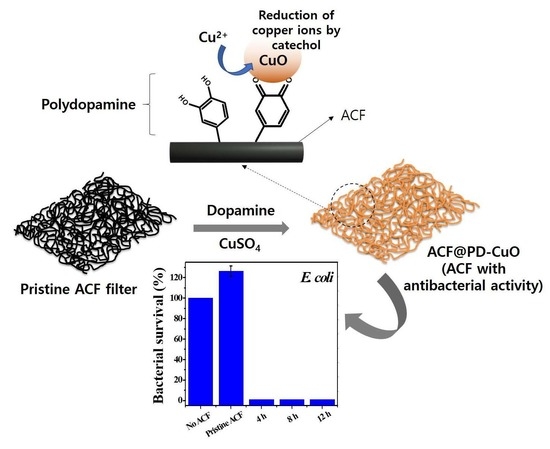
One-Pot Decoration of Cupric Oxide on Activated Carbon Fibers Mediated by Polydopamine for Bacterial Growth Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Characterization
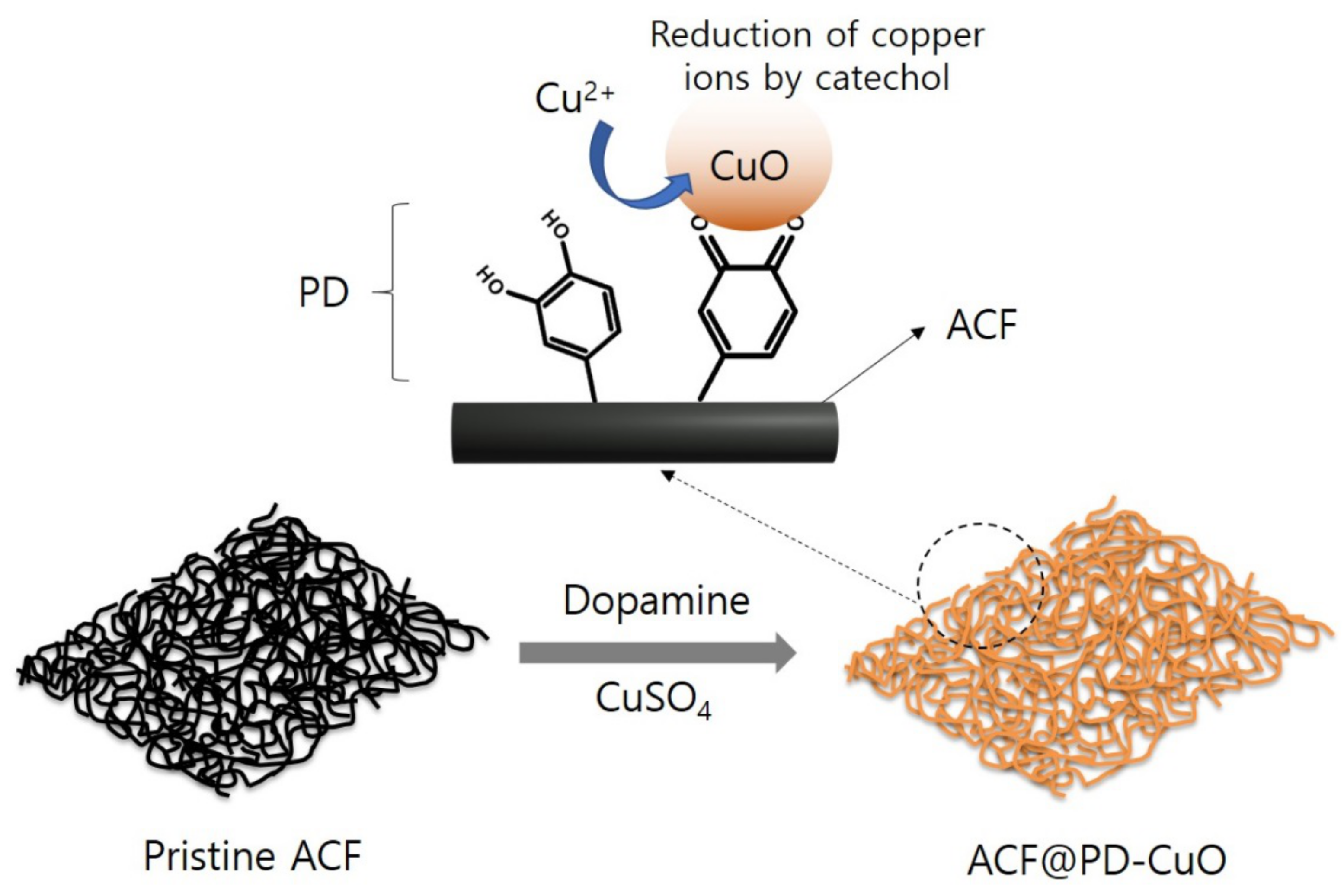
2.3. Preparation of activated carbon fiber (ACF) Coated with Polydopamine and Copper(II) Oxide (ACF@PD-CuO)
2.4. Antimicrobial Test
2.5. Pressure Drop Measurement
2.6. Mechanical Property Measurement
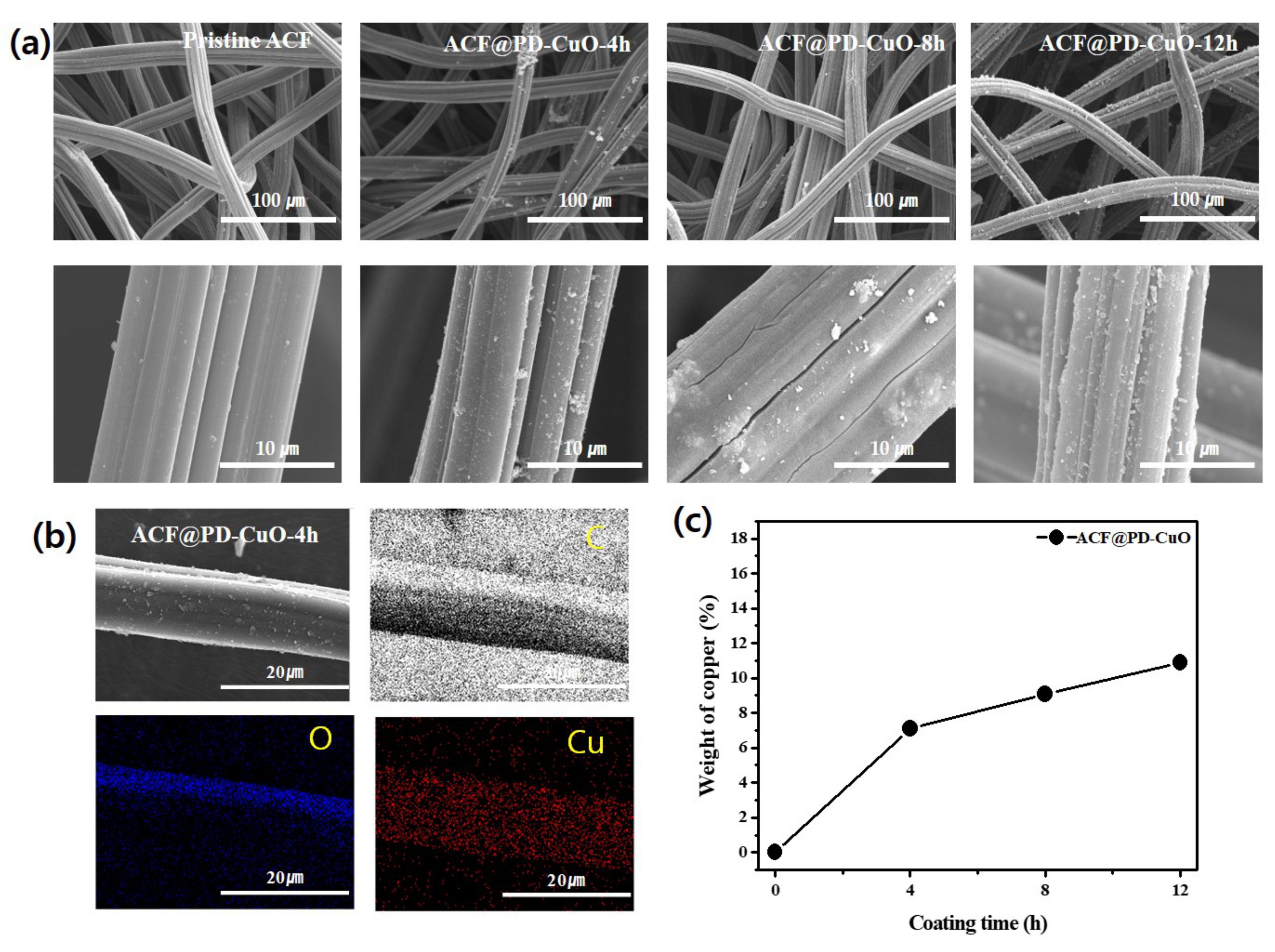
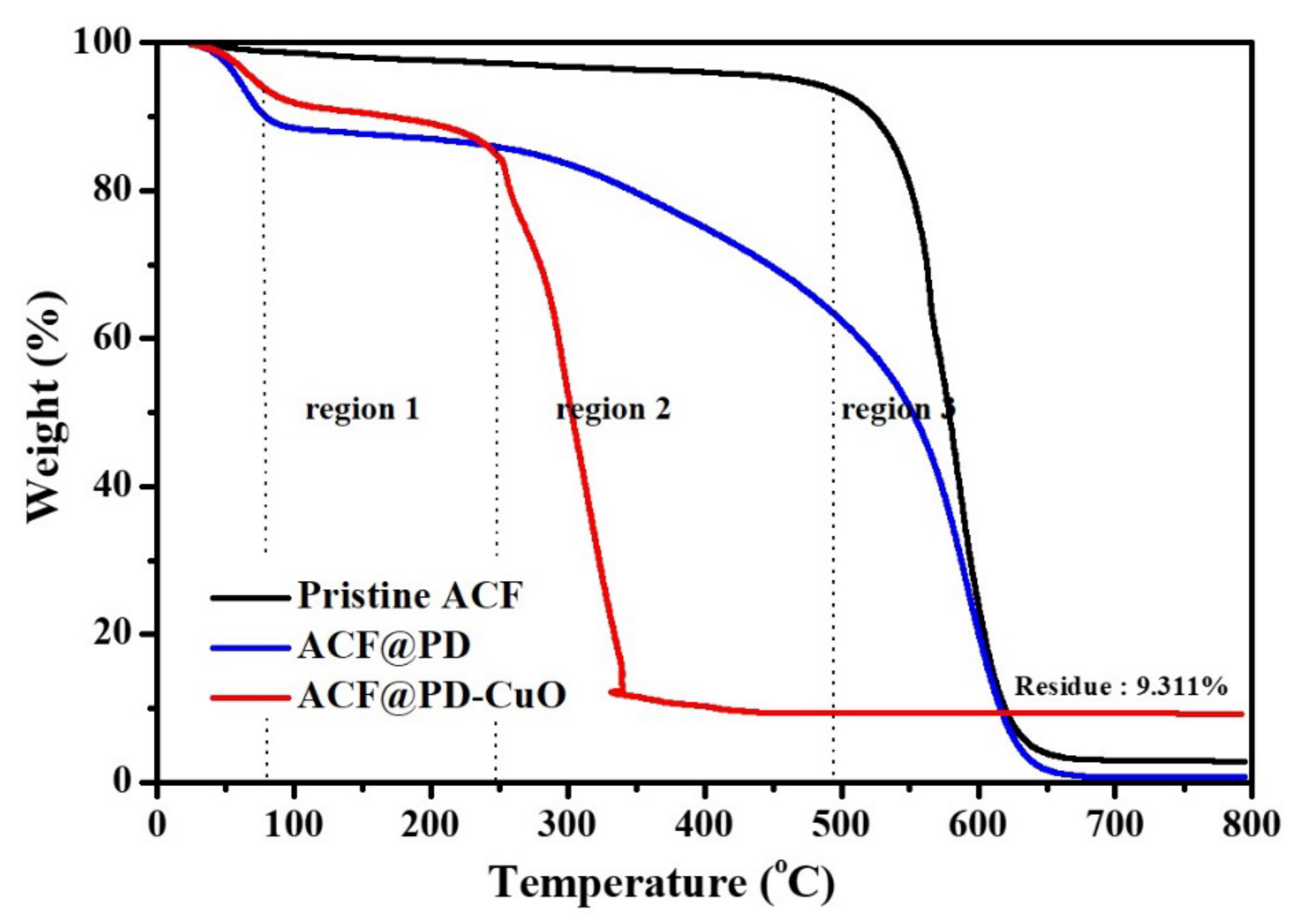
3. Results and Discussion
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Suzuki, M. Activated carbon fiber: Fundamentals and applications. Carbon 1994, 32, 577–586. [Google Scholar] [CrossRef]
- Ali, N.; Kamal, T.; Ul-Islam, M.; Khan, A.; Shah, S.J.; Zada, A. Chitosan-coated cotton cloth supported copper nanoparticles for toxic dye reduction. Int. J. Biol. Macromol. 2018, 111, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-C.; Chiang, P.-C.; Huang, C.-P. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Yoo, P.; Amano, Y.; Machida, M. Adsorption of nitrate onto nitrogen-doped activated carbon fibers prepared by chemical vapor deposition. Korean J. Chem. Eng. 2018, 35, 2468–2473. [Google Scholar] [CrossRef]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar]
- Li, C.Y.; Wan, Y.; Wang, J.; Wang, Y.; Jiang, X.; Han, L. Antibacterial pitch-based activated carbon fiber supporting silver. Carbon 1998, 36, 61–65. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Byeon, J.H.; Park, C.W.; Hwang, J. Antimicrobial effect of silver particles on bacterial contamination of activated carbon fibers. Environ. Sci. Technol. 2008, 42, 1251–1255. [Google Scholar] [CrossRef]
- Anita, S.; Ramachandran, T.; Rajendran, R.; Koushik, C.; Mahalakshmi, M. A study of the antimicrobial property of encapsulated copper oxide nanoparticles on cotton fabric. Text. Res. J. 2011, 81, 1081–1088. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.; Karuppiah, P.; Al-Dhabi, N.A. Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J. Nanomater. 2014, 2014, 17. [Google Scholar] [CrossRef]
- Ivask, A.; Juganson, K.; Bondarenko, O.; Mortimer, M.; Aruoja, V.; Kasemets, K.; Blinova, I.; Heinlaan, M.; Slaveykova, V.; Kahru, A. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: A comparative review. Nanotoxicology 2014, 8, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huo, Y. The comparison of electrochemical migration mechanism between electroless silver plating and silver electroplating. J. Mater. Sci. Mater. Electron. 2016, 27, 931–941. [Google Scholar] [CrossRef]
- Ohno, I. Electrochemistry of electroless plating. Mater. Sci. Eng. 1991, 146, 33–49. [Google Scholar] [CrossRef]
- Grujicic, D.; Pesic, B. Electrodeposition of copper: The nucleation mechanisms. Electrochim. Acta 2002, 47, 2901–2912. [Google Scholar] [CrossRef]
- Djokić, S.; Nikolić, N.; Živković, P.; Popov, K.; Djokić, N. Electrodeposition and electroless deposition of metallic powders: A comparison. ECS Trans. 2011, 33, 7–31. [Google Scholar]
- Lu, Z.; Xiao, J.; Wang, Y.; Meng, M. In situ synthesis of silver nanoparticles uniformly distributed on polydopamine-coated silk fibers for antibacterial application. J. Colloid Interface Sci. 2015, 452, 8–14. [Google Scholar] [CrossRef]
- Ponzio, F.; Barthès, J.; Bour, J.R.M.; Michel, M.; Bertani, P.; Hemmerlé, J.; d’Ischia, M.; Ball, V. Oxidant control of polydopamine surface chemistry in acids: A mechanism-based entry to superhydrophilic-superoleophobic coatings. Chem. Mater. 2016, 28, 4697–4705. [Google Scholar] [CrossRef]
- Mrówczyński, R.; Markiewicz, R.; Liebscher, J. Chemistry of polydopamine analogues. Polym. Int. 2016, 65, 1288–1299. [Google Scholar] [CrossRef]
- Son, H.Y.; Ryu, J.H.; Lee, H.; Nam, Y.S. Silver-Polydopamine Hybrid Coatings of Electrospun Poly (vinyl alcohol) Nanofibers. Macromol. Mater. Eng. 2013, 298, 547–554. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Liang, R.P.; Meng, X.Y.; Liu, C.M.; Qiu, J.D. PDMS microchip coated with polydopamine/gold nanoparticles hybrid for efficient electrophoresis separation of amino acids. Electrophoresis 2011, 32, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial application. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; van der Westen, R.; Postma, A.; Städler, B. Polydopamine—A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Trizio, A.D.; Srisuk, P.; Costa, R.R.; Fraga, A.G.; Modena, T.; Genta, I.; Dorati, R.; Pedtosa, J.; Conti, B.; Correlo, V.M.; et al. Natural based eumelanin nanoparticles functionalization and preliminary evaluation as carrier for gentamicin. React. Funct. Polym. 2017, 114, 38–48. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y.J.L. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly (dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef]
- Massardier, V.; Voron, L.; Esnouf, C.; Merlin, J. Identification of the nitrides formed during the annealing of a low-carbon low-aluminium steel. J. Mater. Sci. 2001, 36, 1363–1371. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Y.; Feng, J. Polydopamine as an efficient and robust platform to functionalize carbon fiber for high-performance polymer composites. ACS Appl. Mater. Interfaces 2013, 6, 349–356. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Y.; Wang, Y.; Zhang, L.; Wang, W. Preparation and characterization of dopamine-decorated hydrophilic carbon black. Appl. Surf. Sci. 2012, 258, 5387–5393. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, W.-J. Hierarchically mesoporous CuO/carbon nanofiber coaxial shell-core nanowires for lithium ion batteries. Sci. Rep. 2015, 5, 09754. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, Y.; Miao, Y.-E.; Tjiu, W.W.; Liu, T. Polydopamine-coated electrospun poly (vinyl alcohol)/poly (acrylic acid) membranes as efficient dye adsorbent with good recyclability. J. Hazard. Mater. 2015, 283, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Rim, N.G.; Kim, S.J.; Shin, Y.M.; Jun, I.; Lim, D.W.; Park, J.H.; Shin, H. Mussel-inspired surface modification of poly (L-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells. Colloids Surf. B Biointerfaces 2012, 91, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zong, X.; Zhou, X.; Li, C. Cu2O/CuO photocathode with improved stability for photoelectrochemical water reduction. RSC Adv. 2015, 5, 10790–10794. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Chen, J.-M.; Lin, H.-H.; Shih, H.-C. Field emission from various CuO nanostructures. Appl. Phys. Lett. 2003, 83, 3383–3385. [Google Scholar] [CrossRef]
- Tahir, D.; Tougaard, S. Electronic and optical properties of Cu, CuO and Cu2O studied by electron spectroscopy. J. Phys. Condens. Matter 2012, 24, 175002. [Google Scholar] [CrossRef]
- Viji, S.; Anbazhagi, M.; Ponpandian, N.; Mangalaraj, D.; Jeyanthi, S.; Santhanam, P.; Devi, A.S.; Viswanathan, C. Diatom-based label-free optical biosensor for biomolecules. Appl. Biochem. Biotechnol. 2014, 174, 1166–1173. [Google Scholar] [CrossRef]
- Li, S.Y.; Ma, W.; Zhou, Y.; Chen, X.; Ma, M.; Xu, Y.; Ding, Z.; Wu, X. 3-aminopropyltriethoxysilanes modified porous silicon as a voltammetric sensor for determination of silver ion. Int. J. Electrochem. Sci. 2013, 8, 1802–1812. [Google Scholar]
- Ren, S.; Tao, J.; Cui, Y.; Gao, J.; Li, X.; Tan, F. Preparation and characterization of hydrophilic polydopamine-coated Fe 3 O 4/oxide graphene imprinted nanocomposites for removal of bisphenol A in waters. Korean J. Chem. Eng. 2018, 35, 1836–1843. [Google Scholar] [CrossRef]
- Raul, P.K.; Senapati, S.; Sahoo, A.K.; Umlong, I.M.; Devi, R.R.; Thakur, A.J.; Veer, V. CuO nanorods: A potential and efficient adsorbent in water purification. RSC Adv. 2014, 4, 40580–40587. [Google Scholar] [CrossRef]
- Sundar, S.; Venkatachalam, G.; Kwon, S. Biosynthesis of Copper Oxide (CuO) Nanowires and Their Use for the Electrochemical Sensing of Dopamine. Nanomaterials 2018, 8, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papo, N.; Shai, Y. A molecular mechanism for lipopolysaccharide protection of Gram-negative bacteria from antimicrobial peptides. J. Biol. Chem. 2005, 280, 10378–10387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzilai, A.; Zilkha-Falb, R.; Daily, D.; Stern, N.; Offen, D.; Ziv, I.; Melamed, E.; Shirvan, A. The molecular mechanism of dopamine-induced apoptosis: Identification and characterization of genes that mediate dopamine toxicity. In Advances in Research on Neurodegeneration; Springer: Berlin/Heidelberg, Germany, 2000; pp. 59–76. [Google Scholar]
- Wang, C.; Li, J.; Sun, S.; Li, X.; Wu, G.; Wang, Y.; Xie, F.; Huang, Y. Controlled growth of silver nanoparticles on carbon fibers for reinforcement of both tensile and interfacial strength. RSC Adv. 2016, 6, 14016–14026. [Google Scholar] [CrossRef]


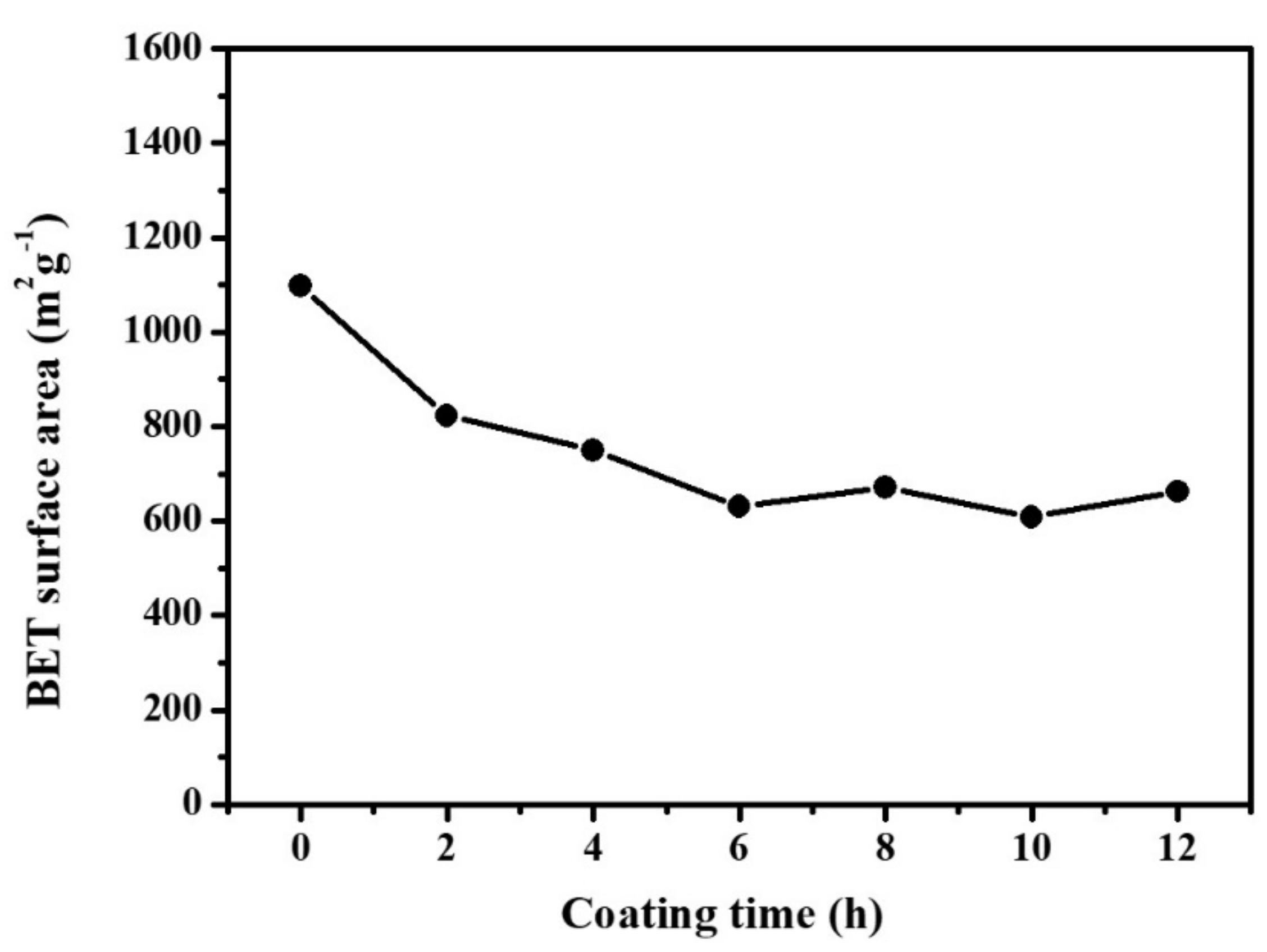


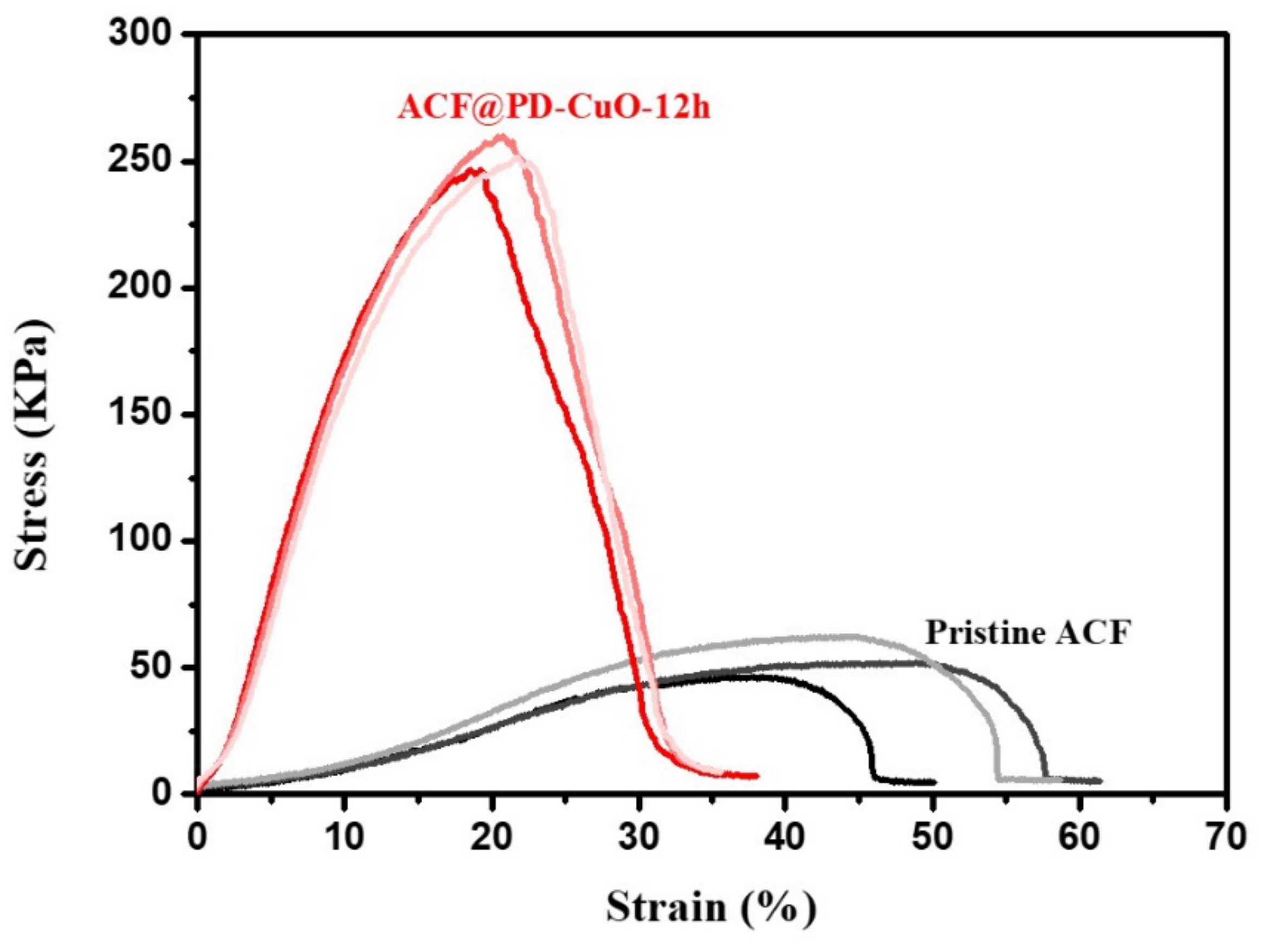
| Young’s Modulus (MPa) | Tensile Strength (kPa) | Elongation (%) | Tensile Strain(%) | |
|---|---|---|---|---|
| Pristine ACF | 0.21 ± 0.03 | 53.73 ± 7.97 | 43.20 ± 5.46 | 56.74 ± 5.93 |
| ACF@PD-CuO-12h | 2.09 ± 0.06 | 252.82 ± 6.61 | 20.63 ± 1.26 | 36.35 ± 1.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, H.; Lee, Y.-C.; Hur, J. One-Pot Decoration of Cupric Oxide on Activated Carbon Fibers Mediated by Polydopamine for Bacterial Growth Inhibition. Materials 2020, 13, 1158. https://doi.org/10.3390/ma13051158
Moon H, Lee Y-C, Hur J. One-Pot Decoration of Cupric Oxide on Activated Carbon Fibers Mediated by Polydopamine for Bacterial Growth Inhibition. Materials. 2020; 13(5):1158. https://doi.org/10.3390/ma13051158
Chicago/Turabian StyleMoon, Hangil, Young-Chul Lee, and Jaehyun Hur. 2020. "One-Pot Decoration of Cupric Oxide on Activated Carbon Fibers Mediated by Polydopamine for Bacterial Growth Inhibition" Materials 13, no. 5: 1158. https://doi.org/10.3390/ma13051158