The Distribution of Furfuryl Alcohol (FA) Resin in Bamboo Materials after Surface Furfurylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FA Solution
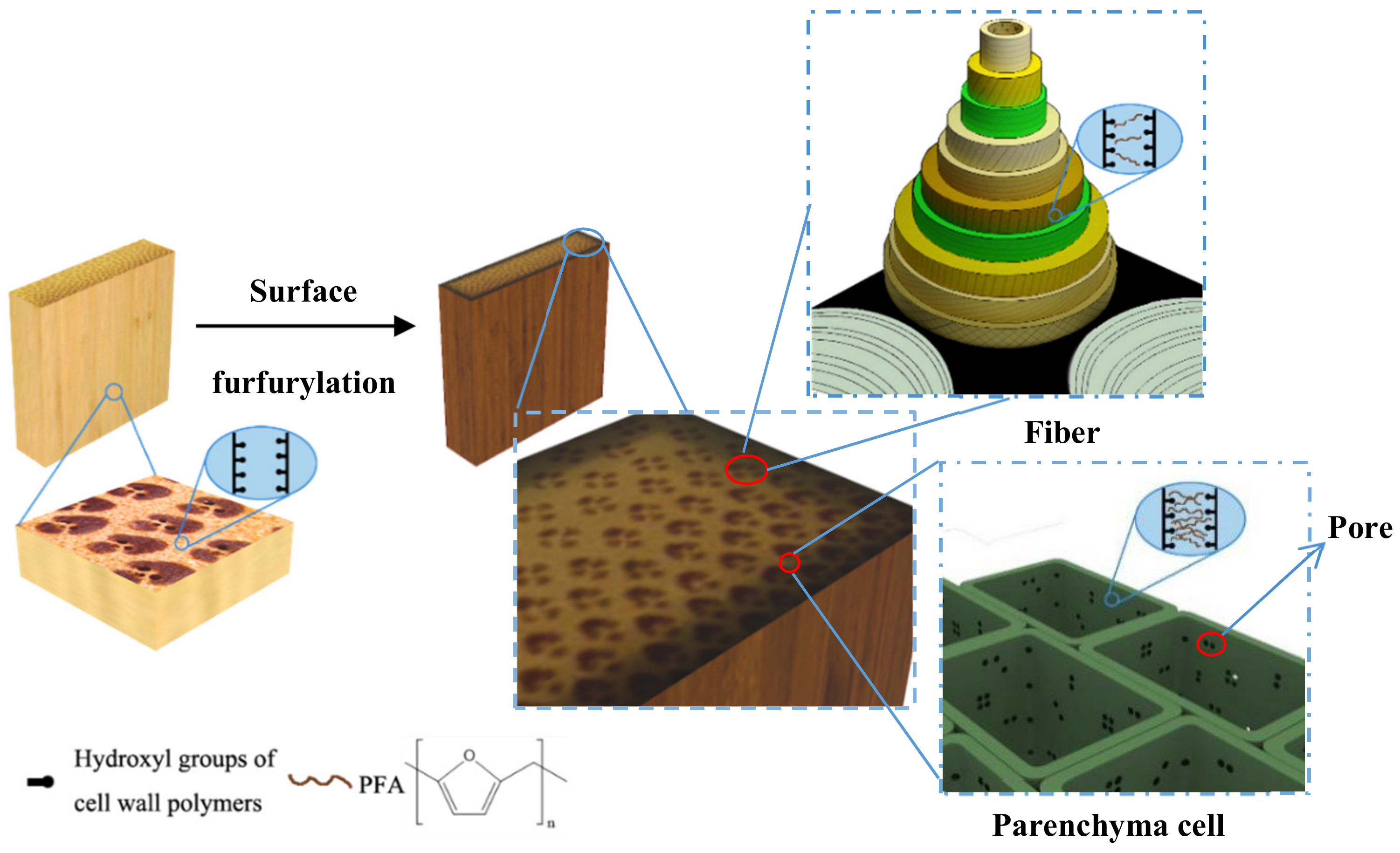
2.3. Furfurylation of Bamboo
2.4. Physical and Mechanical Properties
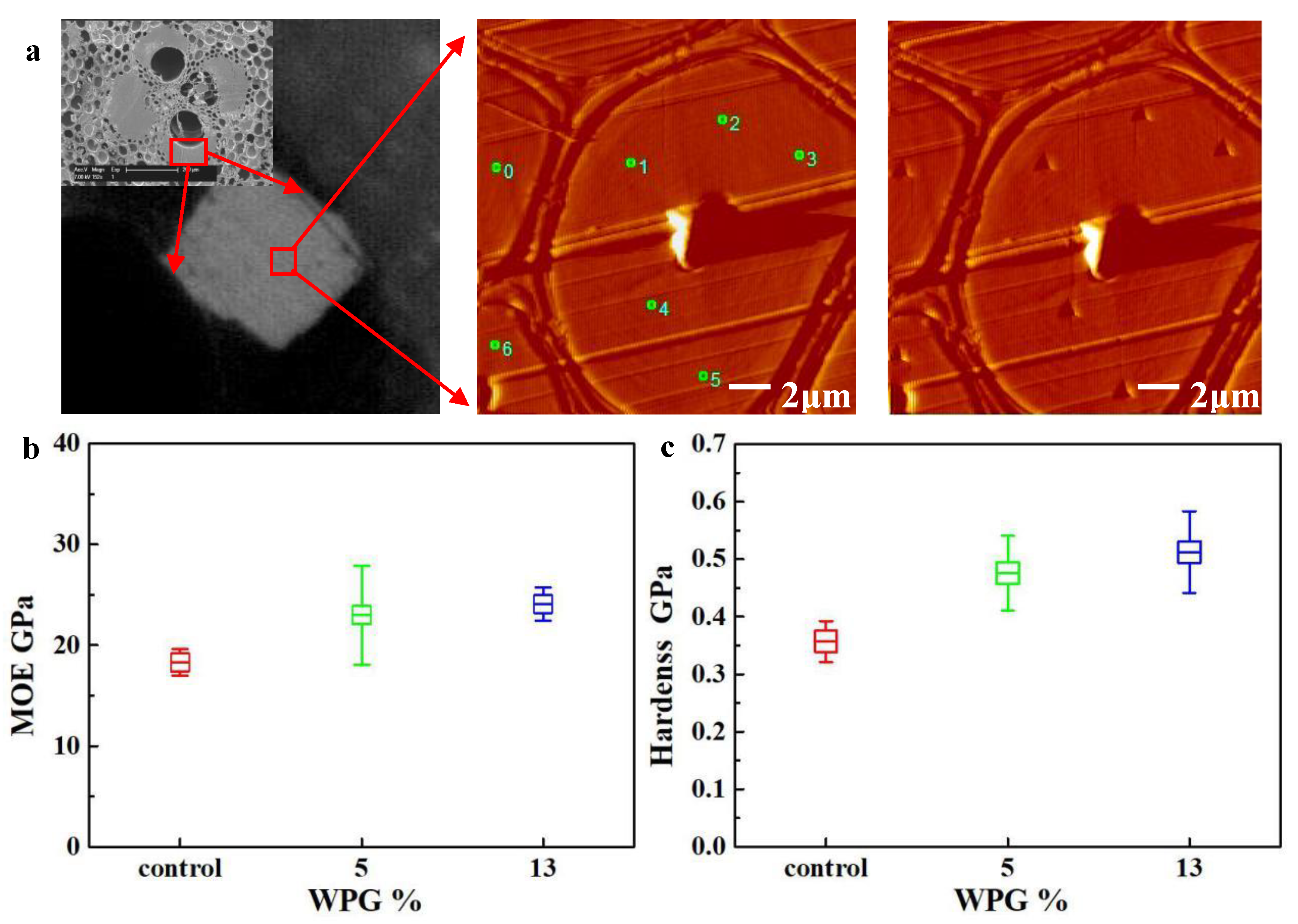
2.5. Nanoindentation
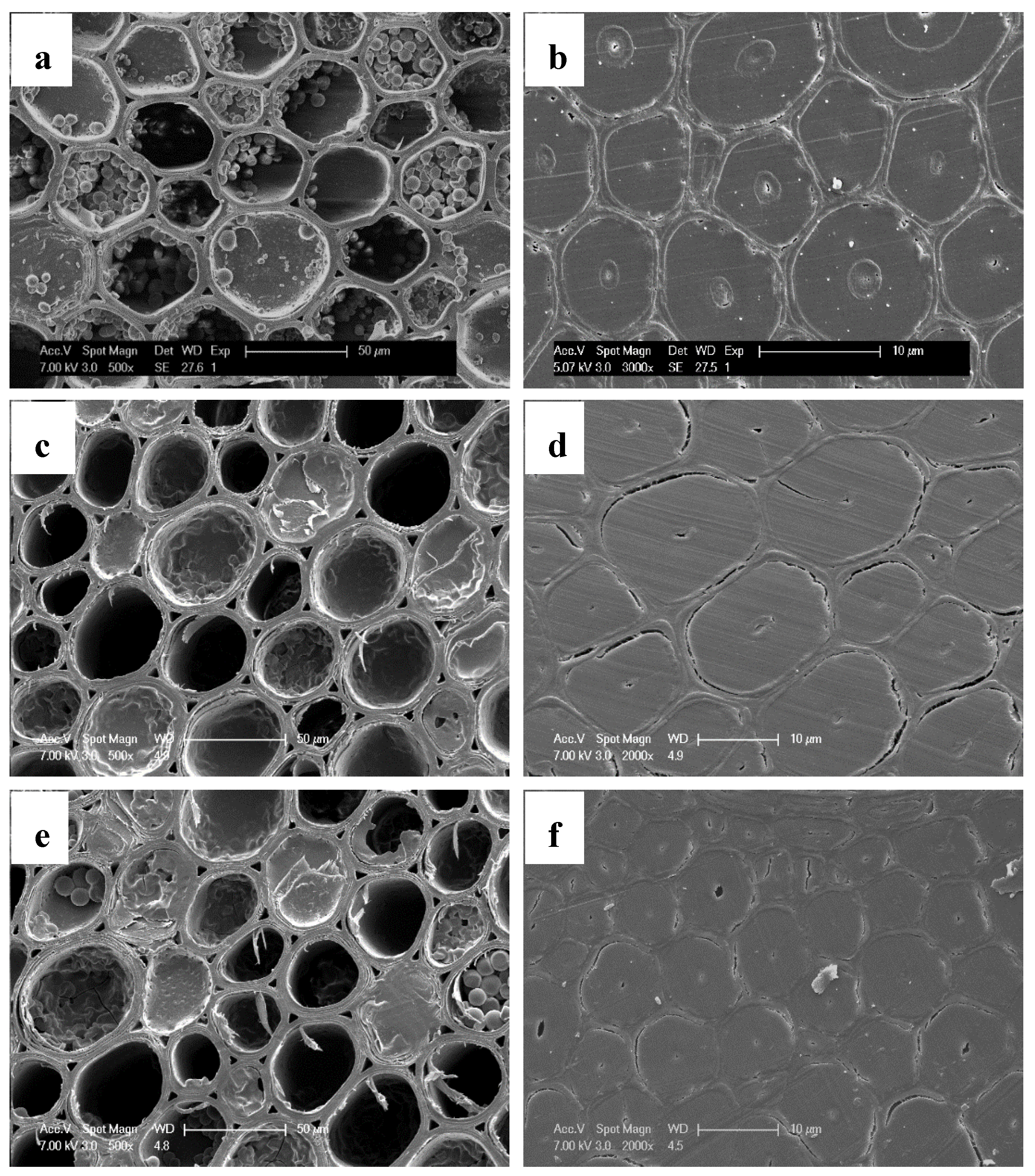
2.6. SEM Observation
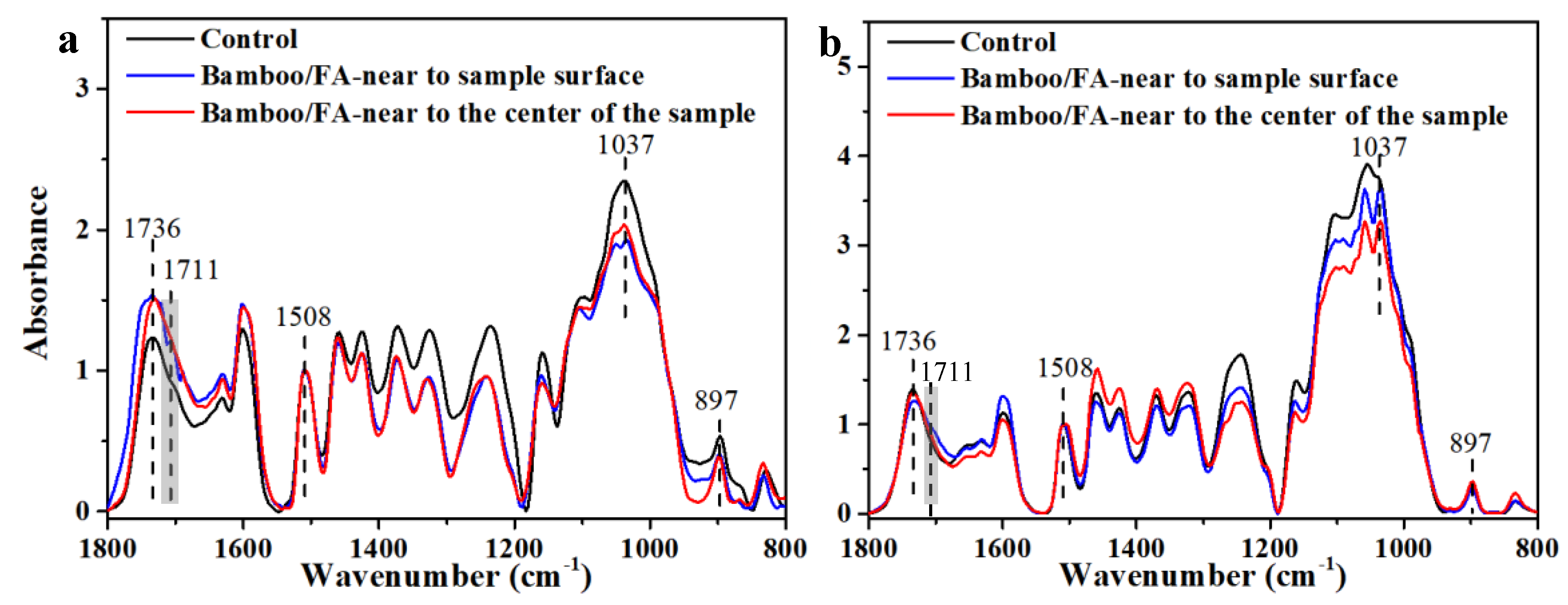
2.7. Imaging FTIR Microscopy
3. Results
3.1. Physical and Mechanical Properties
3.2. The Biological Durability of Surface Furfurylated Bamboo
3.3. The Distribution of FA Resin in Bamboo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scurlock, J.M.O.; Dayton, D.C.; Hames, B. Bamboo: An overlooked biomass resource? Biomass Bioenergy 2000, 19, 229–244. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Lu, Y.; Li, L.; Zhao, Q.; Feng, Q.; Gao, Z.; Lu, H.; Hu, T.; Yao, N.; Liu, K.; et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat. Genet. 2013, 45, 456. [Google Scholar] [CrossRef] [Green Version]
- Dixon, P.G.; Gibson, L.J. The structure and mechanics of moso bamboo material. J. R. Soc. Interface 2014, 11, 20140321. [Google Scholar] [CrossRef]
- Verma, C.S.; Sharma, N.K.; Chariar, V.M.; Maheshwari, S.; Hada, M.K. Comparative study of mechanical properties of bamboo laminae and their laminates with woods and wood based composites. Compos. Part B Eng. 2014, 60, 523–530. [Google Scholar] [CrossRef]
- Sharma, B.; Gatóo, A.; Ramage, M.H. Effect of processing methods on the mechanical properties of engineered bamboo. Constr. Build. Mater. 2015, 83, 95–101. [Google Scholar] [CrossRef]
- Song, J.; Surjadi, J.U.; Hu, D.; Lu, Y. Fatigue characterization of structural bamboo materials under flexural bending. Int. J. Fatigue 2017, 100, 126–135. [Google Scholar] [CrossRef]
- Yang, T.H.; Lee, C.H.; Lee, C.J.; Cheng, Y.W. Effects of different thermal modification media on physical and mechanical properties of moso bamboo. Constr. Build. Mater. 2016, 119, 251–259. [Google Scholar] [CrossRef]
- Ermeydan, M.A.; Cabane, E.; Hass, P.; Koetz, J.; Burgert, I. Fully biodegradable modification of wood for improvement of dimensional stability and water absorption properties by poly (ε-caprolactone) grafting into the cell walls. Green Chem. 2014, 16, 3313–3321. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A simple approach to prepare carboxycellulose nanofibers from untreated biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from spinifex as an effective adsorbent to remove cadmium (II) from water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Sun, Q.; Han, S.; Fan, B.; Yao, Q.; Yan, C.; Jin, C. Fabrication of superhydrophobic bamboo timber based on an anatase TiO2 film for acid rain protection and flame retardancy. RSC Adv. 2015, 5, 62265–62272. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Chen, X.; Mi, B.; Wei, P.; Fei, B.; Mu, X. Antimicrobial bamboo materials functionalized with ZnO and graphene oxide nanocomposites. Materials 2017, 10, 239. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, J.; Zhuang, X.; Pan, X.; Yu, H.; Sun, F.; Song, J.; Jin, C.; Jiang, Y. Improved mould resistance and antibacterial activity of bamboo coated with ZnO/graphene. R. Soc. Open Sci. 2018, 5, 180173. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Li, J.; Xu, J.; Wu, Z.; Bao, Y.; Li, N.; Chen, Y. Efficient Antifungal and Flame-Retardant Properties of ZnO-TiO2-Layered Double-Nanostructures Coated on Bamboo Substrate. Coatings 2018, 8, 341. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Fu, Q.; Wang, Q.; Xiao, Z.; Militz, H. Effects of chemical modification on the mechanical properties of wood. Eur. J. Wood Wood Prod. 2013, 71, 401–416. [Google Scholar] [CrossRef]
- Yao, M.; Yang, Y.; Song, J.; Yu, Y.; Jin, Y. Lignin-based catalysts for chinese fir furfurylation to improve dimensional stability and mechanical properties. Industr. Crops Prod. 2017, 107, 38–44. [Google Scholar] [CrossRef]
- Pfriem, A.; Dietrich, T.; Buchelt, B. Furfuryl alcohol impregnation for improved plasticization and fixation during the densification of wood. Holzforschung 2012, 66, 215–218. [Google Scholar] [CrossRef]
- Sejati, P.S.; Imbert, A.; Gérardin-Charbonnier, C.; Dumarçay, S.; Fredon, E.; Masson, E.; Nandika, D.; Priadi, T.; Gérardin, P. Tartaric acid catalyzed furfurylation of beech wood. Wood Sci. Technol. 2018, 51, 379–394. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wang, H.; Ren, D.; Yu, Y.S.; Yu, Y. Wood modification with furfuryl alcohol catalysed by a new composite acidic catalyst. Wood Sci. Technol. 2015, 49, 845–856. [Google Scholar] [CrossRef]
- Li, W.; Ren, D.; Zhang, X.; Wang, H.; Yu, Y. The furfurylation of wood: A nanomechanical study of modified wood cells. BioResources 2016, 11, 3614–3625. [Google Scholar] [CrossRef]
- Iménez-Gómez, C.P.; Cecilia, J.A.; Durán-Martín, D.; Moreno-Tost, R.; Santamaría-González, J.; Mérida-Robles, J.; Mariscal, R.; Maireles-Torres, P. Gas-phase hydrogenation of furfural to furfuryl alcohol over Cu/ZnO catalysts. J. Catal. 2016, 336, 107–115. [Google Scholar] [CrossRef]
- Monien, B.H.; Herrmann, K.; Florian, S.; Glatt, H. Metabolic activation of furfuryl alcohol: Formation of 2-methylfuranyl DNA adducts in Salmonella typhimurium strains expressing human sulfotransferase 1A1 and in FVB/N mice. Carcinogenesis 2011, 32, 1533–1539. [Google Scholar] [CrossRef] [Green Version]
- Lande, S.; Westin, M.; Schneider, M. Properties of furfurylated wood. Scand. J. For. Res. 2004, 19, 22–30. [Google Scholar] [CrossRef]
- Kim, T.; Assary, R.S.; Pauls, R.E.; Marshall, C.L.; Curtiss, L.A.; Stair, P.C. Thermodynamics and reaction pathways of furfuryl alcohol oligomer formation. Catal. Commun. 2014, 46, 66–70. [Google Scholar] [CrossRef]
- Lande, S.; Eikenes, M.; Westin, M. Chemistry and ecotoxicology of furfurylated wood. Scand. J. For. Res. 2004, 19, 14–21. [Google Scholar] [CrossRef]
- Keplinger, T.; Cabane, E.; Chanana, M.; Hass, P.; Merk, V.; Gierlinger, N.; Burgert, I. A versatile strategy for grafting polymers to wood cell walls. Acta Biomater. 2015, 11, 256–263. [Google Scholar] [CrossRef]
- Tang, T.; Chen, X.; Zhang, B.; Liu, X.; Fei, B. Research on the physico-mechanical properties of moso bamboo with thermal treatment in tung oil and its influencing factors. Materials 2019, 12, 599. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Deng, Y.; Li, Y.; Kjoller, K.; Roy, A.; Wang, S. In situ identification of the molecular-scale interactions of phenol-formaldehyde resin and wood cell walls using infrared nanospectroscopy. RSC Adv. 2016, 6, 76318–76324. [Google Scholar] [CrossRef]
- Guo, J.; Song, K.; Salmén, L.; Yin, Y. Changes of wood cell walls in response to hygro-mechanical steam treatment. Carbohydr. Polym. 2015, 115, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Cao, J.; Ma, E. How does delignification influence the furfurylation of wood? Ind. Crops Prod. 2019, 135, 91–98. [Google Scholar] [CrossRef]
- Kong, L.; Guan, H.; Wang, X. In situ polymerization of furfuryl alcohol with ammonium dihydrogen phosphate in poplar wood for improved dimensional stability and flame retardancy. ACS Sustain. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Gindl, W.; Gupta, H.S.; Schöberl, T.; Lichtenegger, H.C.; Fratzl, P. Mechanical properties of spruce wood cell walls by nanoindentation. Appl. Phys. A 2004, 79, 2069–2073. [Google Scholar]
- Sassu, M.; De Falco, A.; Giresini, L.; Puppio, M.L. Structural solutions for low-cost bamboo frames: Experimental tests and constructive assessments. Materials 2016, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Olmstead, J.; Gray, D. Fluorescence spectroscopy of cellulose, lignin and mechanical pulps: A review. J. Pulp Pap. Sci. 1997, 23, 571–581. [Google Scholar]
- Hadi, Y.S.; Westin, M.; Rasyid, E. Resistance of furfurylated wood to termite attack. For. Prod. J. 2005, 5. [Google Scholar]
- Bryne, L.E.; Lausmaa, J.; Ernstsson, M.; Englund, F.; Wålinder, M.E. Ageing of modified wood. Part 2: Determination of surface composition of acetylated, furfurylated, and thermally modified wood by XPS and ToF-SIMS. Holzforschung 2010, 64, 305–313. [Google Scholar] [CrossRef]
- Pranger, L.; Tannenbaum, R. Biobased nanocomposites prepared by in situ polymerization of furfuryl alcohol with cellulose whiskers or montmorillonite clay. Macromolecules 2008, 41, 8682–8687. [Google Scholar] [CrossRef]
- Ahmad, E.E.M.; Luyt, A.S.; Djoković, V. Thermal and dynamic mechanical properties of bio-based poly (furfuryl alcohol)/sisal whiskers nanocomposites. Polym. Bull. 2013, 70, 1265–1276. [Google Scholar] [CrossRef]
- Sun, R.C.; Fang, J.M.; Tomkinson, J.; Geng, Z.C.; Liu, J.C. Fractional isolation, physico-chemical characterization and homogeneous esterification of hemicelluloses from fast-growing poplar wood. Carbohydr. Polym. 2001, 44, 29–39. [Google Scholar] [CrossRef]
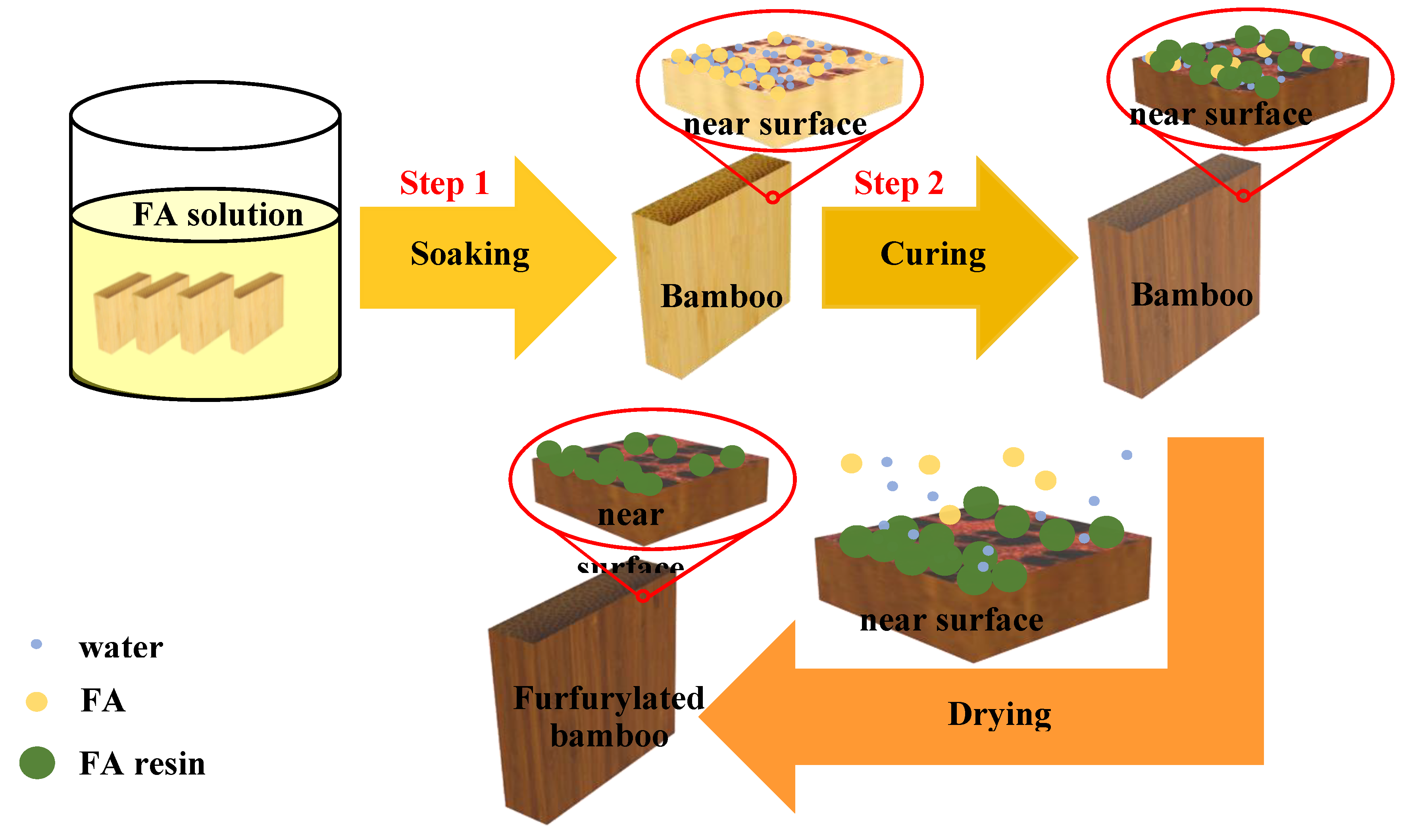

| Items | Size (T × R × L) | Numbers in Each Group |
|---|---|---|
| WPG | 20 mm × 5 mm × 20 mm | 16 |
| EMC and CS | 20 mm × 5 mm × 20 mm | 15 |
| MOR and MOE | 10 mm × 5 mm × 160 mm | 11 |
| Mould test | 20 mm × 5 mm × 50 mm | 24 |
| Decay test | 20 mm × 5 mm × 20 mm | 16 |
| Termites test | 20 mm × 5 mm × 50 mm | 5 |
| Samples | WPG/% | EMC/% | V.ASE/% | MOR/MPa | MOE/GPa | CS/MPa |
|---|---|---|---|---|---|---|
| Furfurylated bamboo | 13.3 ± 2.38 | 6.60 ± 0.69 | 51.1 ± 10.4 | 118.1 ± 20.4 | 8.91 ± 1.48 | 52.08 ± 8.58 |
| Control | - | 9.61 ± 0.28 | - | 109.6 ± 12.6 | 8.72 ± 1.24 | 51.64 ± 7.66 |
| Samples | Infection Value | Resist Effectiveness % | |||
|---|---|---|---|---|---|
| A. niger | P. citrinum | T. viride | B. theobromae | ||
| furfurylated bamboo | 0 * | 0 | 0 | 0 | 100 |
| control | 4 * | 4 | 4 | 4 | 0 |
| Samples | Weight Loss Ratio % | ||
|---|---|---|---|
| Coriolus versicolor | Gloeophyllum trabeum | Formosan subterranean termites | |
| furfurylated bamboo | 7.85 ± 1.00 | 8.48 ± 1.29 | 0.82 ± 0.12 |
| control | 60.48 ± 11.00 | 54.36 ± 12.73 | 12.80 ± 2.77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Li, W.; Wang, H.; Zhang, X.; Yu, Y. The Distribution of Furfuryl Alcohol (FA) Resin in Bamboo Materials after Surface Furfurylation. Materials 2020, 13, 1157. https://doi.org/10.3390/ma13051157
Liu M, Li W, Wang H, Zhang X, Yu Y. The Distribution of Furfuryl Alcohol (FA) Resin in Bamboo Materials after Surface Furfurylation. Materials. 2020; 13(5):1157. https://doi.org/10.3390/ma13051157
Chicago/Turabian StyleLiu, Minghui, Wanju Li, Hankun Wang, Xuexia Zhang, and Yan Yu. 2020. "The Distribution of Furfuryl Alcohol (FA) Resin in Bamboo Materials after Surface Furfurylation" Materials 13, no. 5: 1157. https://doi.org/10.3390/ma13051157
APA StyleLiu, M., Li, W., Wang, H., Zhang, X., & Yu, Y. (2020). The Distribution of Furfuryl Alcohol (FA) Resin in Bamboo Materials after Surface Furfurylation. Materials, 13(5), 1157. https://doi.org/10.3390/ma13051157





