Development of an Ultra-Low Carbon MgO Refractory Doped with α-Al2O3 Nanoparticles for the Steelmaking Industry: A Microstructural and Thermo-Mechanical Study
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
4. Conclusions
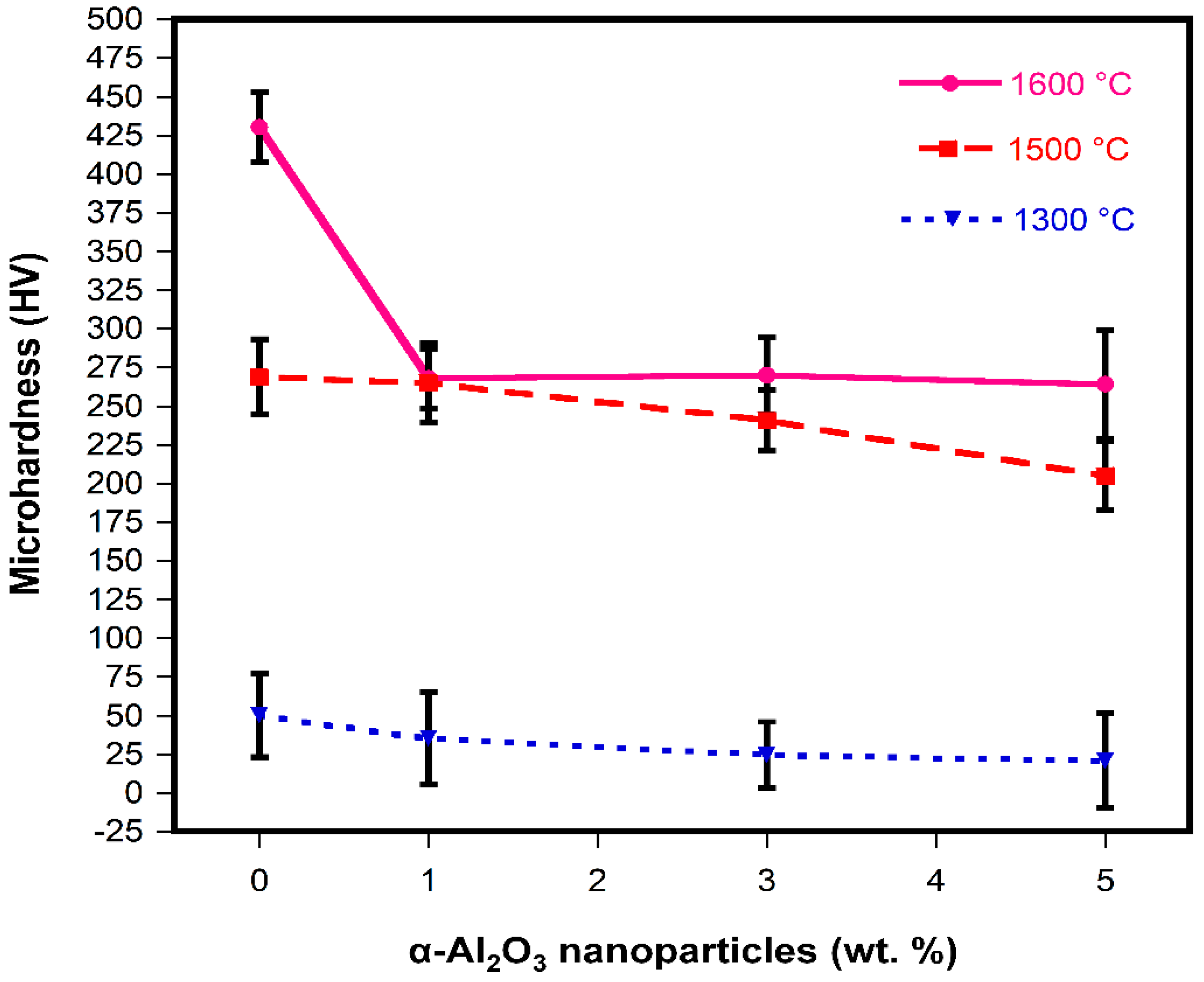
- The sintering temperatures and the α-Al2O3 nanoparticle content had an important role in the physical and mechanical properties of MgO-based refractories.
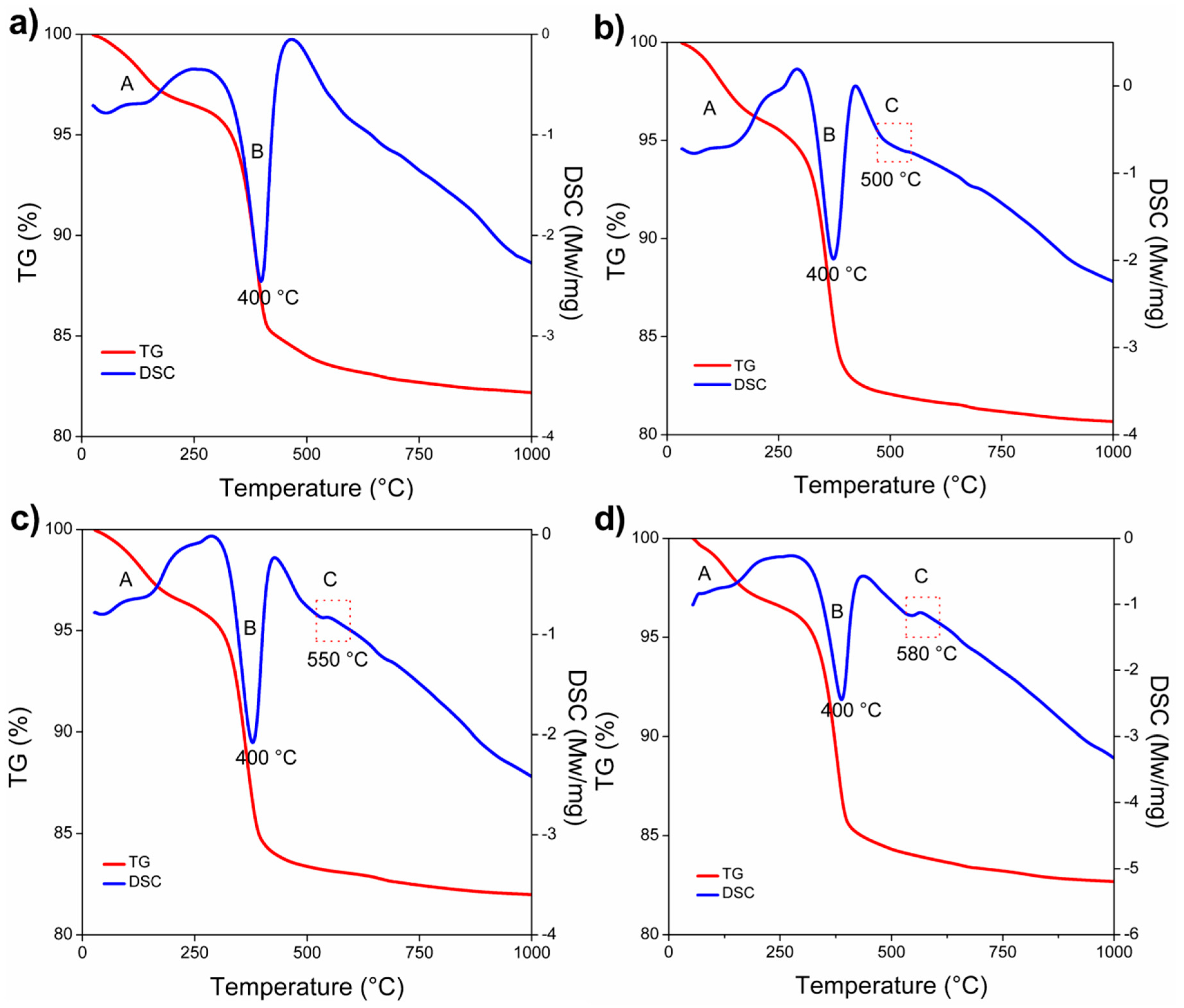
- DSC-TGA analysis allowed to relate a small exothermic peak at 550 °C, with the nucleation and formation of the spinel (MgAl2O4) due to the reaction between α-Al2O3 nanoparticles and magnesia.
- The adequate dispersion and presence in triple points of the spinel phase (MgAl2O4) promoted the densification of the magnesia matrix significantly at sintering temperatures of 1500 and 1600 °C.
- The use of α-Al2O3 nanoparticles played significant roles in precise control in situ spinel formation and to effectively develop microcrack networks around spinel particles.
- The large difference in thermal expansion coefficient between MgO and MgAl2O4 led to the formation of microcracks, which can be beneficial up to a certain limit because they allow dissipating the stored energy in compression loads.
- The maximum CCS value registered was 156 MPa, which corresponded to the addition of 5 wt.% of α-Al2O3 nanoparticles at 1500 °C.
- The size and number of microcracks were increased at the sintering temperature of 1600 °C, which had a detrimental effect since the overall strength and stiffness of the refractory samples were reduced.
- The physical and mechanical properties developed by the refractories studied in this research work are comparable and/or superior to those of MgO-based commercial refractories, which allows them to be considered as an option for application in the steelmaking industry.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sadik, C.; Moudden, O.; El Bouari, A.; El Amrani, I. Review on the elaboration and characterization of ceramics refractories based on magnesite and dolomite. J. Asian Ceram. Soc. 2016, 4, 219–233. [Google Scholar] [CrossRef]
- Schacht, C.A. Refractories Handbook, 1st ed.; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Routschka, G.; Wuthnow, H. Refractory Materials: Pocket Manual, 2nd ed.; Vulkan-Verlag: Essen, Germany, 2004; Volume 9, ISBN 3-8027-3154. [Google Scholar]
- Szczerba, J. Modified magnesia refractory materials. Ceramics 2007, 99, 1–204. (In Polish) [Google Scholar]
- Lee, K.S.; Jo, G.; Jung, Y.; Byeun, Y. Effect of carbon content on the mechanical behavior of MgO-C refractories characterized by Hertzian indentation. Ceram. Int. 2016, 42, 9955–9962. [Google Scholar] [CrossRef]
- Benavidez, E.; Brandaleze, E.; Musante, L.; Galliano, P. Corrosion Study of MgO-C Bricks in Contact with a Steelmaking Slag. Proc. Mat. Sci. 2015, 8, 228–235. [Google Scholar] [CrossRef][Green Version]
- Baudson, H.; Debucquoy, F.; Huger, M.; Gault, C.; Rigaud, M. Ultrasonic measurement of Young’s modulus MgO/C refractories at high temperature. J. Eur. Ceram. Soc. 1999, 19, 1895–1901. [Google Scholar] [CrossRef]
- Hashemi, B.; Nemati, Z.A.; Faghihi-Sani, M.A. Effect of resin and graphite content on density and oxidation behavior of MgO-C refractory brick. Ceram. Int. 2006, 32, 313–319. [Google Scholar] [CrossRef]
- Musante, L.; Martorello, L.F.; Galliano, P.G.; Cavalieri, A.I.; Tomba Martinez, A.G. Mechanical behavior of MgO-C refractory bricks evaluated by stress-strain curves. Ceram. Int. 2012, 38, 4035–4047. [Google Scholar] [CrossRef]
- Mohamed, E.; Ewais, M. Carbon based refractories. J. Ceram. Soc. Jpn. 2004, 112, 517–532. [Google Scholar] [CrossRef]
- Qeintela, M.A.; Santos, F.D.; Pessoa, C.A.; Rodrigues, J.A.; Pandolfelli, V.C. MgO-C refractories for steel ladles slag line. Refract. Appl. News. 2006, 11, 15–19. [Google Scholar]
- Aneziris, C.G.; Borzov, D.; Ulbricht, J. Magnesia carbon bricks a high duty refractory material. Inter. Refract. Man. 2003, 22–27. [Google Scholar]
- Han, B.; Ke, C.; Wei, Y.; Yan, W.; Wang, C.; Chen, F.; Li, N. Degradation of MgO-C refractories corroded by SiO2-Fe2O3-V2O5-TiO2-MnO-MgO slag. Ceram. Int. 2015, 41, 10966–10973. [Google Scholar] [CrossRef]
- Luo, M.; Li, Y.W.; Jin, S.L.; Sang, S.B.; Zhao, L.; Li, Y.B. Microstructures and mechanical properties of Al2O3-C refractories with addition of multi-walled carbon nanotubes. Mater. Sci. Eng. A 2012, 548, 134–141. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Karamian, E. Impact of titania nanoparticles addition on the microstructure and properties of MgO-C refractories. Ceram. Int. 2017, 43, 15472–15477. [Google Scholar] [CrossRef]
- Yua, C.; Dingb, J.; Denga, C.; Zhua, H.; Peng, N. The effects of sintering temperature on the morphology and physical properties of in situ Si3N4 bonded MgO–C refractory. Ceram. Int. 2018, 44, 1104–1109. [Google Scholar] [CrossRef]
- Mahato, S.; Pratihar, S.K.; Behera, S.K. Fabrication and Properties of MgO-C refractories improved with expanded graphite. Ceram. Int. 2014, 40, 16535–16542. [Google Scholar] [CrossRef]
- Bag, M.; Adak, S.; Sarkar, R. Study on low carbon containing MgO-C refractory: use of nanocarbon. Ceram. Int. 2012, 38, 2339–2346. [Google Scholar] [CrossRef]
- Behera, S.; Sarkar, R. Effect of different metal powder anti-oxidants on N220 nano carbon containing low carbon MgO-C refractory: An in -depth investigation. Ceram. Int. 2016, 42, 18484–18494. [Google Scholar] [CrossRef]
- Uchida, S.; Ichikawa, K.; Niihara, K. High-temperature properties of unburned MgO-C bricks containing Al and Si powders. J. Am. Ceram. Soc. 1998, 81, 2910–2916. [Google Scholar] [CrossRef]
- Gokce, A.S.; Gurcan, C.; Ozgen, S.; Aydin, S. The effect of antioxidants on the oxidation behavior of magnesia–carbon refractory bricks. Ceram. Int. 2008, 34, 323–330. [Google Scholar] [CrossRef]
- Ghosh, N.K.; Jagannathan, K.P.; Ghosh, D.N. Oxidation of magnesia–carbon refractories with addition of aluminum and silicon in air. Interceram. 2001, 50, 196–202. [Google Scholar]
- Ghasemi-Kahrizsangi, S.; Gheisari Dehsheikh, H.; Boroujerdnia, M. Effect of micro and nano-Al2O3 addition on the microstructure and properties of MgO-C refractory ceramic composite. Mater. Chem. Phys. 2017, 189, 230–236. [Google Scholar] [CrossRef]
- Bavand-Vandchali, M.; Sarpoolaky, H.; Golestani-Fard, F.; Rezaie, H.R. Atmosphere and carbon effects on microstructure and phase analysis of in situ spinel formation in MgO–C refractories matrix. Ceram. Int. 2009, 35, 861–868. [Google Scholar] [CrossRef]
- Liu, H.; Meng, F.; Li, Q.; Huang, Z.; Fang, M.; Liu, Y.G.; Wu, X. Phase behavior analysis of MgO-C refractory at high temperature: influence of Si powder additives. Ceram. Int. 2015, 41, 5186–5190. [Google Scholar] [CrossRef]
- Yamakuchi, A. Behaviors of SiC and Al added to carbon-containing refractories. Taikabutsu Overseas 1984, 4, 14–18. [Google Scholar]
- Aneziris, C.G.; Hubalkov, J.; Barabas, R. Microstructure evaluation of MgO-C refractories with TiO2- and Al-additions. J. Eur. Ceram. Soc. 2007, 27, 73–78. [Google Scholar] [CrossRef]
- Bitencourta, C.S.; Luzan, A.P.; Pagliosab, C.; Pandolfelli, V.C. Phase and microstructural evolution based on Al, Si and TiO2 reactions with a MgO-C resin-bonded refractory. Ceram. Int. 2016, 42, 16480–16490. [Google Scholar] [CrossRef]
- Nemati, Z.A.; Poya-Mehr, M.R. Effects of aluminum, silicon and ferro-silicon antioxidants in MgO-C refractories. IJE Trans. B: Appl. 2003, 16, 361–372. [Google Scholar]
- Suruga, T. Effect of Mg-B material addition to MgO-C bricks. Taikabutsu Overseas 1995, 15, 25–31. [Google Scholar]
- Wang, T.; Yamaguchi, A. Oxidation protection of MgO-C refractories by means of Al8B4C7. J. Am. Ceram. Soc. 2001, 84, 577–582. [Google Scholar] [CrossRef]
- Sunwoo, S.; Kim, J.H.; Lee, K.G.; Kim, H. Preparation of ZrO2 coated graphite powders. J. Mater. Sci. 2000, 35, 3677–3680. [Google Scholar] [CrossRef]
- Zhang, S.; Yamaguchi, A. Effects of CaO and Al2O3 added to MgO-C refractories on MgO-C reaction. J. Ceram. Soc. Jpn. 1996, 104, 84–88. [Google Scholar] [CrossRef][Green Version]
- Ichikawa, K.; Nishio, H.; Nomura, O.; Hoshiyama, Y. Suppression effects of aluminum on oxidation of MgO–C bricks. Taikabutsu Overseas 1995, 15, 21–24. [Google Scholar]
- Rekha Das, R. Effect of micron and nano MgAl2O4 spinel addition on the properties of magnesia-carbon refractories. Master’s Thesis, National Institute of Technology Rourkela, Rourkela, India, 25 October 2010. [Google Scholar]
- Saberi, A.; Golestani-Fard, F.; Sarpoolaky, H.; Willert-Porada, M.; Gerdes, T.; Simon, R.; Liebscher, C. Development of MgAl2O4 spinel coating on graphite surface to improve its water-wettability and oxidation resistance. Ceram. Int. 2009, 35, 457–461. [Google Scholar] [CrossRef]
- Ganesh, I.; Bhattacherjee, S.; Saha, B.P.; Johnson, R.; Rajeshwari, K.; Sengupta, R.; Ramana, M.V.; Mahajan, Y.R. An efficient MgAl2O4 spinel additive for improved slag erosion and penetration resistance of high-Al2O3 and MgO-C refractories. Ceram. Int. 2002, 28, 245–253. [Google Scholar] [CrossRef]
- Li, Y.W.; Liao, N.; Sang, S.B.; Peng, H. Microstructure and mechanical properties of Al2O3-C refractories using carbon black and multi-walled carbon nanotubes as carbon sources. J. Ceram. Sci. Tech. 2015, 6, 207–214. [Google Scholar] [CrossRef]
- Liao, N.; Li, Y.; Jin, S.; Sang, S.; Harmuth, H. Enhanced mechanical performance of Al2O3-C refractories with nano carbon black and in-situ formed multi-walled carbon nanotubes (MWCNTs). J. Eur. Ceram. Soc. 2015, 3, 867–874. [Google Scholar] [CrossRef]
- Zhu, T.; Li, Y.; Sang, S.; Xie, Z. Fracture behavior of low carbon MgO-C refractories using the wedge splitting test. J. Eur. Ceram. Soc. 2017, 37, 1789–1797. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Li, S.; Xu, N.; Xiang, R.; Yang, O.; Ni, Y. Effects of nano-alumina content on the formation of interconnected pores in porous purging plug materials. Ceram. Int. 2017, 43, 16722–16726. [Google Scholar] [CrossRef]
- Shahraki, A.; Ghasemi-Kahrizsangi, S.; Nemati, A. Performance improvement of MgO-CaO refractories by the addition of nano-sized Al2O3. Mater. Chem. Phys. 2017, 198, 354–359. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Sedeh, M.B.; Dehsheikh, H.G.; Shahraki, A.; Farooghi, M. Densification and properties of ZrO2 nanoparticles added magnesia-doloma refractories. Ceram. Int. 2016, 42, 15658–15663. [Google Scholar] [CrossRef]
- Dehsheikh, H.G.; Ghasemi-Kahrizsangi, S. The influence of silica nanoparticles addition on the physical, mechanical, thermo-mechanical as well as microstructure of Mag-Dol refractory composites. Ceram. Int. 2017, 43, 16780–16786. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Karamian, E.; Nemati, A. A comparative evaluation of the additional impact of nanometer-sized tetravalent oxides on the performance of Doloma-Magnesia ceramic refractories. Ceram. Int. 2018, 44, 2058–2064. [Google Scholar] [CrossRef]
- Yuan, W.; Tang, H.; Zhou, Y.; Zhang, D. Effects of fine reactive alumina powders on properties of alumina-magnesia castables with TiO2 addition. Ceram. Int. 2018, 44, 5032–5036. [Google Scholar] [CrossRef]
- Bag, M.; Adak, S.; Sarkar, R. Nano carbon containing MgO-C refractory: effect of graphite content. Ceram. Int. 2012, 38, 4909–4914. [Google Scholar] [CrossRef]
- Tamura, S.; Ochiai, S.; Takanaga, S.; Kanai, T.; Nakamura, H. Nano-tech. refractories-1: The development of the nano structural matrix. In Proceedings of the UNITECR´03 Congress, Osaka, Japan, 19–22 October 2003; pp. 515–520. [Google Scholar]
- Takanaga, S.; Ochiai, T.; Tamura, T.; Kanai, T.; Nakamura, H. Nano-tech. refractories-2: The application of the nano structural matrix to MgO-C bricks. In Proceedings of the UNITECR´03 Congress, Osaka, Japan, 19–22 October 2003; pp. 521–524. [Google Scholar]
- Zhu, T.B.; Li, Y.W.; Sang, S.B.; Jin, S.L.; Li, Y.B.; Zhao, L.; Liang, X. Effect of nanocarbon sources on microstructure and mechanical properties of MgO-C refractories. Ceram. Int. 2014, 40, 4333–4340. [Google Scholar] [CrossRef]
- Zhu, T.B.; Li, Y.W.; Luo, M.; Sang, S.B.; Wang, Q.H.; Zhao, L.; Li, Y.B.; Li, S.J. Microstructure and mechanical properties of MgO-C refractories containing graphite oxide nanosheets (GONs). Ceram. Int. 2013, 39, 3017–3025. [Google Scholar] [CrossRef]
- Zhu, T.B.; Li, Y.W.; Jin, S.L.; Sang, S.B.; Wang, Q.H.; Zhao, L.; Li, Y.B.; Li, S.J. Microstructure and mechanical properties of MgO-C refractories containing expanded graphite. Ceram. Int. 2013, 39, 4529–4537. [Google Scholar] [CrossRef]
- Mahato, S.; Behera, S.K. Oxidation resistance and microstructural evolution in MgO-C refractories with expanded graphite. Ceram. Int. 2016, 42, 7611–7619. [Google Scholar] [CrossRef]
- Wei, G.P.; Zhu, B.Q.; Li, X.C.; Ma, Z. Microstructure and mechanical properties of low carbon MgO-C refractories bonded by an Fe nanosheet-modified phenolic resin. Ceram. Int. 2015, 41, 1553–1566. [Google Scholar] [CrossRef]
- Matsuo, Y.; Tanaka, M.; Yoshitomi, S.; Yoon, S.; Miyawaki, J. Effect of the carbon nanofiber addition on the mechanical properties of MgO-C brick. In Proceedings of the UNITECR´11 Congress, Kyoto, Japan, 30 October–2 November 2011; pp. 959–962. [Google Scholar]
- Ghasemi-Kahrizsangi, S.; Karamian, E.; Dehsheikh, H.G. The impact of ZrSiO4 nanoparticles addition on the microstructure and properties of dolomite based refractories. Ceram. Int. 2017, 43, 13932–13937. [Google Scholar] [CrossRef]
- Zargar, H.R.; Oprea, C.; Oprea, G.; Troczynski, T. The effect of nano-Cr2O3 on solid-solution assisted sintering of MgO refractories. Ceram. Int. 2012, 38, 6235–6241. [Google Scholar] [CrossRef]
- Azhari, A.; Golestani-Fard, F.; Sarpoolaky, H. Effect of nano iron oxide as an additive on phase and microstructural evolution of Mag-Chrome refractory matrix. J. Eur. Ceram. Soc. 2009, 29, 2679–2684. [Google Scholar] [CrossRef]
- Huizhong, L.H.; Jianxiu, W. Influence of nano-Fe2O3 on sintering and mechanical property of magnesia-chrome refractories. Refractories 2003, 5, 002. [Google Scholar]
- Chen, M.; Lu, C.; Yu, J. Improvement in performance of MgO-CaO refractories by addition of nano-sized ZrO2. J. Eur. Ceram. Soc. 2007, 27, 4633–4638. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Karamian, E.; Boroujerdnia, M.; Payandeh, K. Effect of MgAl2O4 nanoparticles addition on the densification and properties of MgO-CaO refractories. Ceram. Int. 2017, 43, 5014–5019. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Boroujerdnia, M. MgO-CaO-Cr2O3 composition as a novel refractory brick: Use of Cr2O3 nanoparticles. Bol. Soc. Española Cerámica Vidr. 2017, 56, 83–89. [Google Scholar] [CrossRef]
- Rodríguez, E.; Moreno, F.H.; Aguilar-Martínez, J.A.; Montes-Mejía, A.E.; Ruiz-Valdés, J.J.; Puente-Ornelas, R.; Contreras, J.E. Effect of nano-titania (η-TiO2) content on the mechano-physical properties of a magnesia refractory composite. Ceram. Int. 2016, 42, 8445–8452. [Google Scholar] [CrossRef]
- Saberi, A.; Golestani-Fard, F.; Willert-Porada, M.; Negahdari, Z.; Liebscher, C.; Gossler, B. A novel approach to synthesis of nanosized MgAl2O4 spinel powder through sol-gel citrate technique and subsequent heat treatment. Ceram. Int. 2009, 35, 933–937. [Google Scholar] [CrossRef]
- Gómez Rodríguez, C.; Das Roy, T.K.; Shaji, S.; Castillo Rodríguez, G.A.; García Quiñonez, L.; Rodríguez, E.; González, J.O.; Aguilar-Martínez, J.A. Effect of addition of Al2O3 and Fe2O3 nanoparticles on the microstructural and physic-chemical evolution of dense magnesia composite. Ceram. Int. 2015, 41, 7751–7758. [Google Scholar] [CrossRef]
- Estili, M.; Sakka, Y. Recent advances in understanding the reinforcing ability and mechanism of carbon nanotubes in ceramic matrix composites. Sci. Technol. Adv. Mater. 2014, 15, 064902. [Google Scholar] [CrossRef]
- Esawi, A.; Farag, M. Carbon nanotube reinforced composites: Potential and current challenges. Mater. Des. 2007, 28, 2394–2401. [Google Scholar] [CrossRef]
- Burakova, E.A.; Dyachkova, T.P.; Rukhov, A.V.; Tugolukov, E.N.; Galunin, E.V.; Trachev, A.G.; Basheer, A.A.; Ali, I. Novel and economic method of carbon nanotubes synthesis on a nickel magnesium oxide catalyst using microwave radiation. J. Mol. Liq. 2018, 253, 340–346. [Google Scholar] [CrossRef]
- Otroj, S.; Daghighi, A. Microstructure and phase evolution of alumina-spinel self-flowing refractory castables containing nano-alumina particles. Ceram. Int. 2011, 37, 1003–1009. [Google Scholar] [CrossRef]
- Gomez-Rodriguez, C.; Fernández-González, D.; García-Quiñonez, L.V.; Castillo-Rodríguez, G.A.; Aguilar-Martínez, J.A.; Verdeja, L.F. MgO refractory doped with ZrO2 nanoparticles: influence of cold isostatic and uniaxial pressing and sintering temperature in the physical and chemical properties. Metals 2019, 9, 1297. [Google Scholar] [CrossRef]
- Roy, J.; Chandra, S.; Maitra, S. Nanotechnology in castable refractory. Ceram. Int. 2018, 45, 19–29. [Google Scholar] [CrossRef]
- Ghosh, A.; Sarkar, R.; Mukherjee, B.; Das, S.K. Effect of spinel content on the properties of magnesia-spinel composite refractory. J. Eur. Ceram. Soc. 2004, 24, 2079–2085. [Google Scholar] [CrossRef]
- Haines, P.J. Thermal Methods of Analysis, 1st ed.; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Salomao, R.; Bittencourt, L.R.M.; Pandolfelli, V.C. A novel approach for magnesia hydration assessment in refractory castables. Ceram. Int. 2007, 33, 803–810. [Google Scholar] [CrossRef]
- Soady, J.S.; Plint, S. A quantitative thermal shock approach to the development of magnesia-spinel refractories for the cement kiln. In Proceedings of the UNITECR´91 Congress, Aachen, Germany, 23–26 September 1991; pp. 443–449. [Google Scholar]
- Verdeja, L.F.; Sancho, J.P.; Ballester, A.; Gonzalez, R. Refractory and Ceramic Materials, 1st ed.; Síntesis: Madrid, Spain, 2014. [Google Scholar]
- Aksel, C.; Riley, F.L. Magnesia-spinel (MgAl2O4) refractory ceramic composites. Ceram. Matrix Compos. 2006, 359–399. [Google Scholar] [CrossRef]
- Rodríguez, E.A.; Limones, A.K.; Contreras, J.E.; Ruíz-Valdez, J.J.; Puente-Ornelas, R.; Arato, A.M.; Aguilar-Martínez, J.A. Effect of hercynite spinel content on the properties of magnesia-calcium zirconate dense refractory composite. J. Eur. Ceram. Soc. 2015, 35, 2631–2639. [Google Scholar] [CrossRef]
- Zhengzhou Rongsheng Kiln Refractory Co., LTD. Available online: https://zzrsnc.en.alibaba.com/ (accessed on 22 January 2020).
- Hardness Testing of Ceramics. Available online: http://www.spectru.com.br/hardness_testing_ceramics.pdf (accessed on 19 November 2019).
- Sainz, M.A.; Mazzoni, A.D.; Aglietti, E.F.; Caballero, A. Thermochemical stability of spinel (MgO·Al2O3) under strong reducing condition. Mater. Chem. Phys. 2004, 86, 399–408. [Google Scholar] [CrossRef]
- Liu, C.-M.; Chen, J.; Chen, C.-J. The growth of an epitaxial Mg-Al spinel layer on sapphire by solid-state reactions. J. Cryst. Growth 2005, 285, 275–283. [Google Scholar] [CrossRef]
- Saberi, A.; Golestani-Fard, F.; Sarpoolaky, H.; Willert-Porada, M.; Gerdes, T.; Simon, R. Chemical synthesis of nanocrystalline magnesium aluminate spinel via nitrate-citrate combustion route. J. Alloy. Comp. 2008, 462, 142–146. [Google Scholar] [CrossRef]
- Alinejad, B.; Sarpoolaky, H.; Beitollahi, A.; Saberi, A.; Afshar, S. Synthesis and characterization of nanocrystalline MgAl2O4 spinel via sucrose process. Mater. Res. Bull. 2008, 43, 1188–1194. [Google Scholar] [CrossRef]
- Su, X.; Du, X.; Li, S.; Li, J. Synthesis of MgAl2O4 spinel nanoparticles using a mixture of bayerite and magnesium sulfate. J. Nanopart. Res. 2010, 12, 1813–1819. [Google Scholar] [CrossRef]
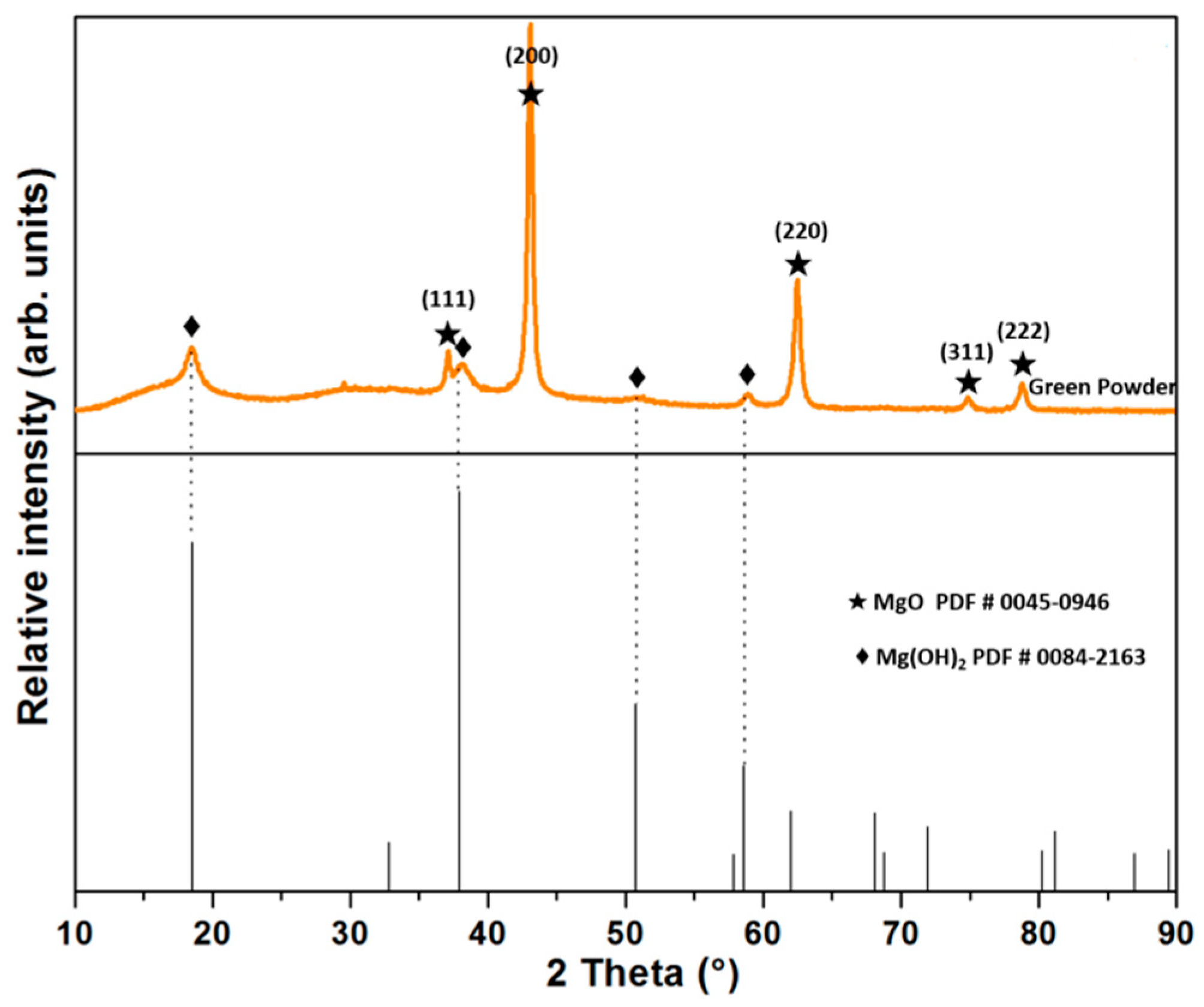
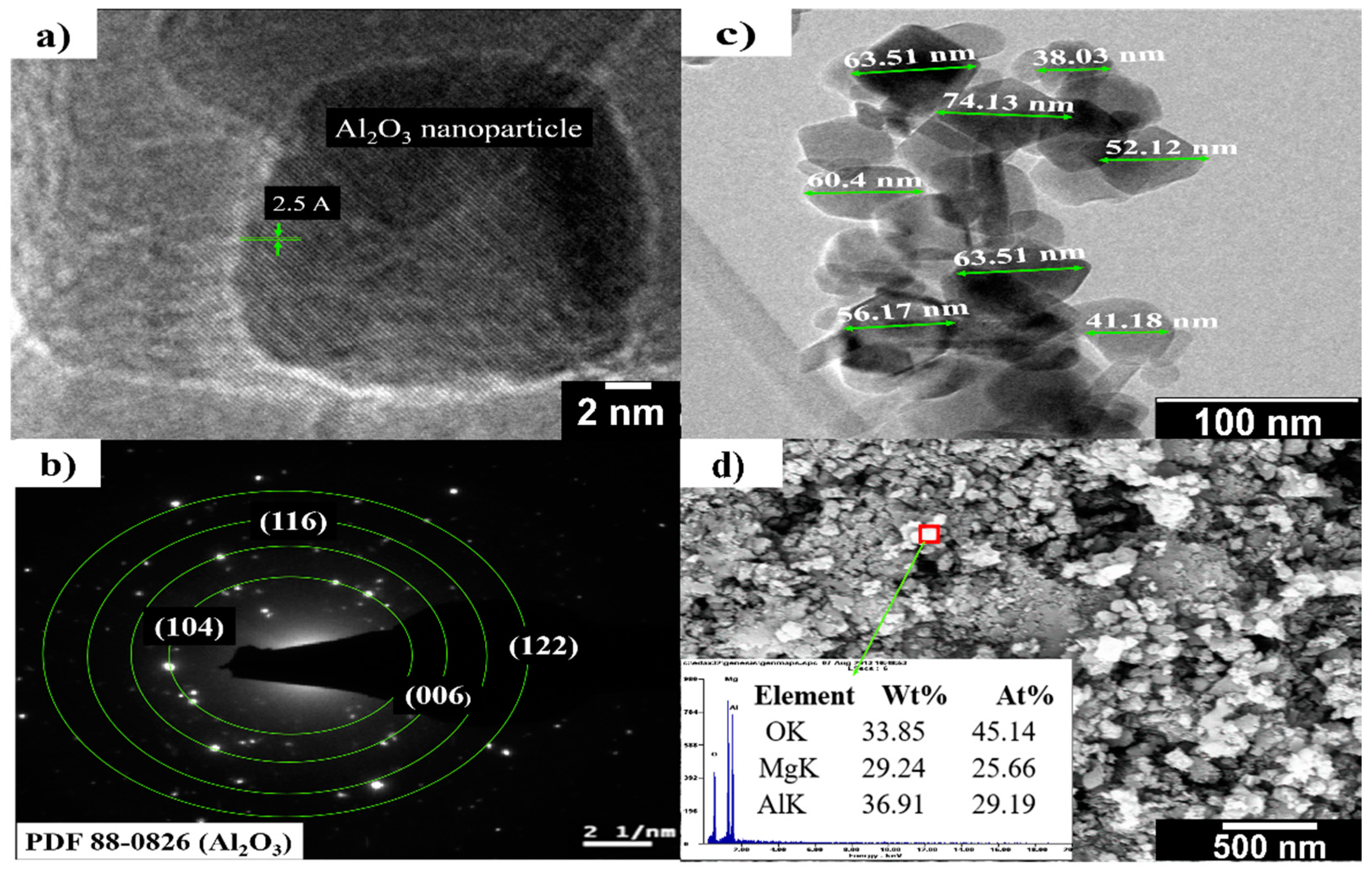

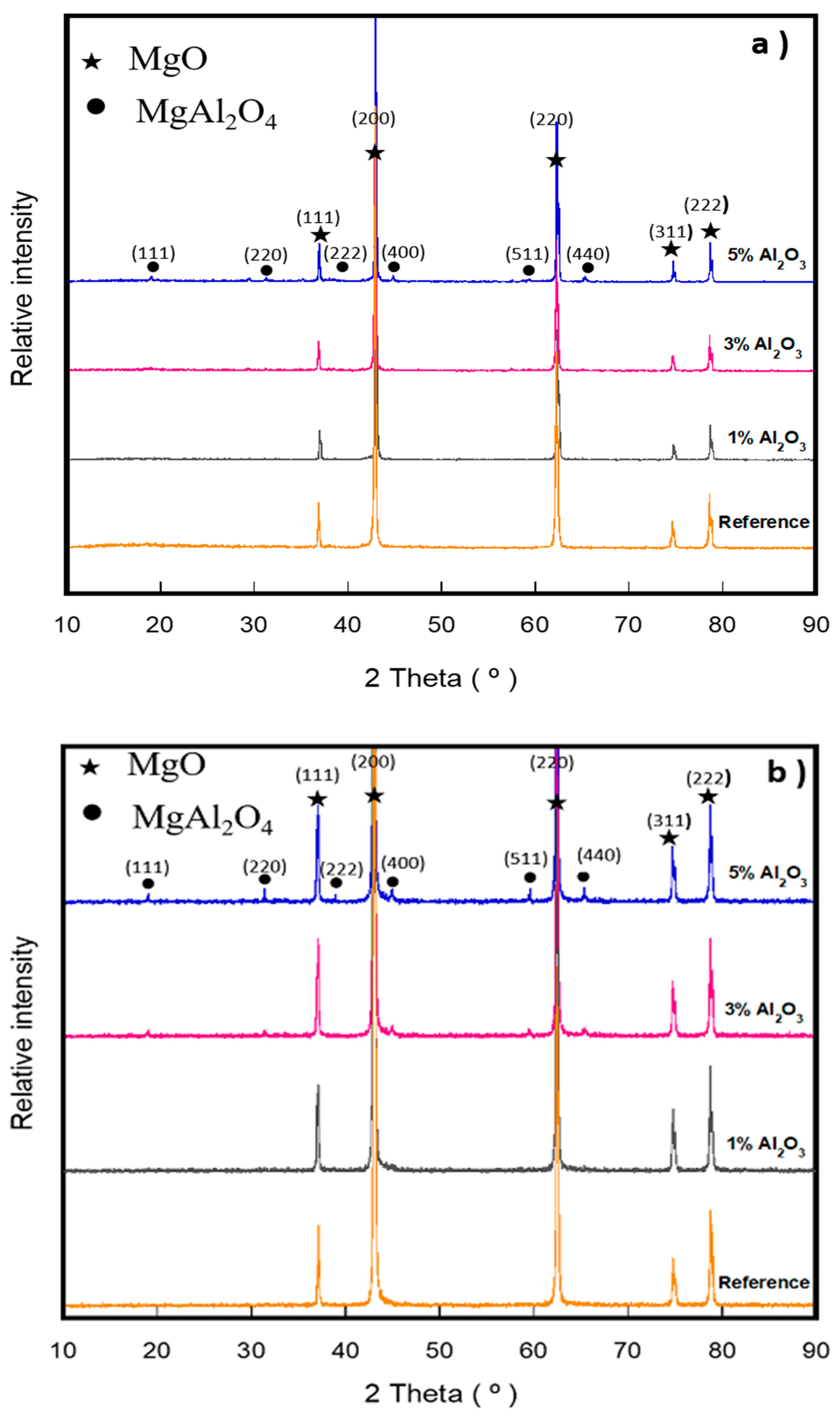

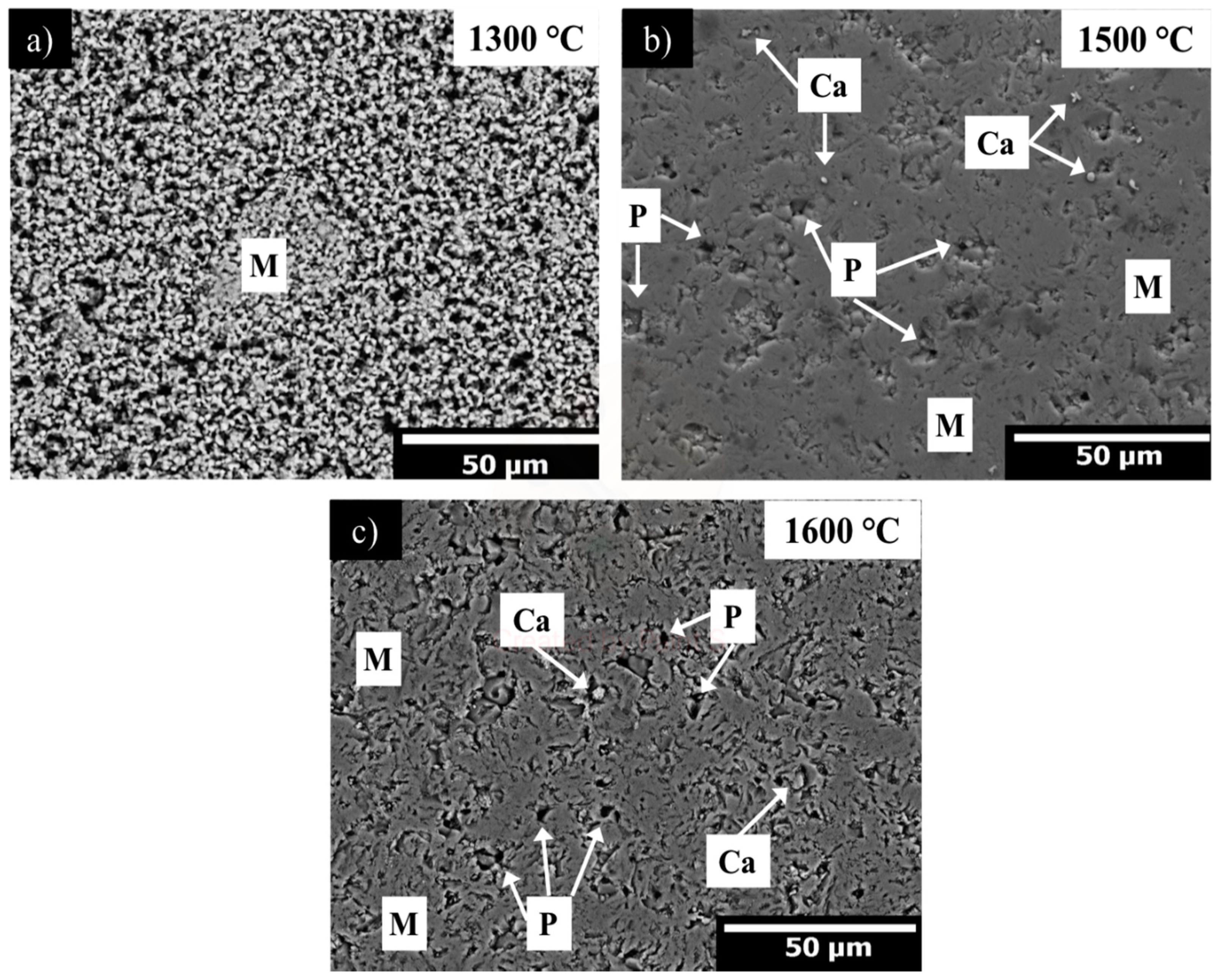
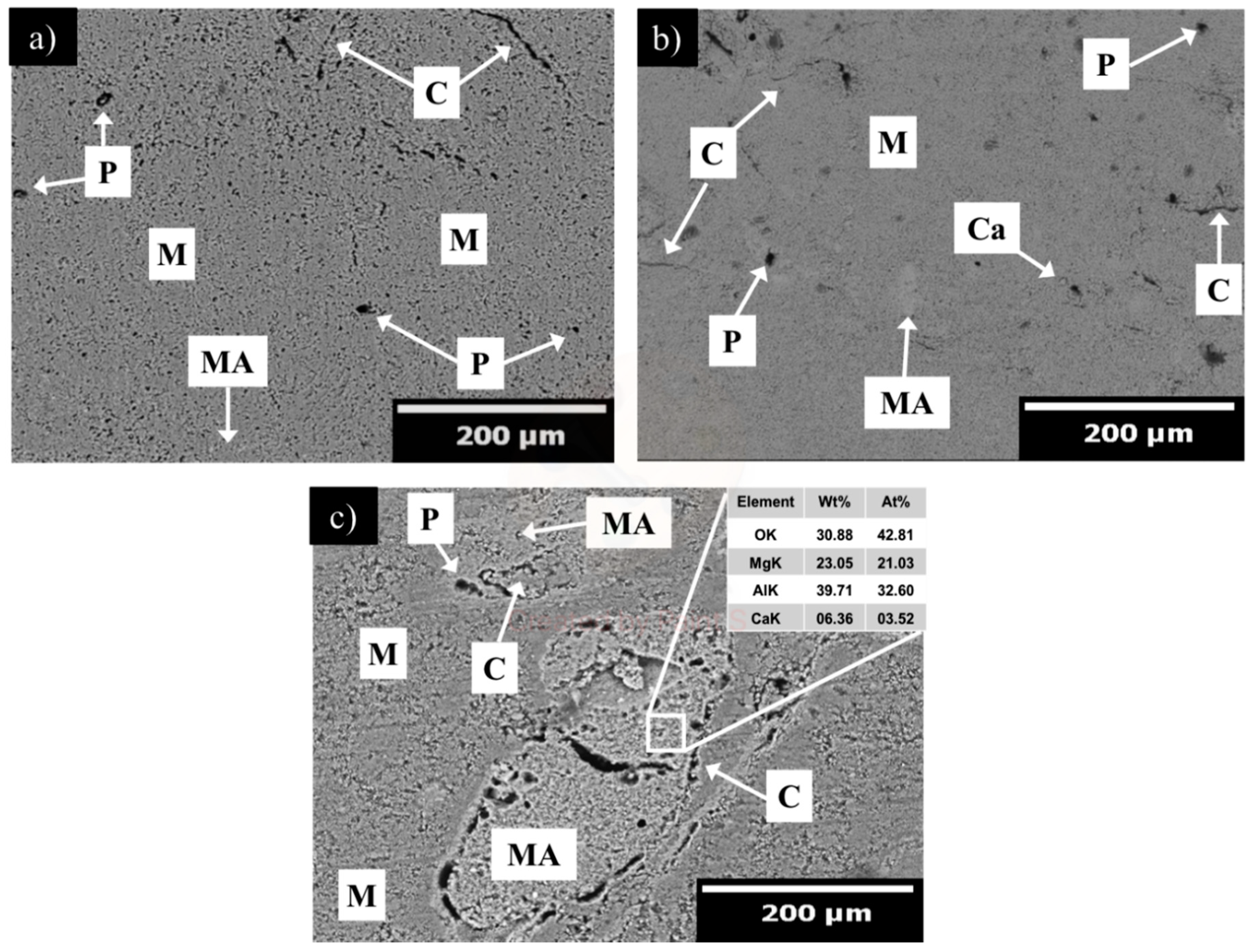
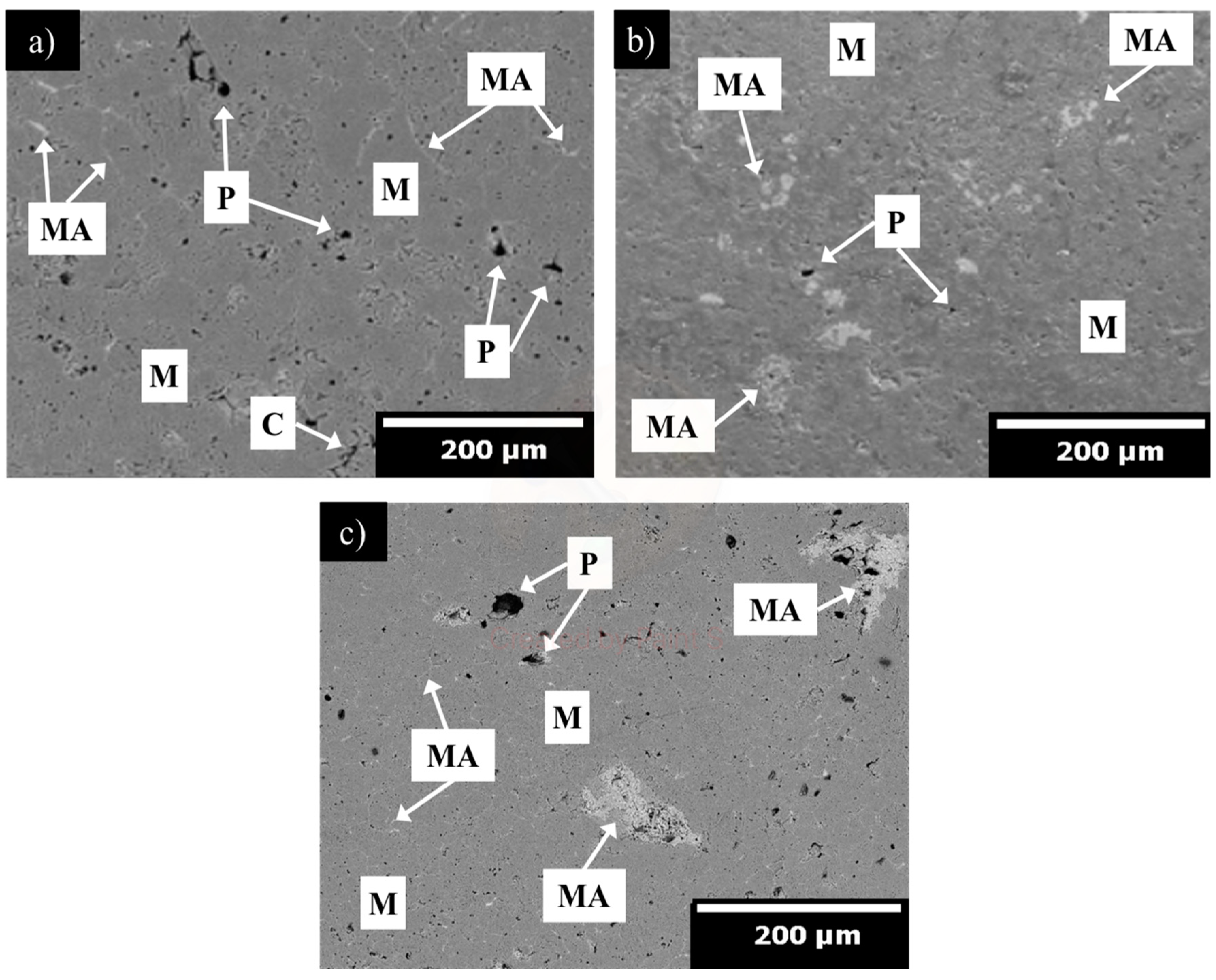
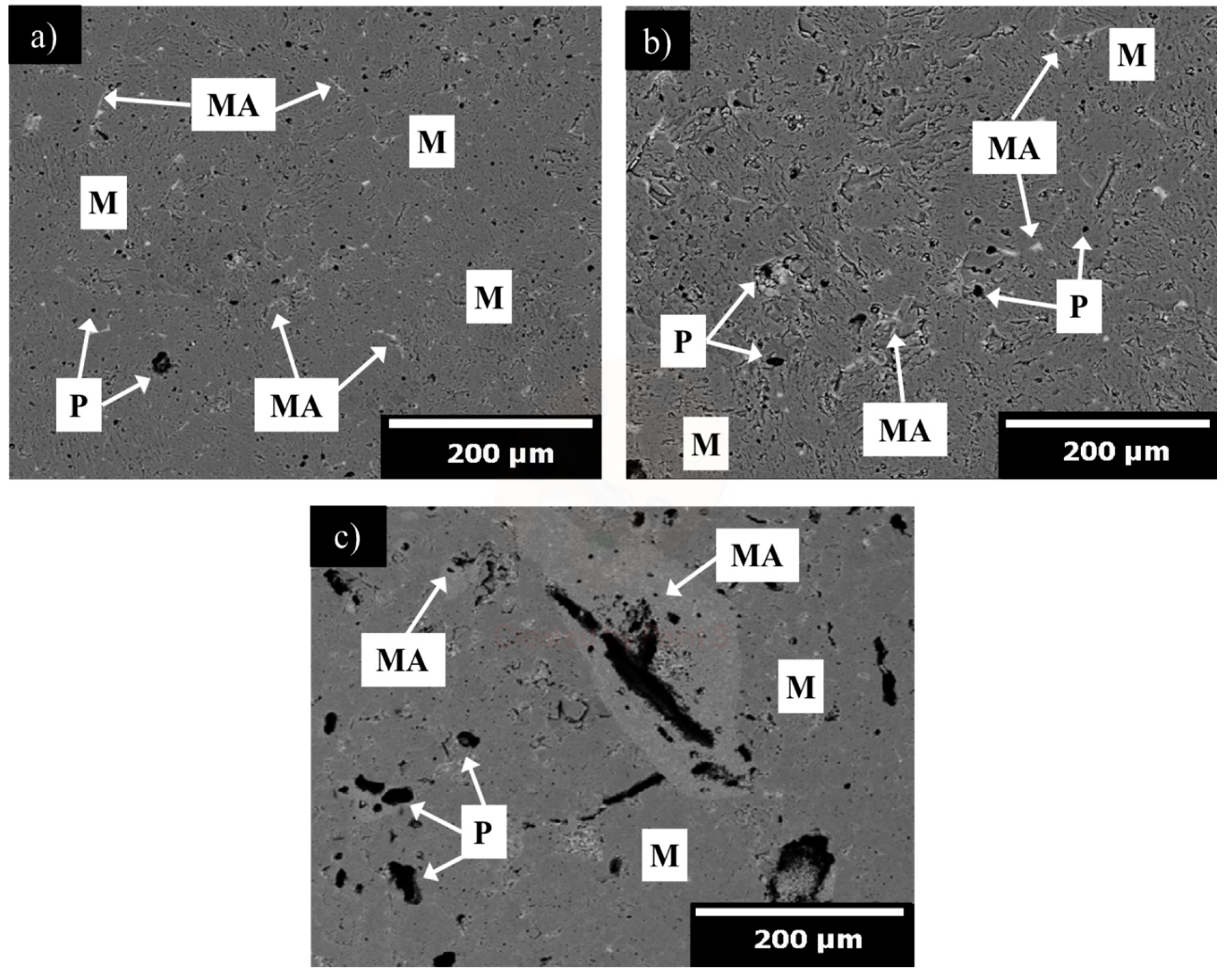

| MgO | CaO | Fe2O3 | SiO2 | LOI |
|---|---|---|---|---|
| 97.43 | 0.9 | 0.06 | 0.6 | 1.01 |
| Purity (wt.%) | Size (nm) | SSA (m2/g) | Color |
|---|---|---|---|
| 99.9 | 50 | 18 | White |
| Temperature | Sample Code | Batch Composition (wt.%) | Green Density (g/cm3) | |
|---|---|---|---|---|
| MgO | Al2O3 | |||
| 1300 °C | A013 | 100 | 0 | 2.29 |
| A113 | 99 | 1 | 2.32 | |
| A313 | 97 | 3 | 2.29 | |
| A513 | 95 | 5 | 2.32 | |
| 1500 °C | A015 | 100 | 0 | 2.32 |
| A115 | 99 | 1 | 2.38 | |
| A315 | 97 | 3 | 2.32 | |
| A515 | 95 | 5 | 2.32 | |
| 1600 °C | A016 | 100 | 0 | 2.31 |
| A116 | 99 | 1 | 2.29 | |
| A316 | 97 | 3 | 2.32 | |
| A516 | 95 | 5 | 2.31 | |
| 1000 °C (DSC) | A0 | 100 | 0 | 2.36 |
| A1 | 99 | 1 | 2.32 | |
| A3 | 97 | 3 | 2.34 | |
| A5 | 95 | 5 | 2.31 | |
| Phase | wt.% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Compositions | ||||||||||||
| A0 | A113 | A313 | A513 | A015 | A115 | A315 | A515 | A016 | A116 | A316 | A516 | |
| MgO | 100 | 99.69 | 99.02 | 96.07 | 100 | 97.22 | 94.19 | 90.03 | 100 | 98.87 | 96.28 | 90.37 |
| MgAl2O4 | 0 | 0.310 | 0.980 | 3.93 | 0 | 2.78 | 5.81 | 9.97 | 0 | 1.13 | 3.72 | 9.63 |
| Refractory Matrix | Modifying Agent | Density (g/cm3) | Apparent Porosity (%) | Maximum CCS (MPa) | Reference/Year |
|---|---|---|---|---|---|
| Laboratory Study | |||||
| MgO | nano-TiO2 | 3.46 | 0.52 | 234.7 | [63]/2016 |
| MgO | nano-Fe2O3/Al2O3 | 3.38/3.18 | 1.87/6.5 | 65.1/42.7 | [65]/2015 |
| MgO-C | nano-TiO2 | 3.32 | 4.97 | 42.6 | [15]/2017 |
| MgO-C | expanded graphite | 3.05 | 3.0 | 59.7 | [17]/2014 |
| MgO-C | nano-Al2O3 | 3.31 | 5.73 | 40.2 | [23]/2016 |
| MgO-C | nano carbon black | 3.12 | 4.25 | 51.0 | [18]/2012 |
| MgO-C | Fe nanosheets | - | - | 92.4 | [54]/2015 |
| MgO-CaO | nano-Al2O3 | 3.5 | 5 | 100 | [42]/2017 |
| MgO-CaO | nano-SiO2 | 3.35 | 5.7 | 58.8 | [44]/2017 |
| MgO-CaO | nano-ZrO2/SiO2/TiO2 | 3.27/3.17/3.2 | 7.2/6.4/7.1 | 49/54/51 | [45]/2018 |
| MgO-CaO | nano-ZrSiO4 | 3.33 | 4.84 | 38.5 | [56]/2017 |
| MgO-CaO | nano-MgAl2O4 | 3.31 | 9.6 | 66.0 | [61]/2017 |
| MgO-CaO | nano-Cr2O3 | 3.35 | 2.9 | 82.3 | [62]/2017 |
| MgO-CaZrO3 | Fe2O4 | 3.2 | 11.4 | 180.3 | [78]/2015 |
| Commercial Refractories | |||||
| MgO-iron spinel | FeAl2O4 | 2.9 | 16–17 | 45–55 | [79]/2020 |
| Magnesium aluminum-spinel | MgAl2O4 | 2.95 | 18–19 | 40–45 | [79]/2020 |
| Magnesia | CaO, SiO2 | 2.9 | 16–18 | 60 | [79]/2020 |
| Magnesia-zirconia | ZrO2 | 2.95–3.05 | 18–19 | 40–50 | [79]/2020 |
| Magnesia-chrome | Cr2O3 | 3.0–3.08 | 14–18 | 50–55 | [79]/2020 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Rodríguez, C.; Castillo-Rodríguez, G.A.; Rodríguez-Castellanos, E.A.; Vázquez-Rodríguez, F.J.; López-Perales, J.F.; Aguilar-Martínez, J.A.; Fernández-González, D.; García-Quiñonez, L.V.; Das-Roy, T.K.; Verdeja, L.F. Development of an Ultra-Low Carbon MgO Refractory Doped with α-Al2O3 Nanoparticles for the Steelmaking Industry: A Microstructural and Thermo-Mechanical Study. Materials 2020, 13, 715. https://doi.org/10.3390/ma13030715
Gómez-Rodríguez C, Castillo-Rodríguez GA, Rodríguez-Castellanos EA, Vázquez-Rodríguez FJ, López-Perales JF, Aguilar-Martínez JA, Fernández-González D, García-Quiñonez LV, Das-Roy TK, Verdeja LF. Development of an Ultra-Low Carbon MgO Refractory Doped with α-Al2O3 Nanoparticles for the Steelmaking Industry: A Microstructural and Thermo-Mechanical Study. Materials. 2020; 13(3):715. https://doi.org/10.3390/ma13030715
Chicago/Turabian StyleGómez-Rodríguez, C., G. A. Castillo-Rodríguez, E. A. Rodríguez-Castellanos, F. J. Vázquez-Rodríguez, J. F. López-Perales, J. A. Aguilar-Martínez, D. Fernández-González, L. V. García-Quiñonez, T. K. Das-Roy, and L. F. Verdeja. 2020. "Development of an Ultra-Low Carbon MgO Refractory Doped with α-Al2O3 Nanoparticles for the Steelmaking Industry: A Microstructural and Thermo-Mechanical Study" Materials 13, no. 3: 715. https://doi.org/10.3390/ma13030715
APA StyleGómez-Rodríguez, C., Castillo-Rodríguez, G. A., Rodríguez-Castellanos, E. A., Vázquez-Rodríguez, F. J., López-Perales, J. F., Aguilar-Martínez, J. A., Fernández-González, D., García-Quiñonez, L. V., Das-Roy, T. K., & Verdeja, L. F. (2020). Development of an Ultra-Low Carbon MgO Refractory Doped with α-Al2O3 Nanoparticles for the Steelmaking Industry: A Microstructural and Thermo-Mechanical Study. Materials, 13(3), 715. https://doi.org/10.3390/ma13030715









