Metallization and Diffusion Bonding of CoSb3-Based Thermoelectric Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Skutterudite Alloys
2.2. The Electroplating Process
2.3. Characterizations
3. Results and Discussion
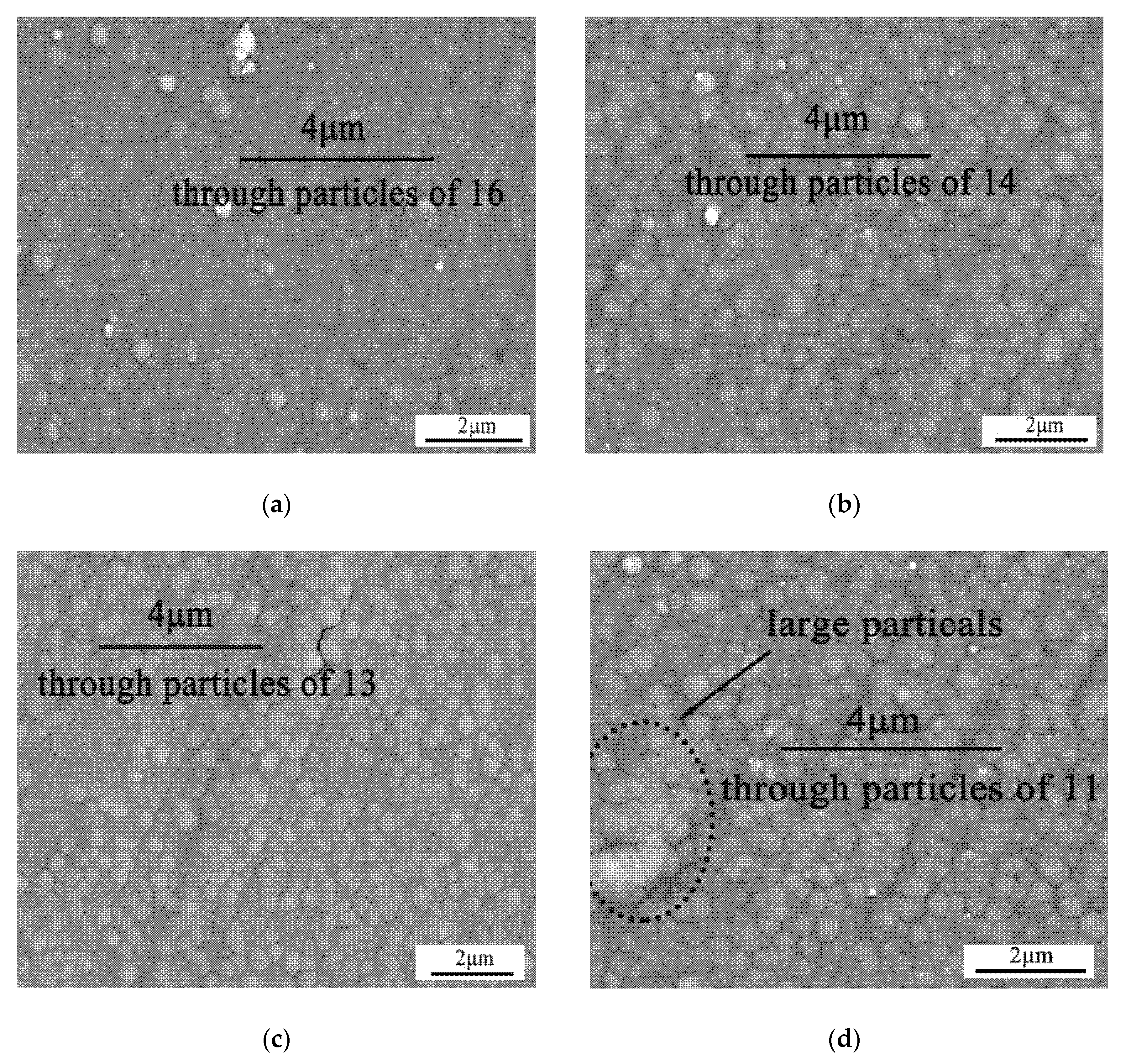
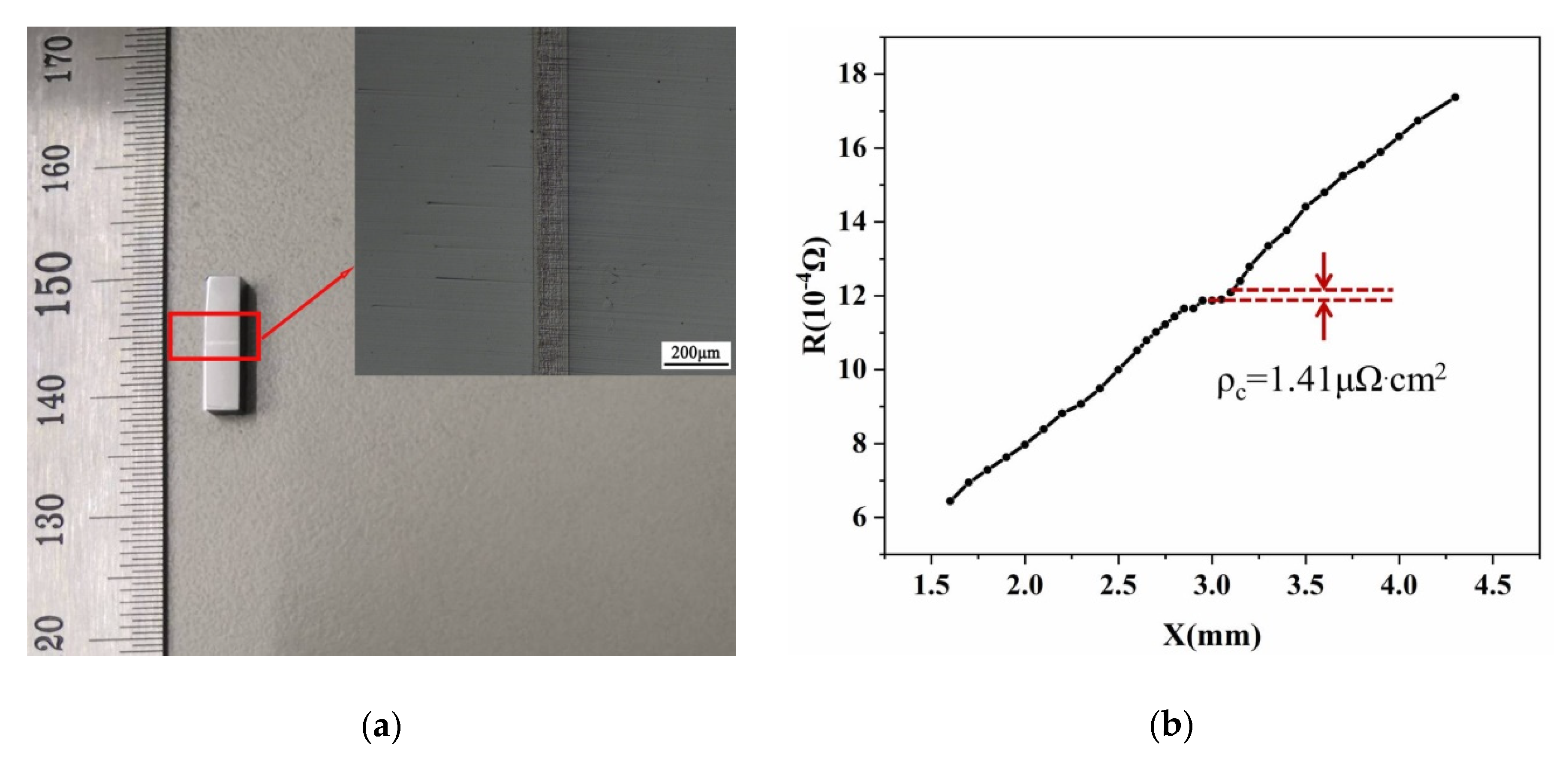
3.1. Metallization of Skutterudite Alloys
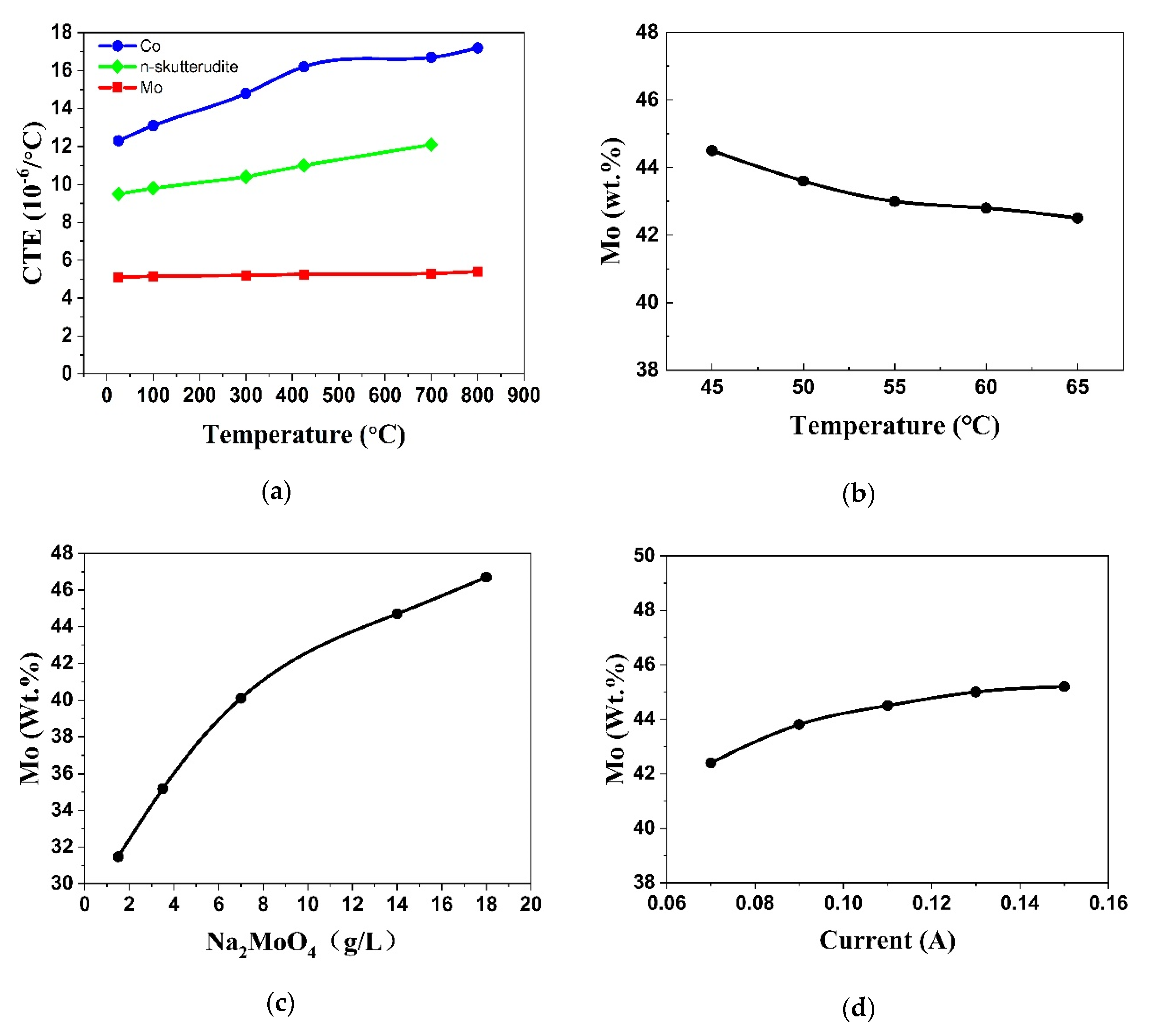
3.2. Tuning the CTE of the Barrier Layer
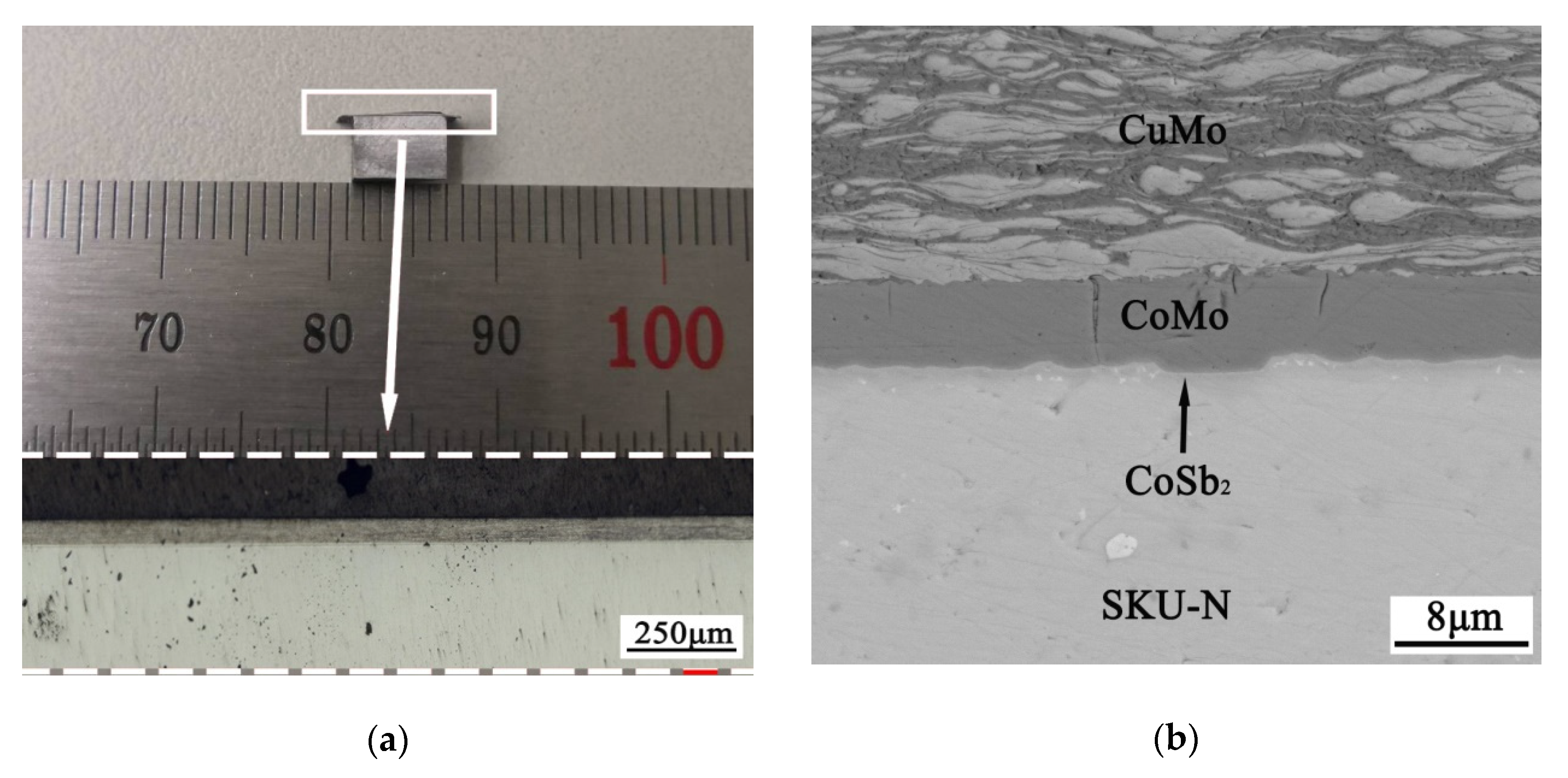
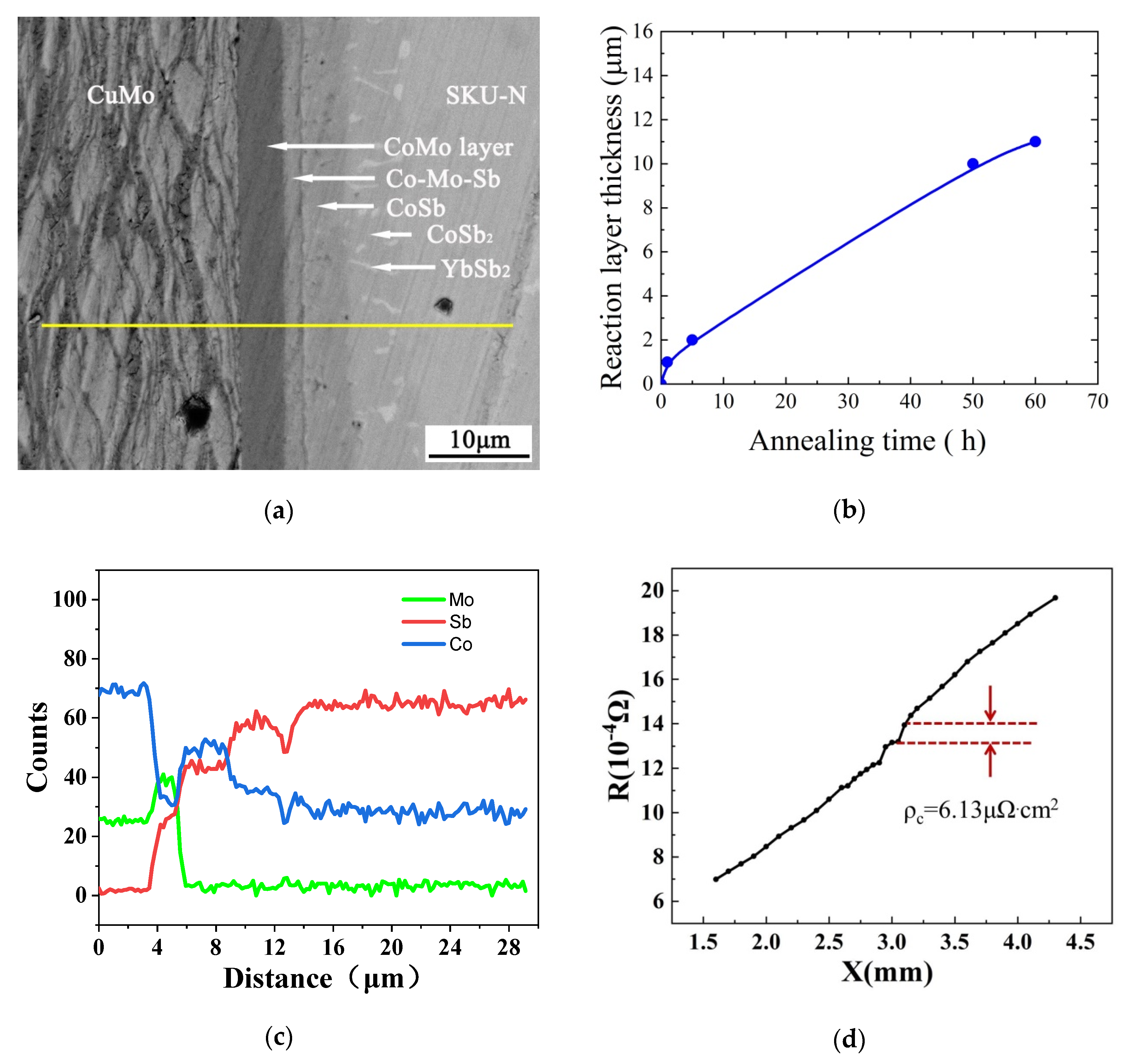
3.3. Diffusion Bonding between the Skutterudite Compound and the Cu-Mo Electrode
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Beretta, D.; Neophytou, N.; Hodges, J.M. Thermoelectrics: From history, a window to the future. Mater. Sci. Eng. R Rep. 2019, 138, 100501. [Google Scholar] [CrossRef]
- Chen, L.; Backhaus-Ricoult, M.; He, L. Fabrication Method for Thermoelectric Device. U.S. Patent 8,198,116, 12 June 2012. [Google Scholar]
- Wu, T.; Bai, S.Q.; Shi, X. Enhanced thermoelectric properties of BaxEuyCo (4) Sb (12) with very high filling fraction. Wuji Cailiao Xuebao J. Inorg. Mater. 2013, 28, 224–228. [Google Scholar] [CrossRef]
- Muto, A.; Yang, J.; Poudel, B.; Ren, Z.; Chen, G. Skutterudite unicouple characterization for energy harvesting applications. Adv. Energy Mater. 2013, 3, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Alleno, E.; Lamquembe, N.; Cardoso-Gil, R. A thermoelectric generator based on an n-type clathrate and ap-type skutterudite unicouple. Phys. Status Solidi a 2014, 211, 1293–1300. [Google Scholar] [CrossRef]
- Salvador, J.R.; Cho, J.Y.; Ye, Z.; Moczygemba, J.E.; Thompson, A.J.; Sharp, J.W.; Koenig, J.; Sakamoto, J. Conversion efficiency of skutterudite-based thermoelectric modules. Phys. Chem. Chem. Phys. 2014, 16, 12510–12520. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Kanno, T.; Sakai, A. Bifunctional thermoelectric tube made of tilted multilayer material as an alternative to standard heat exchangers. Sci. Rep. 2013, 3, 1501. [Google Scholar] [CrossRef]
- Choi, S.; Kurosaki, K.; Li, G. Enhanced thermoelectric properties of Ga and In Co-added CoSb3-based skutterudites with optimized chemical composition and microstructure. AIP Adv. 2016, 6, 125015. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Tritt, T.M. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, eaak9997. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowski, K.T.; Zybala, R.; Mania, R. High temperature CoSb3-Cu junctions. Microelectron. Reliab. 2011, 51, 1198–1202. [Google Scholar] [CrossRef]
- Park, S.H.; Jin, Y.; Cha, J. High-power-density skutterudite-based thermoelectric modules with ultralow contact resistivity using Fe–Ni metallization layers. ACS Appl. Energy Mater. 2018, 1, 1603–1611. [Google Scholar] [CrossRef]
- Fan, X.C.; Gu, M.; Shi, X. Fabrication and reliability evaluation of Yb0.3Co4Sb12/Mo–Ti/Mo–Cu/Ni thermoelectric joints. Ceram. Int. 2015, 41, 7590–7595. [Google Scholar] [CrossRef]
- Gu, M.; Bai, S.; Xia, X. Study on the high temperature interfacial stability of Ti/Mo/Yb0.3Co4Sb12 thermoelectric joints. Appl. Sci. 2017, 7, 952. [Google Scholar] [CrossRef]
- Guo, J.Q.; Geng, H.Y.; Ochi, T. Development of skutterudite thermoelectric materials and modules. J. Electron. Mater. 2012, 41, 1036–1042. [Google Scholar] [CrossRef]
- Geng, H.; Ochi, T.; Suzuki, S. Thermoelectric properties of multifilled skutterudites with La as the main filler. J. Electron. Mater. 2013, 42, 1999–2005. [Google Scholar] [CrossRef]
- Nie, G.; Li, W.; Guo, J. High performance thermoelectric module through isotype bulk heterojunction engineering of skutterudite materials. Nano Energy 2019, 66, 104193. [Google Scholar] [CrossRef]
- Gu, M.; Xia, X.; Li, X. Microstructural evolution of the interfacial layer in the Ti–Al/Yb0.6Co4Sb12 thermoelectric joints at high temperature. J. Alloy. Compd. 2014, 610, 665–670. [Google Scholar] [CrossRef]
- Feng, S.; Chang, Y.; Yang, J.; Poudel, B.; Yu, B.; Ren, Z.; Chen, G. Reliable contact fabrication on nanostructured Bi2Te3-based thermoelectric materials. Phys. Chem. Chem. Phys. 2013, 15, 6757–6762. [Google Scholar] [CrossRef]
- Chen, S.; Chu, A.H.; Wong, D.S.H. Interfacial reactions at the joints of CoSb3-based thermoelectric devices. J. Alloy. Compd. 2017, 699, 448–454. [Google Scholar] [CrossRef]
- Song, B.; Lee, S.; Cho, S. The effects of diffusion barrier layers on the microstructural and electrical properties in CoSb3 thermoelectric modules. J. Alloy. Compd. 2014, 617, 160–162. [Google Scholar] [CrossRef]
- Fan, J.; Chen, L.; Bai, S.; Shi, X. Joining of Mo to CoSb3 by spark plasma sintering by inserting a Ti interlayer. Mater. Lett. 2004, 58, 3876–3878. [Google Scholar] [CrossRef]
- Zhao, D.; Li, X.; He, L.; Jiang, W.; Chen, L. Interfacial evolution behavior and reliability evaluation of CoSb3/Ti/Mo-Cu thermoelectric joints during accelerated thermal aging. Alloy. Compd. 2009, 477, 425–431. [Google Scholar] [CrossRef]
- Zhao, D.; Li, X.; He, L.; Jiang, W.; Chen, L. High temperature reliability evaluation of CoSb3/electrode thermoelectric joints. Intermetallics 2009, 17, 136–141. [Google Scholar] [CrossRef]
- Song, J.; Kim, Y.; Cho, B.J. Thermal diffusion barrier metallization based on Co–Mo powder-mixed composites for n-type skutterudite ((Mm, Sm)yCo4Sb12) thermoelectric devices. J. Alloy. Compd. 2019, 818, 152917. [Google Scholar] [CrossRef]
- Yong, H.; Na, S.K.; Gang, J.G.; Shin, H.S.; Jeon, S.J.; Hyun, S.; Lee, H.J. Study on the contact resistance of various metals (Au, Ti, and Sb) on Bi−Te and Sb−Te thermoelectric films. Jpn. J. Appl. Phys. 2016, 55, 06JE03. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, T.; Wang, Q.; Zhu, Y.; Alshareef, H.N.; Kim, M.J.; Gnade, B.E. Contact resistance and stability study for Au, Ti, Hf and Ni contacts on thin-film Mg2Si. Alloy. Compd. 2017, 699, 1134–1139. [Google Scholar] [CrossRef] [Green Version]
- Ngan, P.H.; Van Nong, N.; Hung, L.T. On the challenges of reducing contact resistances in thermoelectric generators based on half-Heusler alloys. J. Electron. Mater. 2016, 45, 594–601. [Google Scholar] [CrossRef]
- Jie, Q.; Ren, Z.; Chen, G. Fabrication of Stable Electrode/Diffusion Barrier Layers for Thermoelectric Filled Skutterudite Devices. U.S. Patent 9,722,164, 2 October 2015. [Google Scholar]
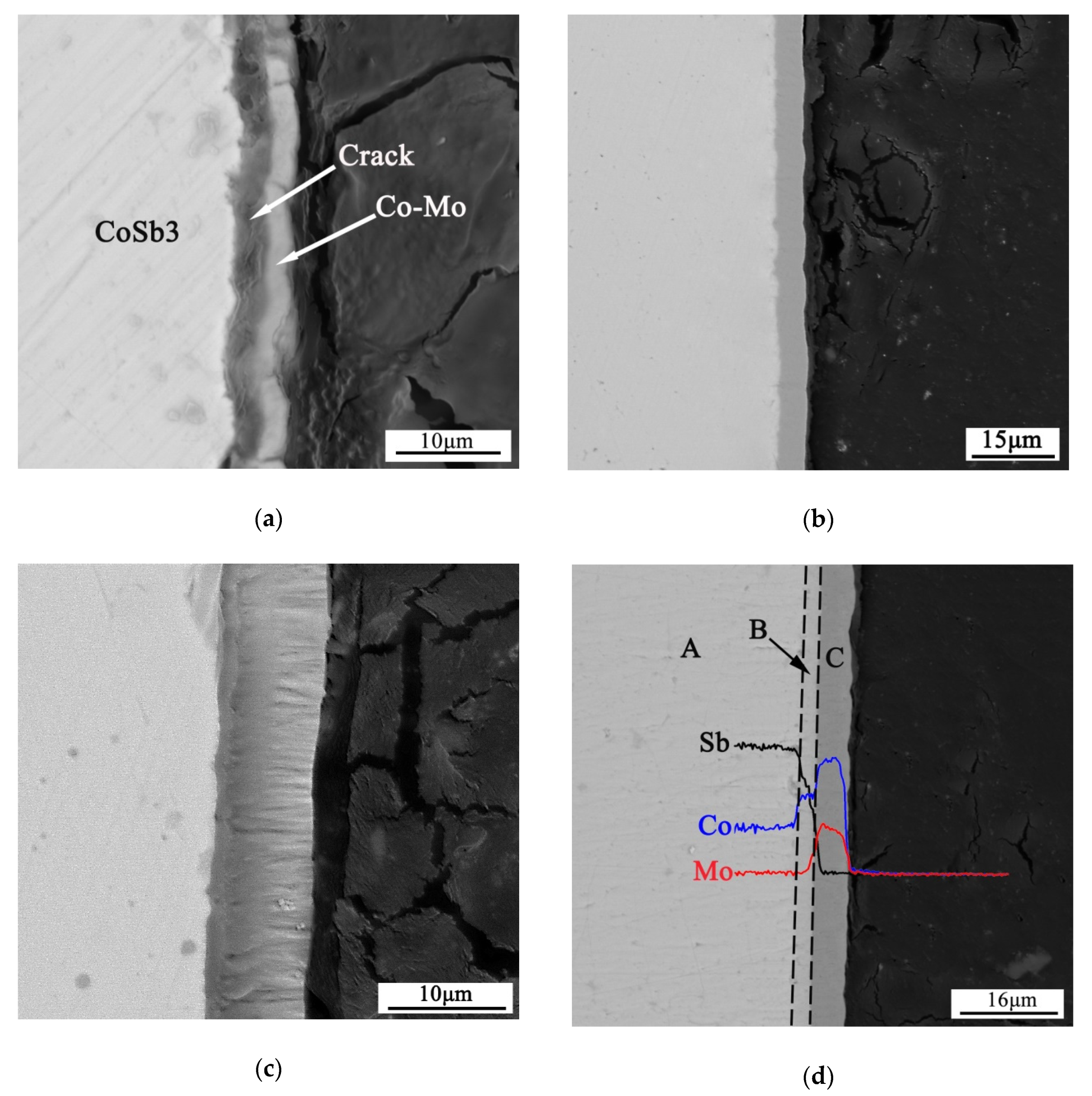


| Co | Sb | Mo | Possible Phase | |
|---|---|---|---|---|
| A | 26.10 | 73.87 | 0.03 | CoSb3 |
| B | 33.01 | 66.93 | 0.06 | CoSb2 |
| C | 74.90 | 0.25 | 24.85 | Co-Mo intermetallic phases |
| Thermoelectric Materials | Metallization Layer | Specific Contact Resistivity (μΩ·cm2) |
|---|---|---|
| Yb0.3Co4Sb12 [14] | Mo-Ti | 9 |
| Bi2Te3 [27] | Au | 2.73 |
| Mg2Si [28] | Ni | 210 |
| Hf0.5Zr0.5CoSn0.2Sb0.8 [29] | Ag | 38 |
| (Mm,Sm)yCo4Sb12 [26] | Fe-Ni | 2 |
| n-type skutterudite [30] | CoSi2 | 0.4 |
| This work | Co-Mo | 1.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Zhang, L.; Zhang, J.; Gou, W.; Zhong, S.; Zhang, G.; Geng, H.; Feng, J. Metallization and Diffusion Bonding of CoSb3-Based Thermoelectric Materials. Materials 2020, 13, 1130. https://doi.org/10.3390/ma13051130
Feng H, Zhang L, Zhang J, Gou W, Zhong S, Zhang G, Geng H, Feng J. Metallization and Diffusion Bonding of CoSb3-Based Thermoelectric Materials. Materials. 2020; 13(5):1130. https://doi.org/10.3390/ma13051130
Chicago/Turabian StyleFeng, Hangbin, Lixia Zhang, Jialun Zhang, Wenqin Gou, Sujuan Zhong, Guanxing Zhang, Huiyuan Geng, and Jicai Feng. 2020. "Metallization and Diffusion Bonding of CoSb3-Based Thermoelectric Materials" Materials 13, no. 5: 1130. https://doi.org/10.3390/ma13051130
APA StyleFeng, H., Zhang, L., Zhang, J., Gou, W., Zhong, S., Zhang, G., Geng, H., & Feng, J. (2020). Metallization and Diffusion Bonding of CoSb3-Based Thermoelectric Materials. Materials, 13(5), 1130. https://doi.org/10.3390/ma13051130




