Mechanical Properties of Solder-Jointed Copper Rods with Electrodeposited Sn-Zn Alloy Films
Abstract
:1. Introduction
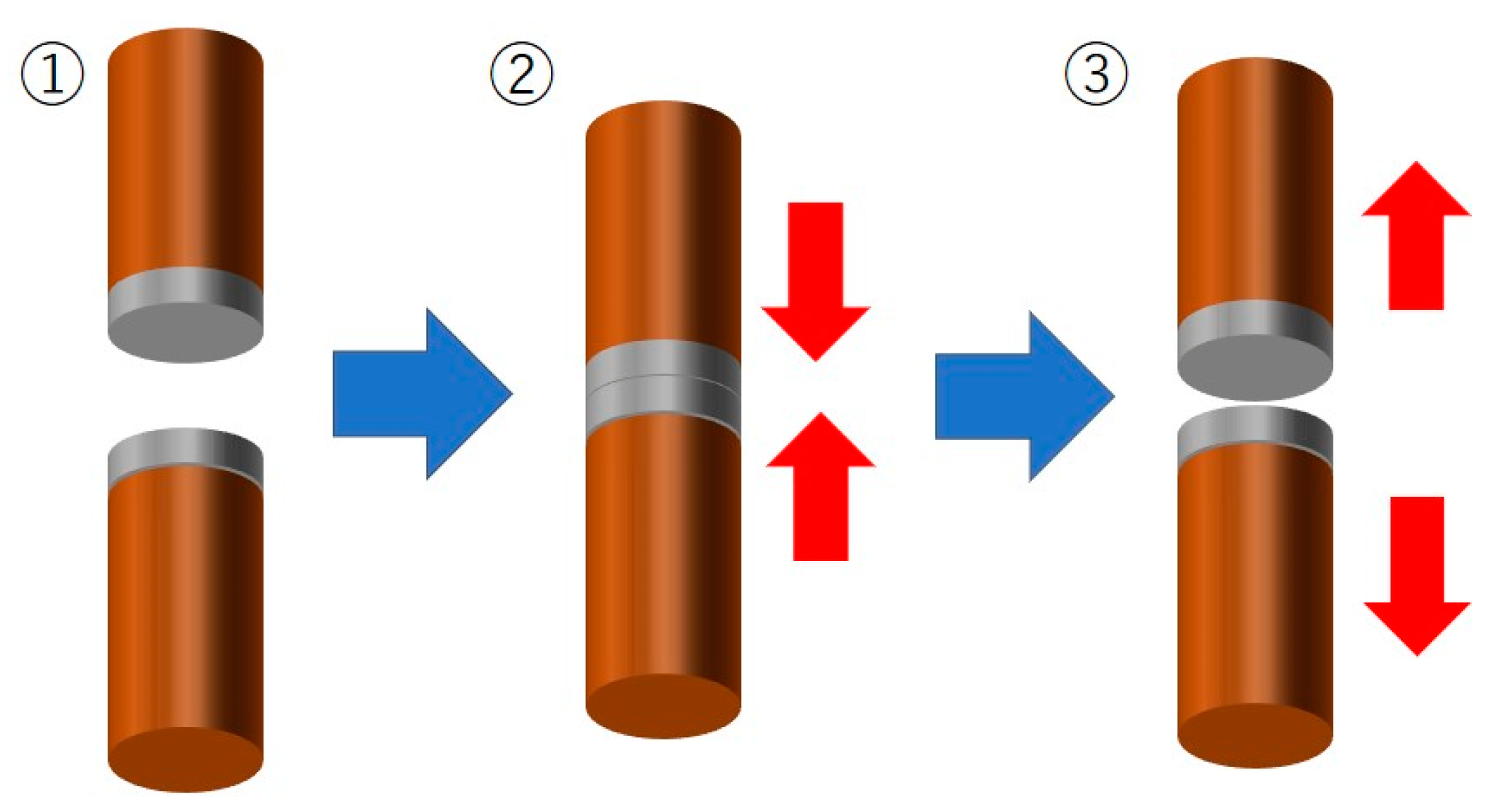
2. Materials and Methods
3. Results and Discussion
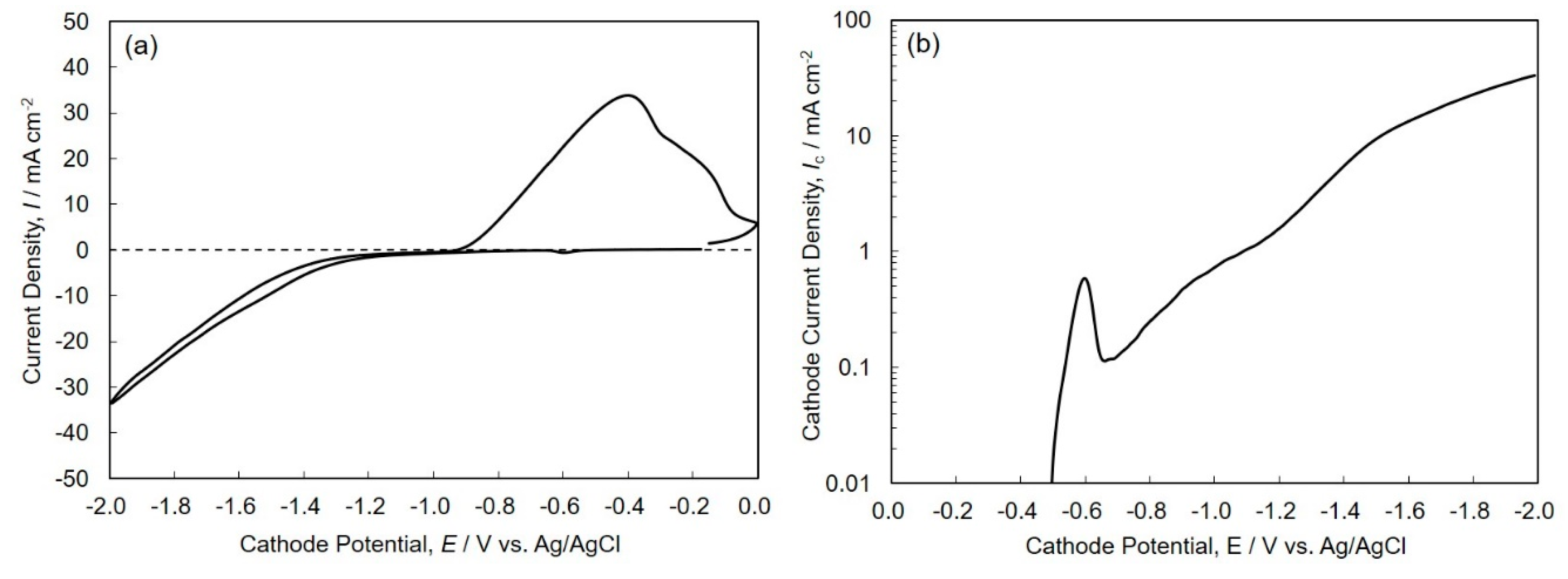
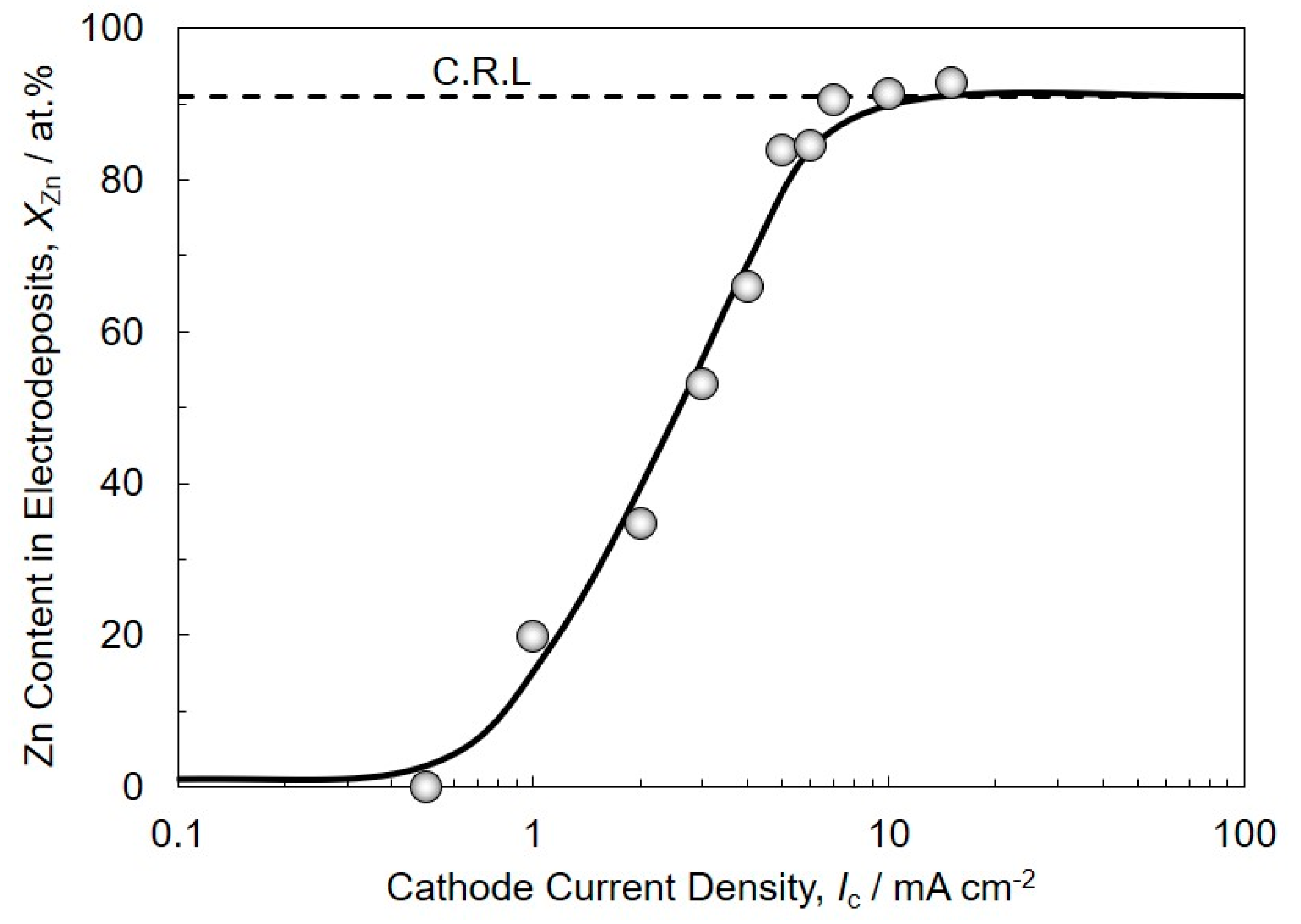
3.1. Electrodeposition Process of Sn-Zn Alloy Films
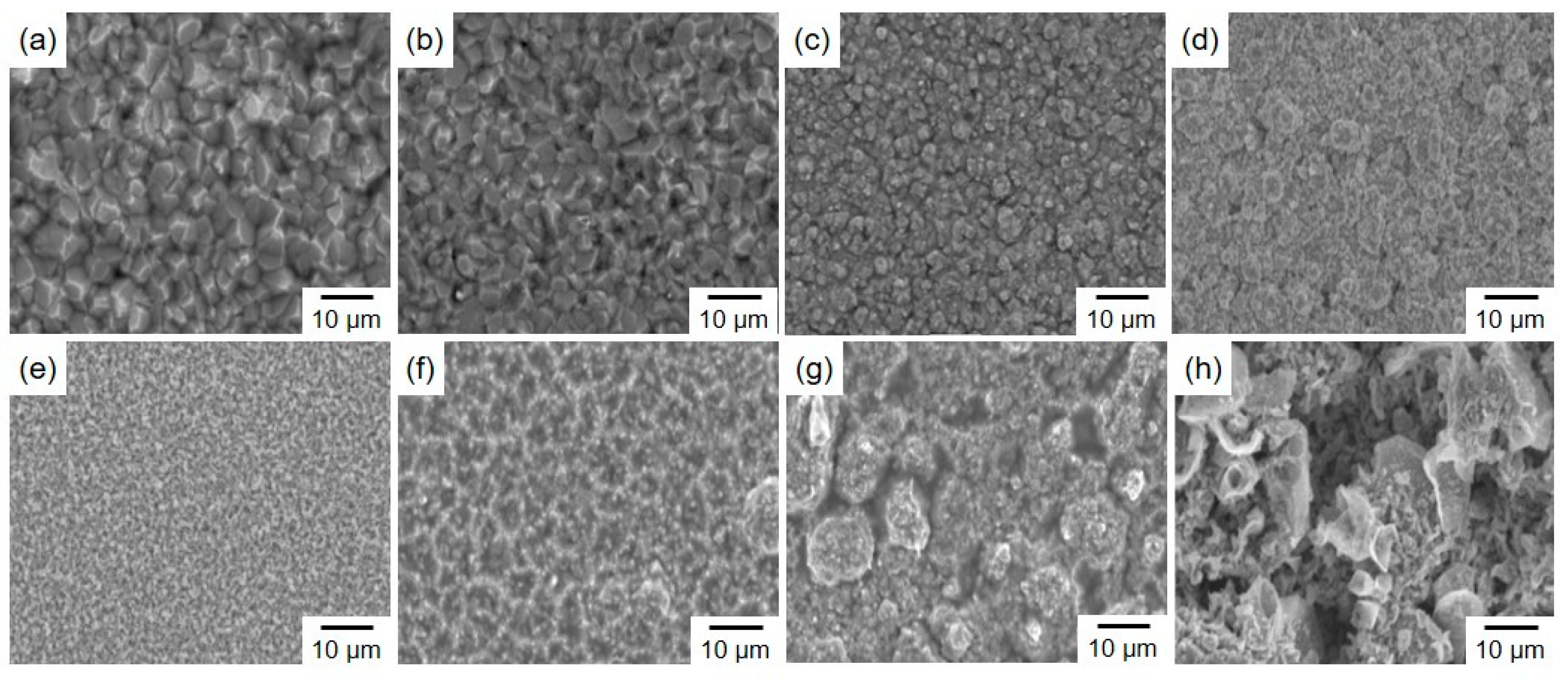
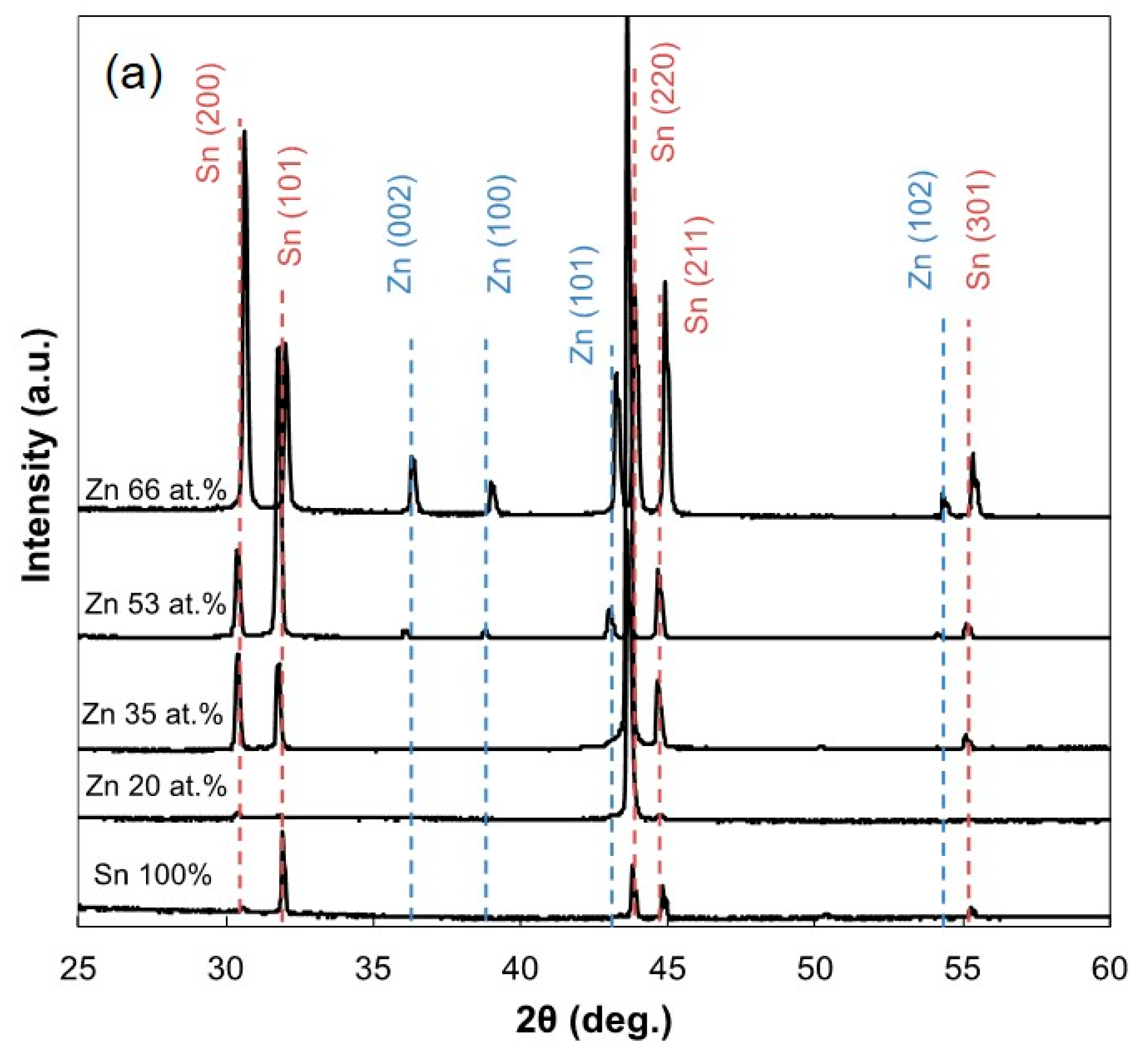
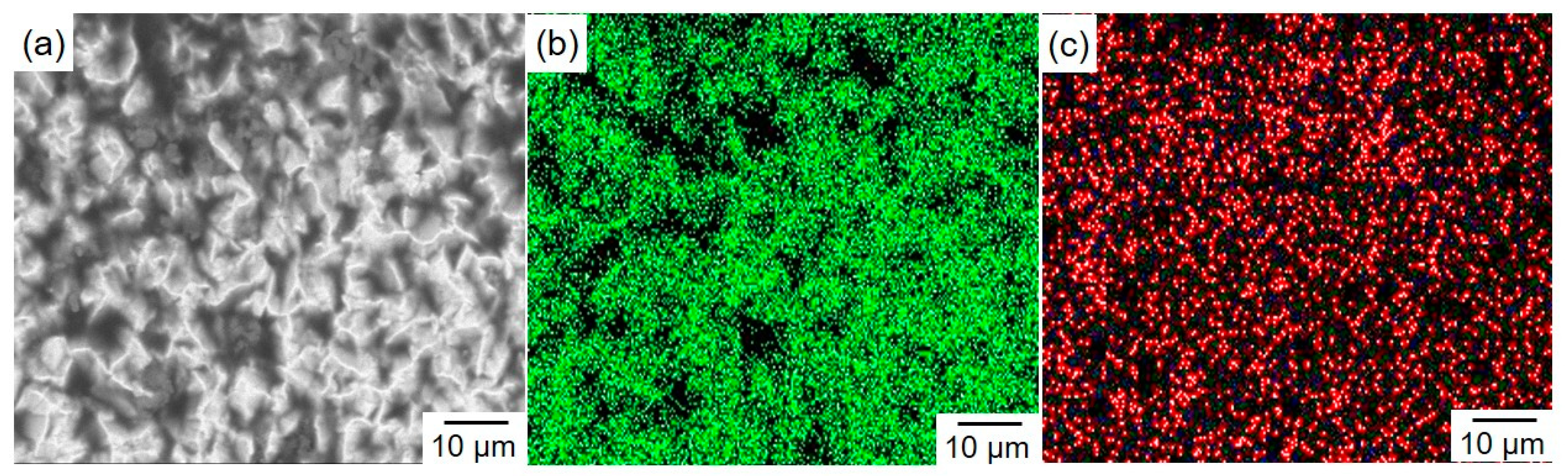
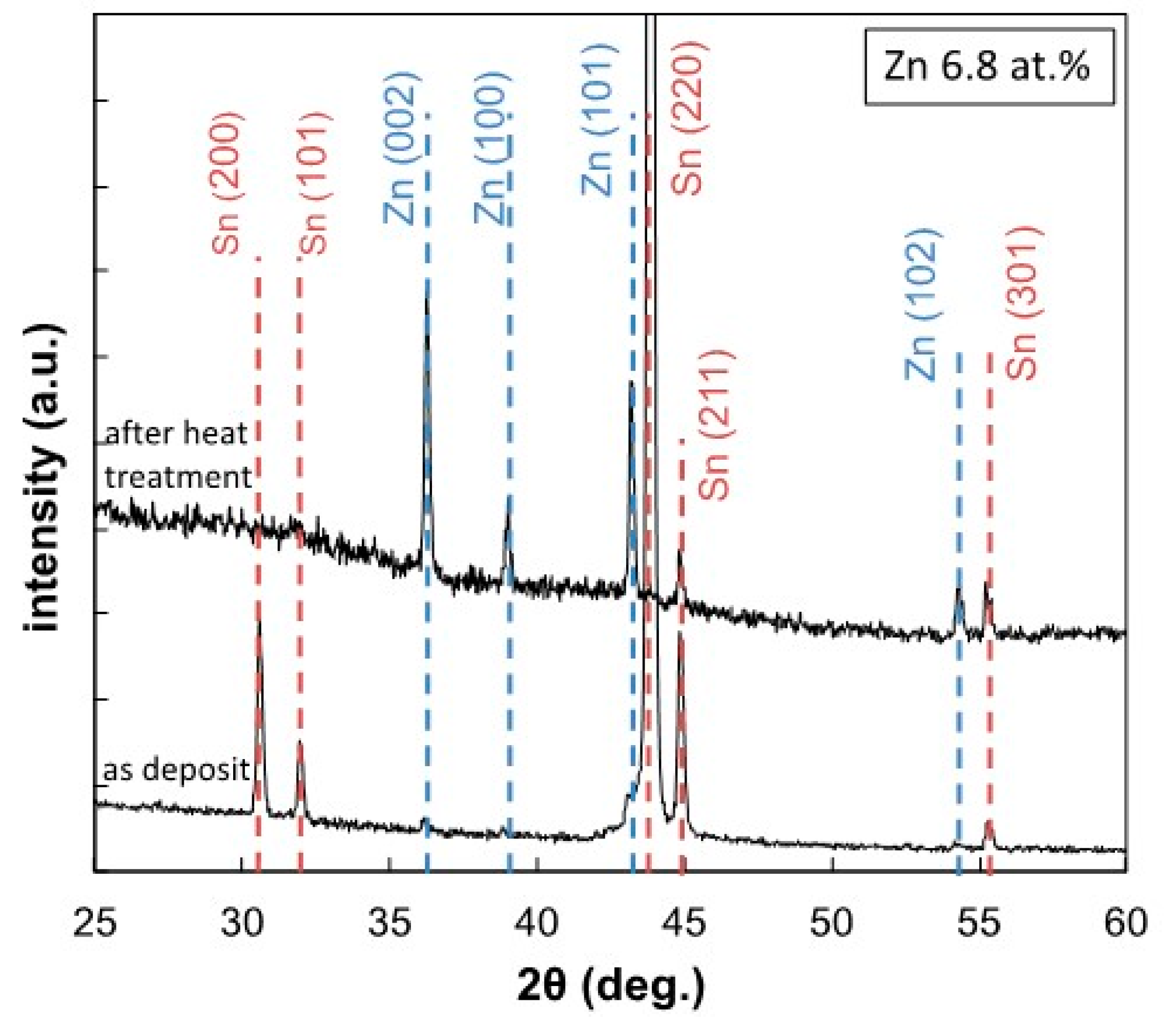
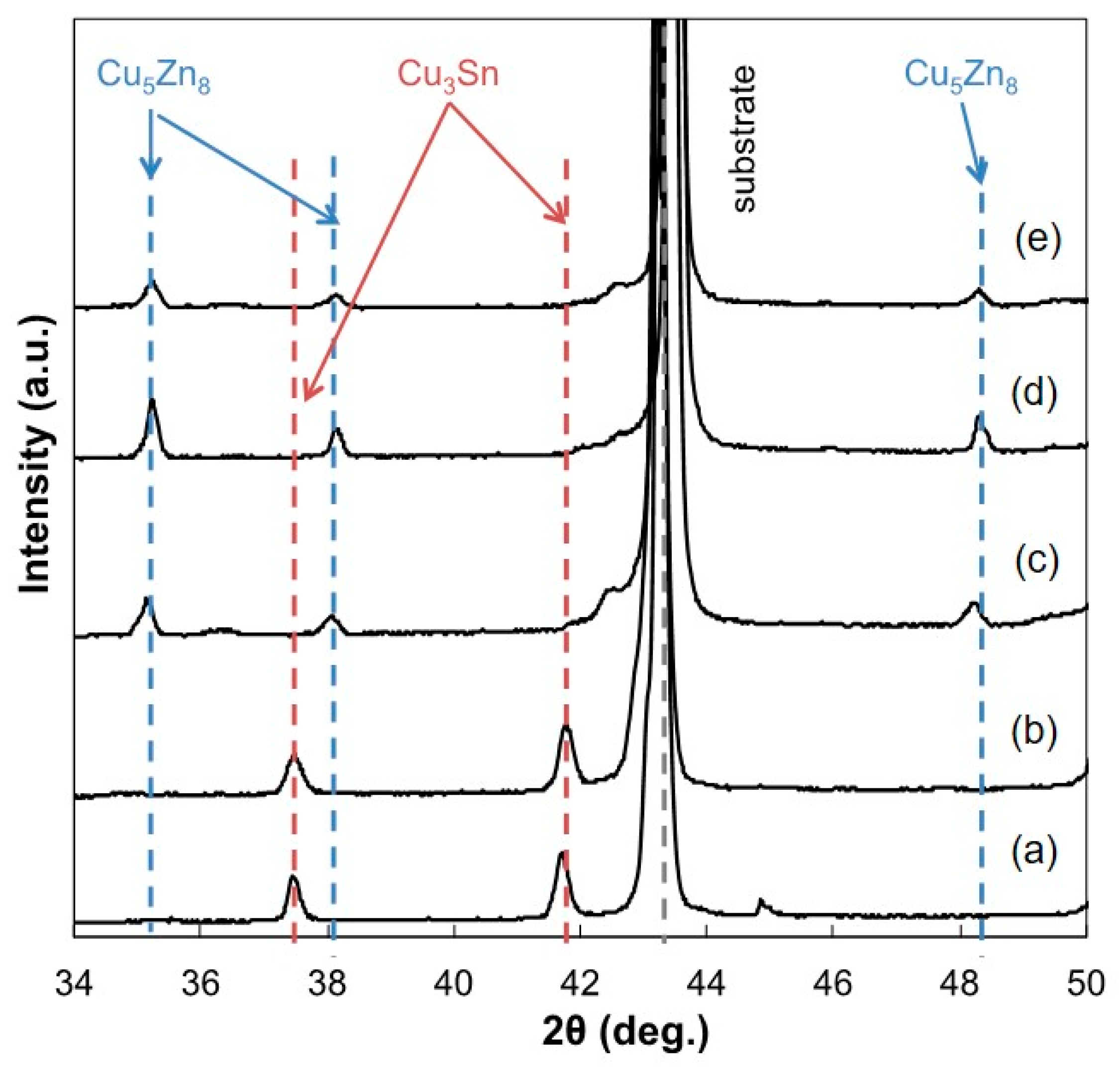
3.2. Surface Morphology and Constituent Phases of Electrodeposited Sn-Zn Alloy Films
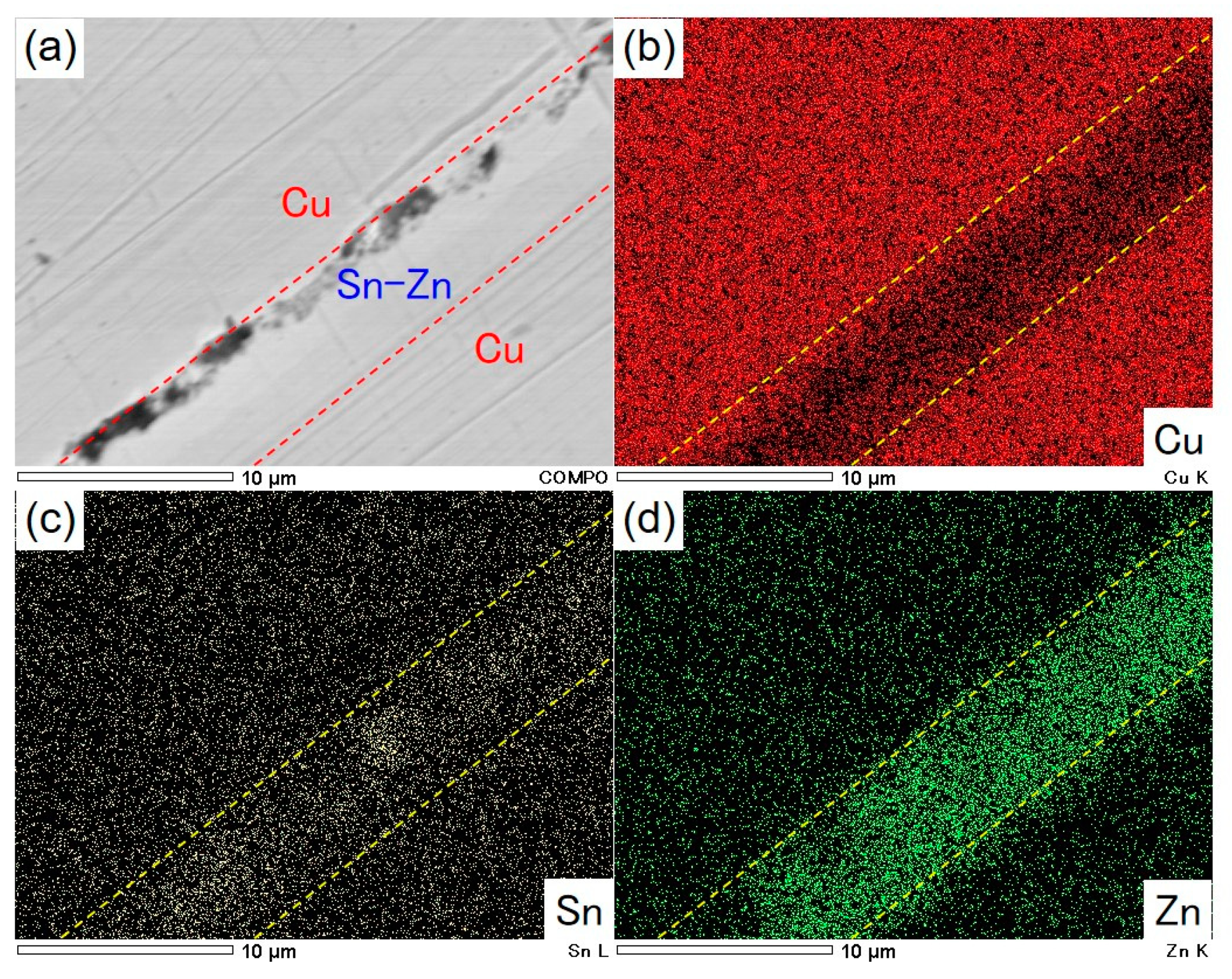
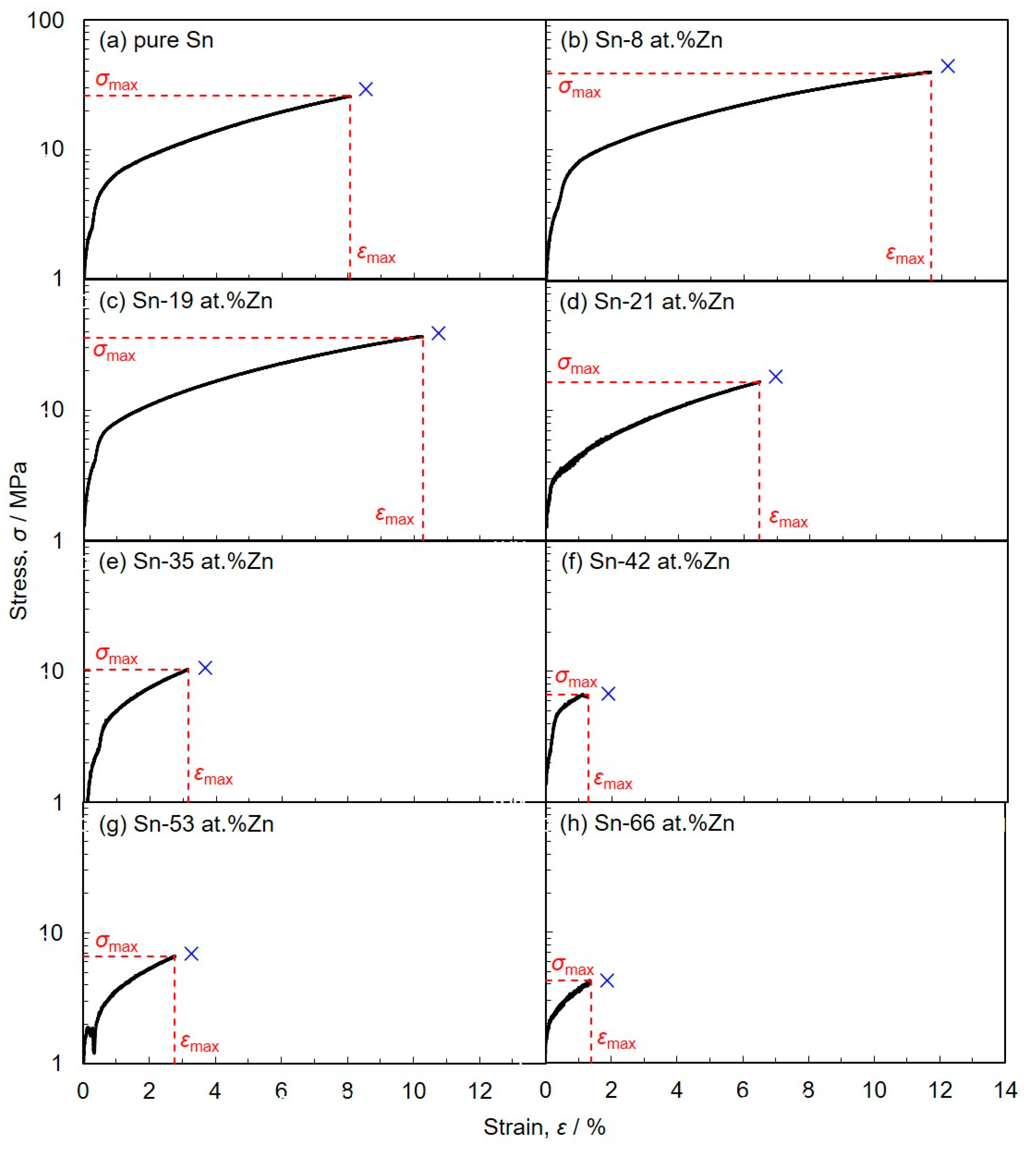
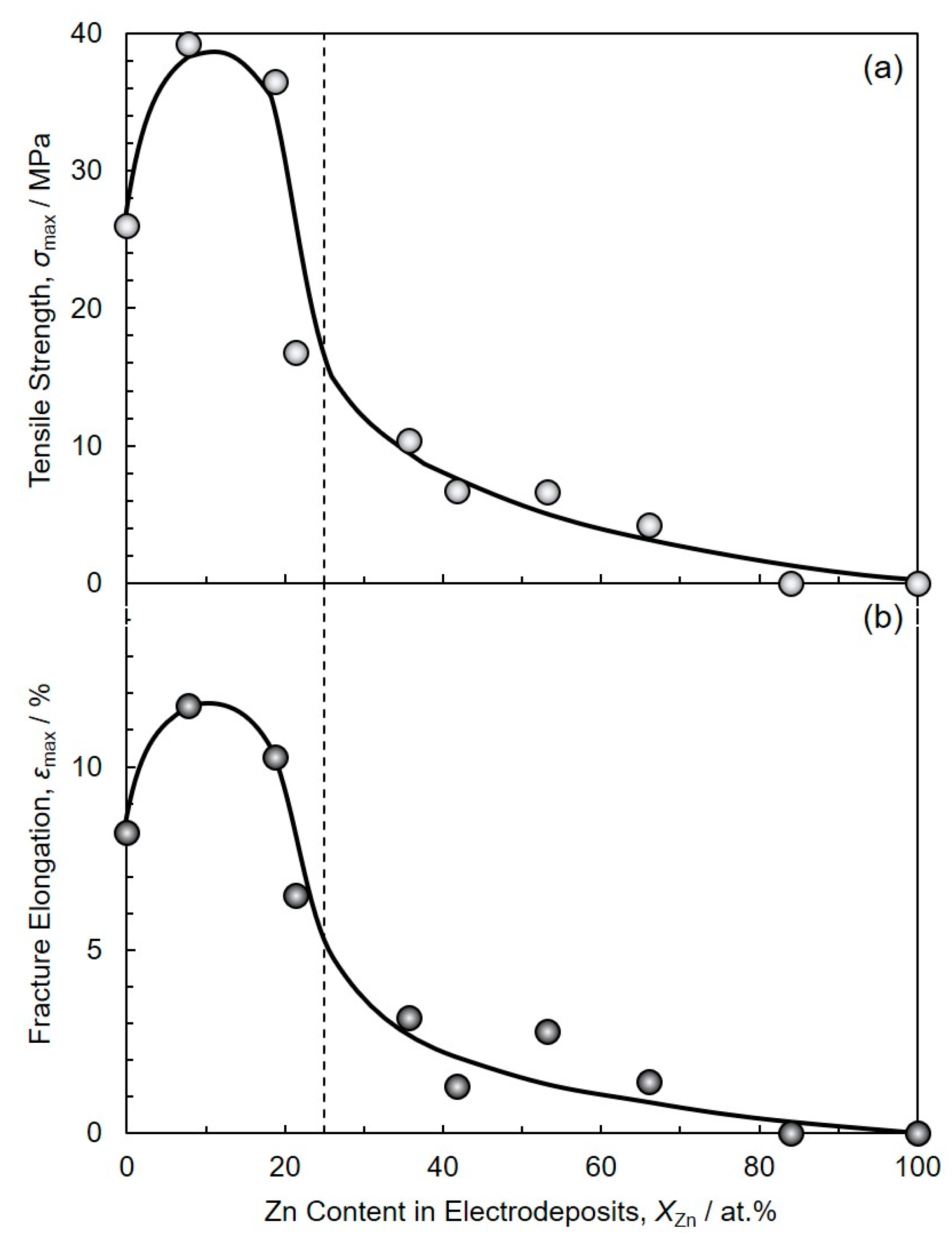
3.3. Soldering Performance of Electrodeposited Sn-Zn Alloy Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goh, Y.; Haseeb, A.S.M.A.; Sabri, M.F.M. Effects of hydroquinone and gelatin on the electrodeposition of Sn-Bi low temperature Pb-free solder. Electrochim. Acta 2013, 90, 265–273. [Google Scholar] [CrossRef]
- Suh, M.S.; Park, C.J.; Kwon, H.S. Effects of plating parameters on alloy composition and microstructure of Sn–Bi electrodeposits from methane sulphonate bath. Surf. Coat. Technol. 2006, 200, 3527–3532. [Google Scholar] [CrossRef]
- Qin, Y.; Wilcox, G.D.; Liu, C. Electrodeposition and characterisation of Sn-Ag-Cu solder alloys for flip-chip interconnection. Electrochim. Acta 2010, 56, 183–192. [Google Scholar] [CrossRef]
- Joseph, S.; Phatak, G.J. Effect of surfactant on the bath stability and electrodeposition of Sn-Ag-Cu films. Surf. Coat. Technol. 2008, 202, 3023–3028. [Google Scholar] [CrossRef]
- Bengoa, L.N.; Pary, P.; Conconi, M.S.; Egli, W.A. Electrodeposition of Cu-Sn alloys from a methanesulfonic acid electrolyte containing benzyl alcohol. Electrochim. Acta 2017, 256, 211–219. [Google Scholar] [CrossRef]
- Correia, A.N.; Façanha, M.X.; Lima-Neto, P.D. Cu-Sn coatings obtained from pyrophosphate-based electrolytes. Surf. Coat. Technol. 2007, 201, 7216–7221. [Google Scholar] [CrossRef]
- Han, C.; Liu, Q.; Ivey, D.G. Nucleation of Sn and Sn-Cu alloys on Pt during electrodeposition from Sn-citrate and Sn-Cu-citrate solutions. Electrochim. Acta 2009, 54, 3419–3427. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, L.; Huang, H.; Xiao, H. Viscosity transition of ZnO-containing rosin. Mater. Lett. 2005, 59, 1889–1892. [Google Scholar] [CrossRef]
- Wei, X.; Huang, H.; Zhou, L.; Zhang, M.; Liu, X. On the advantages of using a hypoeutectic Sn–Zn as lead-free solder material. Mater. Lett. 2007, 61, 655–658. [Google Scholar] [CrossRef]
- Hamada, N.; Hamada, M.; Uesugi, T.; Takigawa, Y.; Higashi, K. Effect of small addition of zinc on creep behavior of tin. Mater. Trans. 2010, 51, 1747–1752. [Google Scholar] [CrossRef] [Green Version]
- Nawafune, H.; Nakatani, T.; Akamatsu, K.; Uchida, E.; Obata, K. Electrodeposition of Tin-Zinc Eutectics Alloy and Codeposition Mechanism of Zinc for Low Temperature Lead-Free Soldering. J. Jpn. Inst. Electron. Pack. 2003, 6, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Moser, Z.; Dutkiewicz, J.; Gasior, W.; Salawa, J. The Sn-Zn (Tin-Zinc) system. J. Phase Equilib. 1985, 6, 330–334. [Google Scholar] [CrossRef]
- Motoyama, Y.; Ozasa, T.; Okane, T. Accurate Evaluation of Copper Alloy Fluidity Using Automatic Pouring Equipment with Improved Pouring Cup Heat Insulation. Mater. Trans. 2017, 58, 629–634. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; McKenzie, K.J.; Ryder, K.S. Electrodeposition of zinc-tin alloys from deep eutectic solvents based on choline chloride. J. Electroanal. Chem. 2007, 599, 288–294. [Google Scholar] [CrossRef]
- Guaus, E.; Torrent-Burgués, J. Tin-zinc electrodeposition from sulphate-tartrate baths. J. Electroanal. Chem. 2005, 575, 301–309. [Google Scholar] [CrossRef]
- Budman, E.; Stevens, D. Tin-zinc plating. Anti-Corro. Meth. Mater. 1998, 45, 327–332. [Google Scholar] [CrossRef]
- Kazimierczak, H.; Ozga, P. Electrodeposition of Sn-Zn and Sn-Zn-Mo layers from citrate solutions. Surf. Sci. 2013, 607, 33–38. [Google Scholar] [CrossRef]
- Salhi, Y.; Cherrouf, S.; Cherkaoui, M.; Abdelouahdi, K. Electrodeposition of nanostructured Sn-Zn coatings. Appl. Surf. Sci. 2016, 367, 64–69. [Google Scholar] [CrossRef]
- Kazimierczak, H.; Ozga, P.; Jałowiec, A.; Kowalik, R. Tin–zinc alloy electrodeposition from aqueous citrate baths. Surf. Coat. Technol. 2014, 240, 311–319. [Google Scholar] [CrossRef]
- Ivanova, V.; Raichevsky, G. Electrodeposition of low tin content zinc-tin alloys. Surf. Coat. Technol. 1996, 82, 226–231. [Google Scholar]
- Guaus, E.; Torrent-Burgués, J. Tin-zinc electrodeposition from sulphate-gluconate baths. J. Electroanal. Chem. 2003, 549, 25–36. [Google Scholar] [CrossRef]
- Hu, C.C.; Wang, C.K.; Lee, G.L. Composition control of tin-zinc deposits using experimental strategies. Electrochim. Acta 2006, 51, 3692–3698. [Google Scholar] [CrossRef]
- Dubent, S.; De Petris-Wery, M.; Saurat, M.; Ayedi, H.F. Composition control of tin–zinc electrodeposits through means of experimental strategies. Mater. Chem. Phy. 2007, 104, 146–152. [Google Scholar] [CrossRef]
- Ohgai, T.; Tanaka, Y.; Fujimaru, T. Soft magnetic properties of Ni-Cr and Co-Cr alloy thin films electrodeposited from aqueous solutions containing trivalent chromium ions and glycine. J. Appl. Electrochem. 2012, 42, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Saeki, R.; Ohgai, T. Determination of Crystal Growth Geometry Factors and Nucleation Site Densities of Electrodeposited Ferromagnetic Cobalt Nanowire Arrays. Crystals 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Ohgai, T.; Tanaka, Y.; Washio, R. Nanocrystalline structure and soft magnetic properties of nickel-molybdenum alloy thin films electrodeposited from acidic and alkaline aqueous solutions. J. Solid State Electrochem. 2013, 17, 743–750. [Google Scholar] [CrossRef]
- Hou, K.H.; Chang, Y.F.; Chang, S.M.; Chang, C.H. The heat treatment effect on the structure and mechanical properties of electrodeposited nano grain size Ni-W alloy coatings. Thin Solid Film. 2010, 518, 7535–7540. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.-Q.; Yang, F.; Zhong, S.-J. Cu6Sn5 Whiskers Precipitated in Sn3.0Ag0.5Cu/Cu Interconnection in Concentrator Silicon Solar Cells Solder Layer. Materials 2017, 10, 327. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Long, W.; Hu, X.; Fu, Y. Interfacial Reaction and IMC Growth of an Ultrasonically Soldered Cu/SAC305/Cu Structure during Isothermal Aging. Materials 2018, 11, 84. [Google Scholar] [CrossRef] [Green Version]
- Saunders, N.; Miodownik, A.P. The Cu-Sn (Copper-Tin) system. Bulletin of Alloy Phase Diagrams 1990, 11, 278–287. [Google Scholar] [CrossRef]
- Chia, P.Y.; Haseeb, A.S.M.A.; Mannan, S.H. Reactions in Electrodeposited Cu/Sn and Cu/Ni/Sn Nanoscale Multilayers for Interconnects. Materials 2016, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, T.; Guo, W.; Yang, S.; Ma, Z.; He, J.; Yan, J. Effect of intermetallic compounds on mechanical properties of copper joints ultrasonic-soldered with Sn-Zn alloy. J. Mater. Process. Tech. 2017, 248, 123–129. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsurusaki, T.; Ohgai, T. Mechanical Properties of Solder-Jointed Copper Rods with Electrodeposited Sn-Zn Alloy Films. Materials 2020, 13, 1330. https://doi.org/10.3390/ma13061330
Tsurusaki T, Ohgai T. Mechanical Properties of Solder-Jointed Copper Rods with Electrodeposited Sn-Zn Alloy Films. Materials. 2020; 13(6):1330. https://doi.org/10.3390/ma13061330
Chicago/Turabian StyleTsurusaki, Tatsuya, and Takeshi Ohgai. 2020. "Mechanical Properties of Solder-Jointed Copper Rods with Electrodeposited Sn-Zn Alloy Films" Materials 13, no. 6: 1330. https://doi.org/10.3390/ma13061330





