Zhang, W.; An, Z.; Chen, D. Dynamic adsorption of toluene on amino-functionalized SBA-15 type spherical mesoporous silica. RSC Adv. 2019, 9, 7196–7202. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.W.; Yang, D.A.; Kim, J.; Kim, J.; Ahn, W.S. Facile synthesis of MOF-177 by a sonochemical method using 1-methyl-2-pyrrolidinone as a solvent. Dalton Trans. 2010, 39, 2883–2887. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Reinhard, M. Sorption of Trichloroethylene in Hydrophobic Micropores of Dealuminated Y Zeolites and Natural Minerals. Environ. Sci. Technol. 2006, 40, 7694–7701. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.-H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Bellarosa, L.; Calero, S.; López, N. Early stages in the degradation of metal-organic frameworks in liquid water from first-principles molecular dynamics. Phys. Chem. Chem. Phys. 2012, 14, 7240–7245. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for post-combustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef]
- Cheng, J.; Jin, S.; Zhang, R.; Shao, X.; Jin, M. Enhanced adsorption selectivity of dibenzothiophene on ordered mesoporous carbon-silica nanocomposites via copper modification. Microporous Mesoporous Mater. 2015, 212, 137–145. [Google Scholar] [CrossRef]
- Cao, F.; Chen, J.; Ni, M.; Song, H.; Xiao, G.; Wu, W.; Gao, X.; Cen, K. Adsorption of NO on ordered mesoporous carbon and its improvement by cerium. RSC Adv. 2014, 4. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, X.; Chen, Y.; Huang, J.; Luo, H.; Deng, S. Adsorption of CO(2), CH(4), and N(2) on ordered mesoporous carbon: approach for greenhouse gases capture and biogas upgrading. Environ. Sci. Technol. 2013, 47, 5474–5480. [Google Scholar] [CrossRef]
- Wang, G.; Dou, B.; Zhang, Z.; Wang, J.; Liu, H.; Hao, Z. Adsorption of benzene, cyclohexane and hexane on ordered mesoporous carbon. J. Environ. Sci. (China) 2015, 30, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.L.; Yilmaz, G.; Ong, W.L.; Ho, G.W. One-step activation towards spontaneous etching of hollow and hierarchical porous carbon nanospheres for enhanced pollutant adsorption and energy storage. Appl. Catal. B Environ. 2018, 220, 533–541. [Google Scholar] [CrossRef]
- Choi, S.W.; Tang, J.; Pol, V.G.; Lee, K.B. Pollen-derived porous carbon by KOH activation: Effect of physicochemical structure on CO2 adsorption. J. CO2 Util. 2019, 29, 146–155. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wang, Y.H.; Dai, Z.; Lu, Z.W.; Gu, X.Y.; Zhao, H.; Sun, G.Z.; Lan, W.; Zhang, Z.X.; Pan, X.J.; et al. Nature of improved double-layer capacitance by KOH activation on carbon nanotube-carbon nanofiber hierarchical hybrids. Carbon 2019, 146, 610–617. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Rash, T.; Firlej, L.; Kuchta, B.; Yu, P.; Suppes, G.; Wexler, C.; Pfeifer, P. Nanospace engineering of KOH activated carbon. Nanotechnology 2012, 23, 015401. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Preparation of highly microporous carbons from fir wood by KOH activation for adsorption of dyes and phenols from water. Sep. Purif. Technol. 2005, 47, 10–19. [Google Scholar] [CrossRef]
- Liu, Y.; Huo, Z.; Song, Z.; Zhang, C.; Ren, D.; Zhong, H.; Jin, F. Preparing a magnetic activated carbon with expired beverage as carbon source and KOH as activator. J. Taiwan Inst. Chem. Eng. 2019, 96, 575–587. [Google Scholar] [CrossRef]
- Silvestre-Albero, A.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. Ethanol removal using activated carbon: Effect of porous structure and surface chemistry. Microporous Mesoporous Mater. 2009, 120, 62–68. [Google Scholar] [CrossRef]
- Kim, S.-S.; Pauly, T.R.; Pinnavaia, T.J. Non-ionic surfactant assembly of wormhole silica molecular sieves from water soluble silicates. Chem. Commun. 2000, 835–836. [Google Scholar] [CrossRef]
- Mitome, T.; Uchida, Y.; Egashira, Y.; Hayashi, K.; Nishiura, A.; Nishiyama, N. Adsorption of indole on KOH-activated mesoporous carbon. Colloids Surf. A Physicochem. Eng. Asp. 2013, 424, 89–95. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Ma, R.; Zhou, Y.; Liu, Q.; Dong, X.; Wang, J. KOH activation of biomass-derived nitrogen-doped carbons for supercapacitor and electrocatalytic oxygen reduction. Electrochim. Acta 2018, 261, 49–57. [Google Scholar] [CrossRef]
- Jianhua, H.; Chuanbao, C.; Faryal, I.; Xilan, M. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556. [Google Scholar]
- Wang, Y.; Yang, R.; Li, M.; Zhao, Z. Hydrothermal preparation of highly porous carbon spheres from hemp (Cannabis sativa L.) stem hemicellulose for use in energy-related applications. Ind. Crops Prod. 2015, 65, 216–226. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, P.; Luo, D.; Xiao, W.; Deng, L.; Qiao, F. Direct production of porous carbon nanosheets/particle composites from wasted litchi shell for supercapacitors. J. Alloy. Compd. 2019, 788, 677–684. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Sing, K.S.W. Adsorption by Powders, Porous Solids: Principles, Methodology, Applications; Academic press: Cambridge, MA, USA, 2013. [Google Scholar]
- Zhai, Y.; Dou, Y.; Liu, X.; Park, S.S.; Ha, C.-S.; Zhao, D. Soft-template synthesis of ordered mesoporous carbon/nanoparticle nickel composites with a high surface area. Carbon 2011, 49, 545–555. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption-desorption on activated carbons with varying pore structure. J. Environ. Sci. 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Brosillon, S.; Manero, M.H.; Foussard, J.N. Mass transfer in VOC adsorption on zeolite: experimental and theoretical breakthrough curves. Environ. Sci. Technol. 2001, 35, 3571–3575. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zeng, X.; Qin, Y.; Li, X.; Zhu, T.; Tang, X. An experimental and theoretical study of the adsorption removal of toluene and chlorobenzene on coconut shell derived carbon. Chemosphere 2018, 206, 285–292. [Google Scholar] [CrossRef]
- Janus, R.; Wądrzyk, M.; Natkański, P.; Cool, P.; Kuśtrowski, P. Dynamic adsorption–desorption of methyl ethyl ketone on MCM-41 and SBA-15 decorated with thermally activated polymers. J. Ind. Eng. Chem. 2019, 71, 465–480. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Y.; Wu, F.; Peng, S.; Li, J.; Liu, Z. Tubular activated carbons made from cotton stalk for dynamic adsorption of airborne toluene. J. Taiwan Inst. Chem. Eng. 2017, 80, 399–405. [Google Scholar] [CrossRef]
- Cheng, B.; Le, Y.; Cai, W.; Yu, J. Synthesis of hierarchical Ni(OH)2 and NiO nanosheets and their adsorption kinetics and isotherms to Congo red in water. J. Hazard. Mater. 2011, 185, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S.; Ryoo, R.; Choi, M. Revisiting side-chain alkylation of toluene to styrene: Critical role of microporous structures in catalysts. J. Catal. 2019, 373, 25–36. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Chiang, P.-C.; Huang, C.-P. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.; Cazorla-Amorós, D.; Linares-Solano, A. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 2005, 43, 1758–1767. [Google Scholar] [CrossRef]

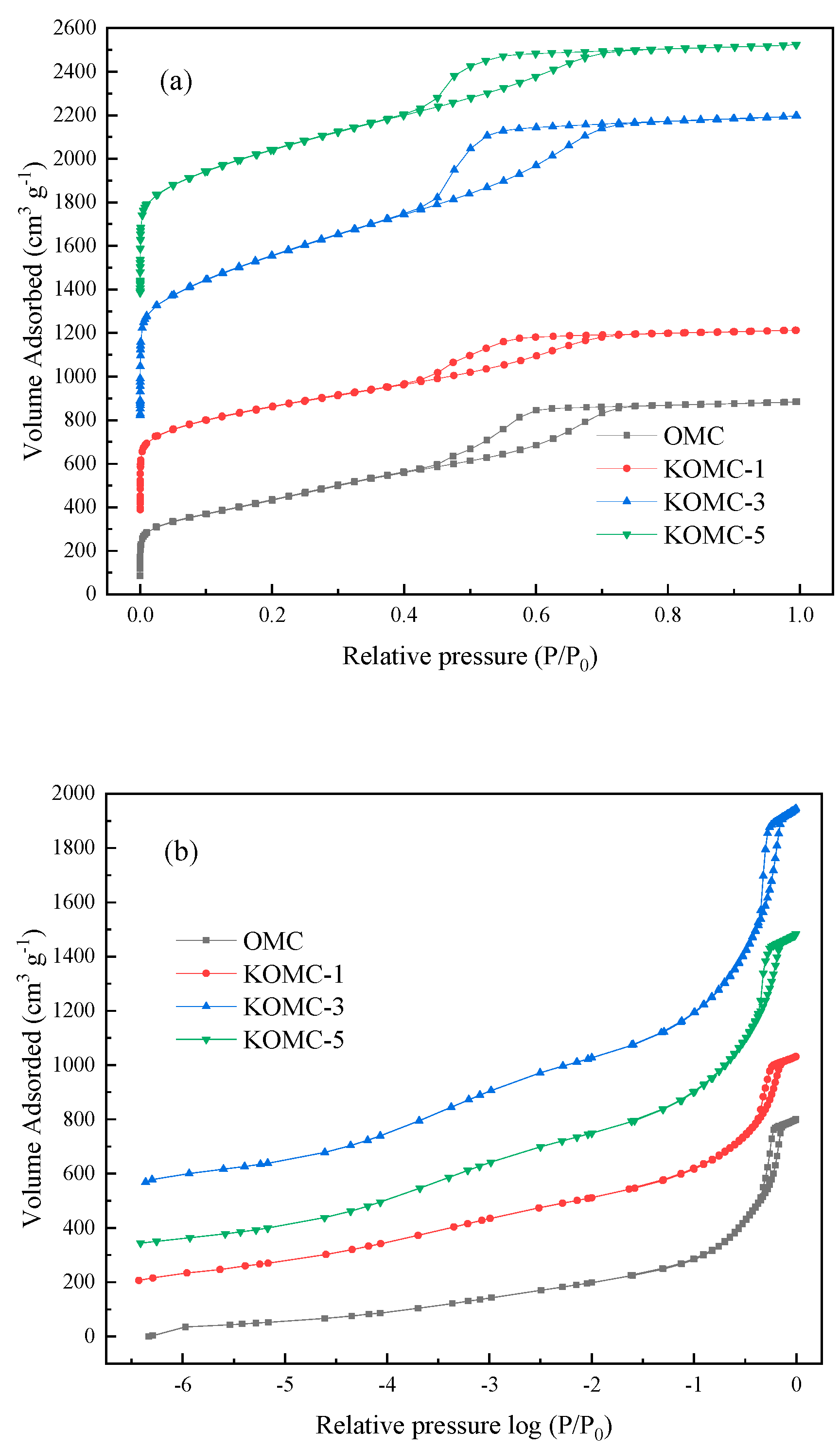


| Sample | SBET a (m2 g−1) | Vt b (cm3 g−1) | Vmic c (cm3 g−1) | Smic d (m2 g−1) |
|---|---|---|---|---|
| OMC | 1343 | 1.237 | 0.099 | 225 |
| KOMC-1 | 1792 | 1.324 | 0.253 | 594 |
| KOMC-3 | 2456 | 1.809 | 0.393 | 848 |
| KOMC-5 | 2661 | 2.139 | 0.281 | 629 |
| Sample | Adsorption Capacity | Y-N Model Parameters | |||
|---|---|---|---|---|---|
| q (mg g−1) | q′ (mmol g−1) | k | R2 | ||
| OMC | 104.61 | 1.135 | 0.287 | 39 | 0.984 |
| KOMC-1 | 242.89 | 2.636 | 0.155 | 94 | 0.988 |
| KOMC-3 | 355.67 | 3.916 | 0.129 | 139 | 0.975 |
| KOMC-5 | 286.76 | 3.449 | 0.128 | 122 | 0.960 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Z.; Kong, S.; Zhang, W.; Yuan, M.; An, Z.; Chen, D. Synthesis and Adsorption Performance of a Hierarchical Micro-Mesoporous Carbon for Toluene Removal under Ambient Conditions. Materials 2020, 13, 716. https://doi.org/10.3390/ma13030716
An Z, Kong S, Zhang W, Yuan M, An Z, Chen D. Synthesis and Adsorption Performance of a Hierarchical Micro-Mesoporous Carbon for Toluene Removal under Ambient Conditions. Materials. 2020; 13(3):716. https://doi.org/10.3390/ma13030716
Chicago/Turabian StyleAn, Zhaohui, Shulin Kong, Wenwen Zhang, Ming Yuan, Zhihao An, and Donghui Chen. 2020. "Synthesis and Adsorption Performance of a Hierarchical Micro-Mesoporous Carbon for Toluene Removal under Ambient Conditions" Materials 13, no. 3: 716. https://doi.org/10.3390/ma13030716




