Photocatalytic Degradation of Diclofenac Using Al2O3-Nd2O3 Binary Oxides Prepared by the Sol-Gel Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
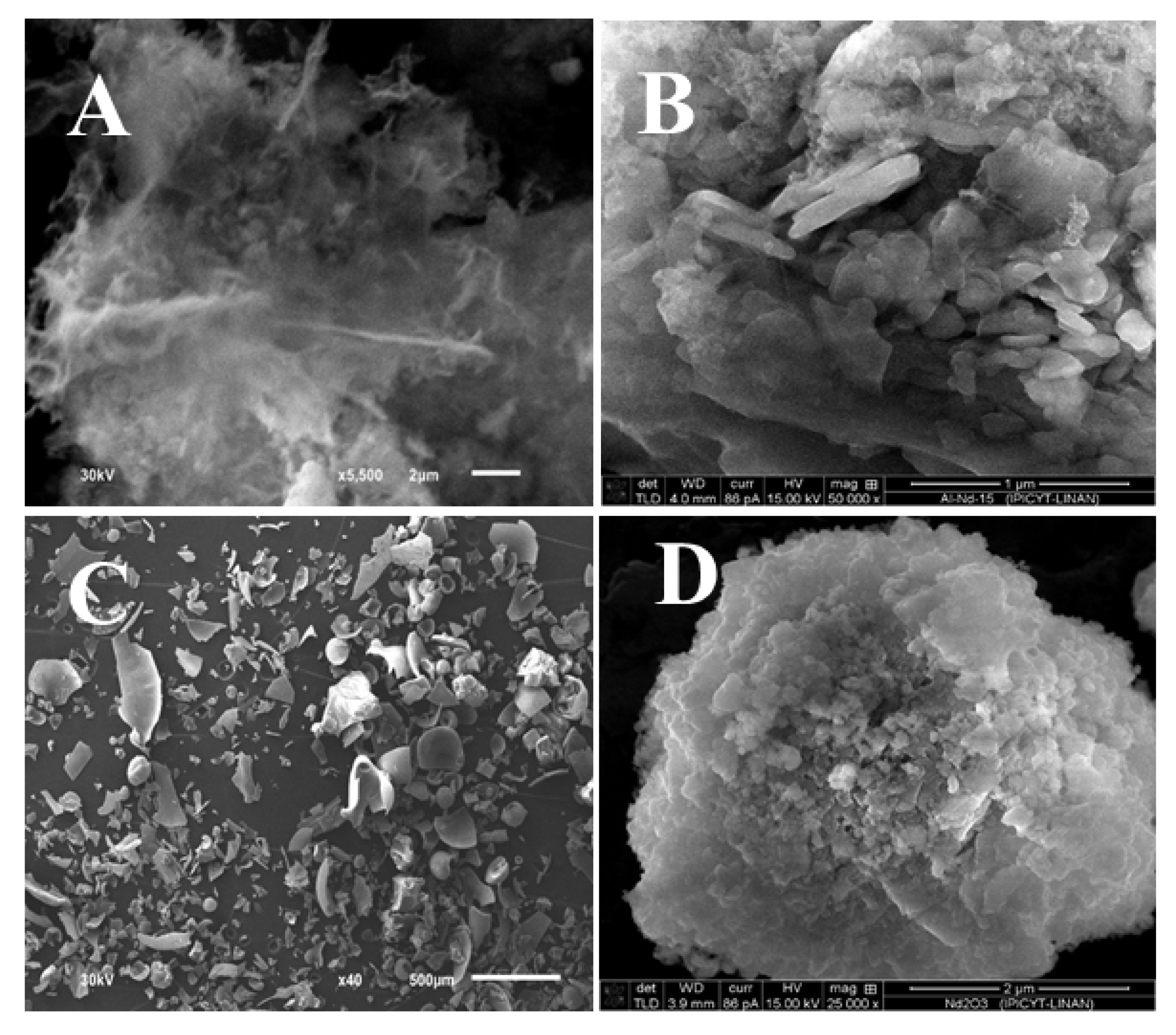
2.2. Solid State Characterization
2.3. Photocatalytic Activity
3. Results and Discussion
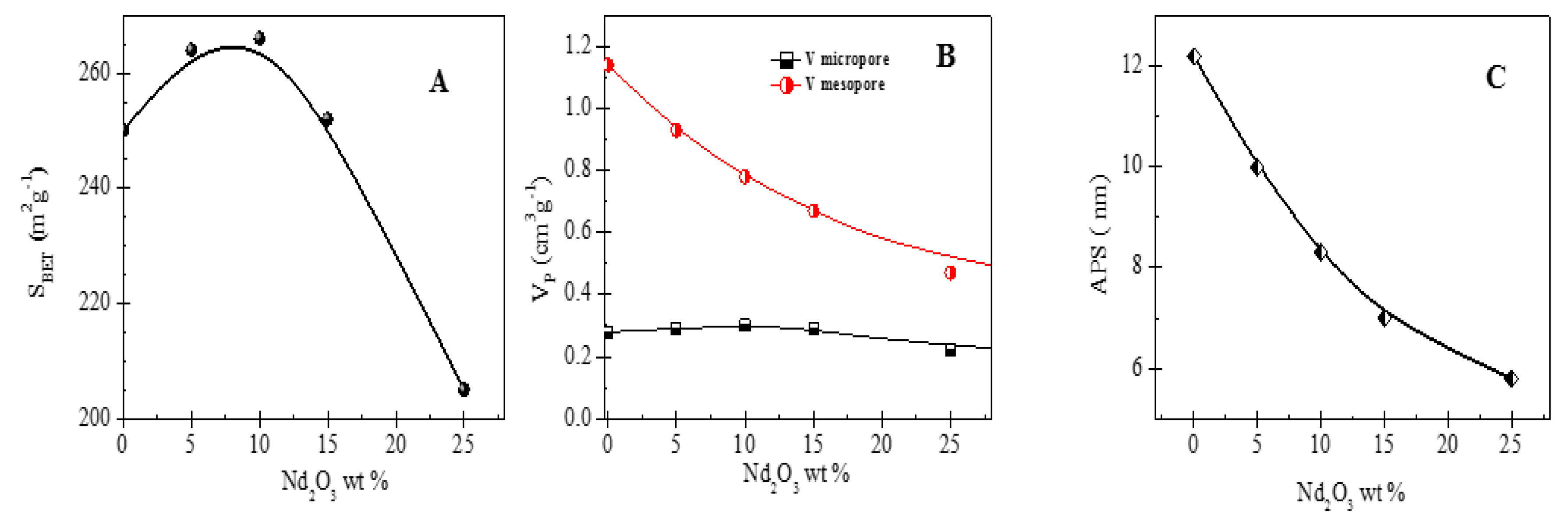
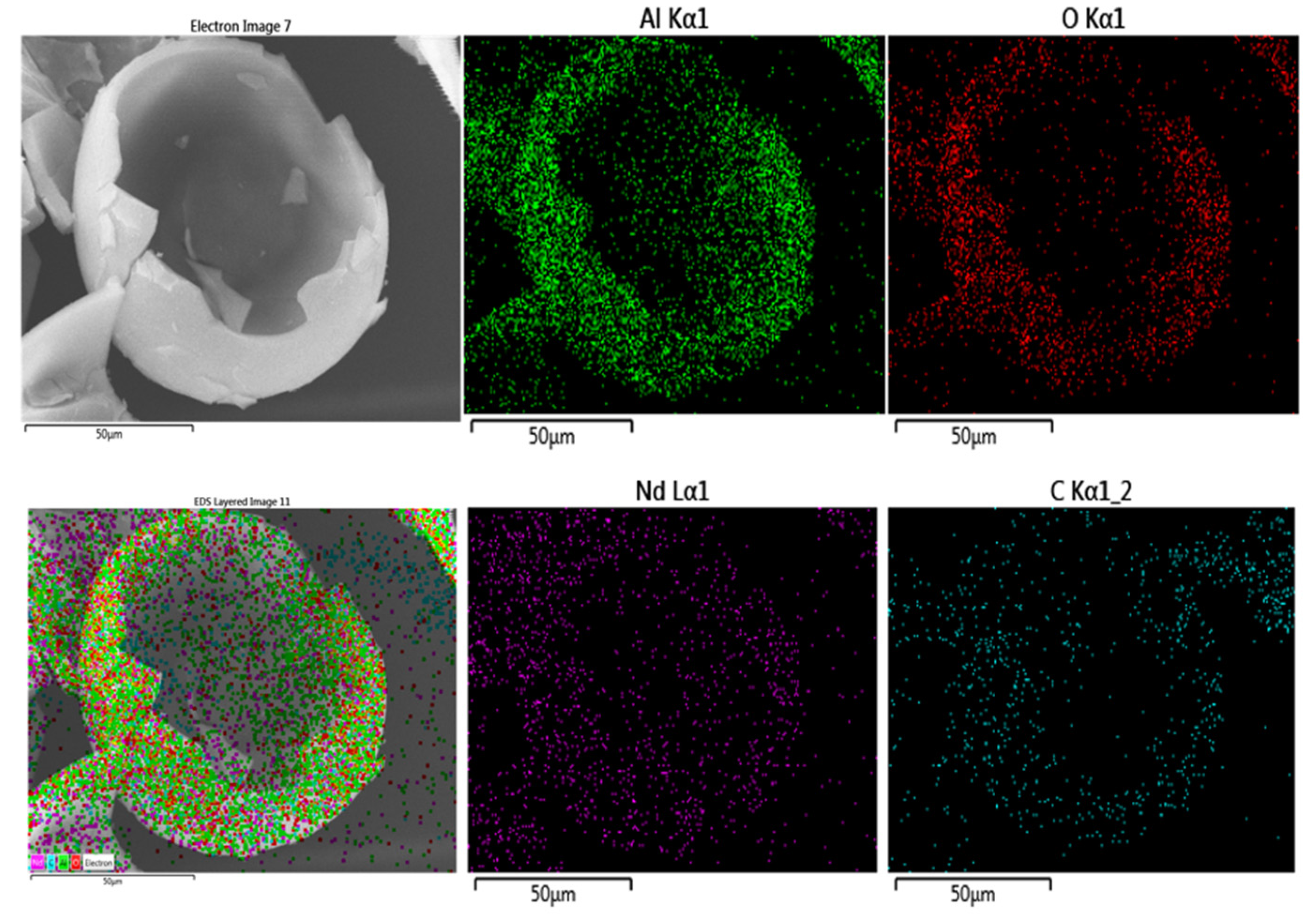
3.1. Textural Properties of Photocatalysts
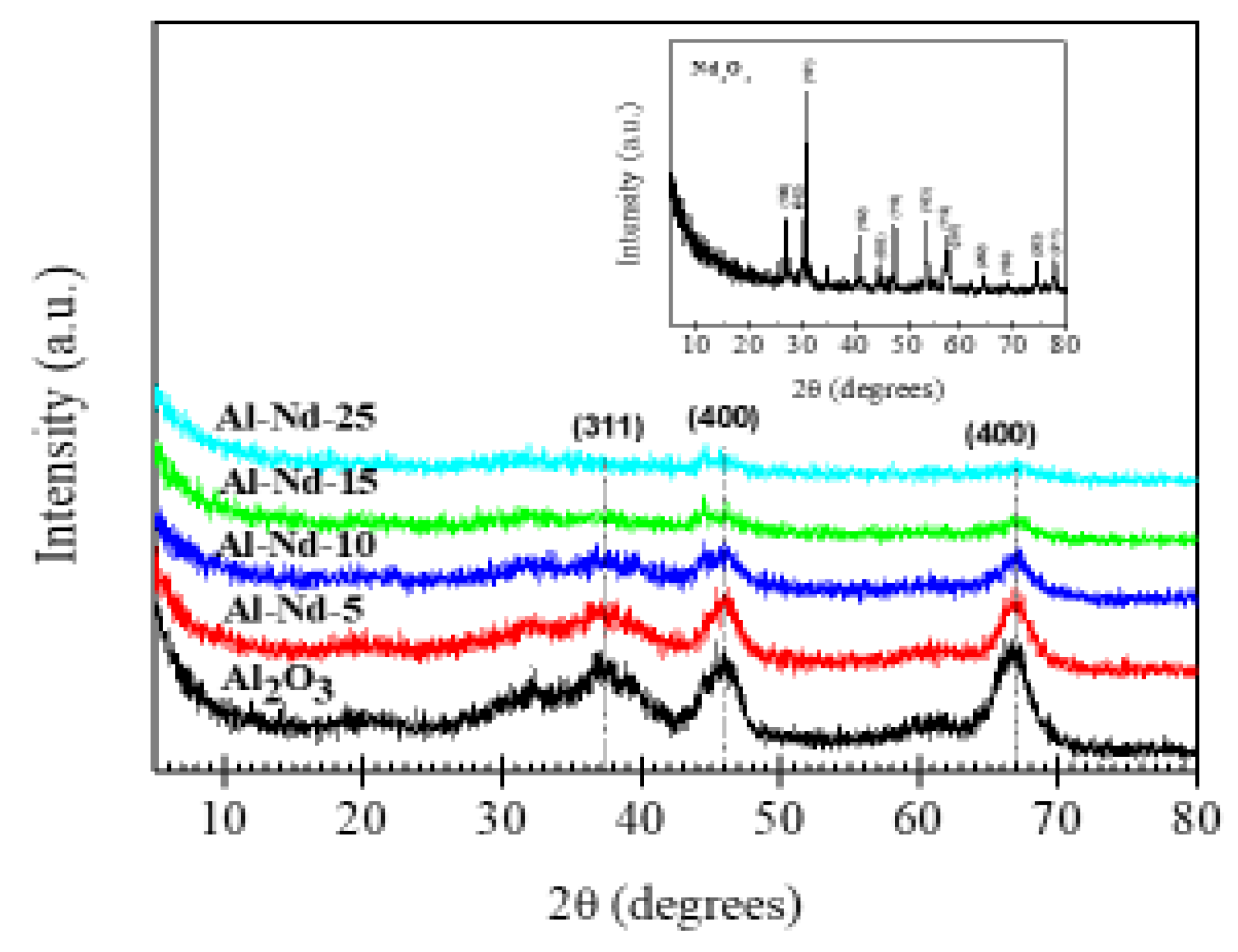
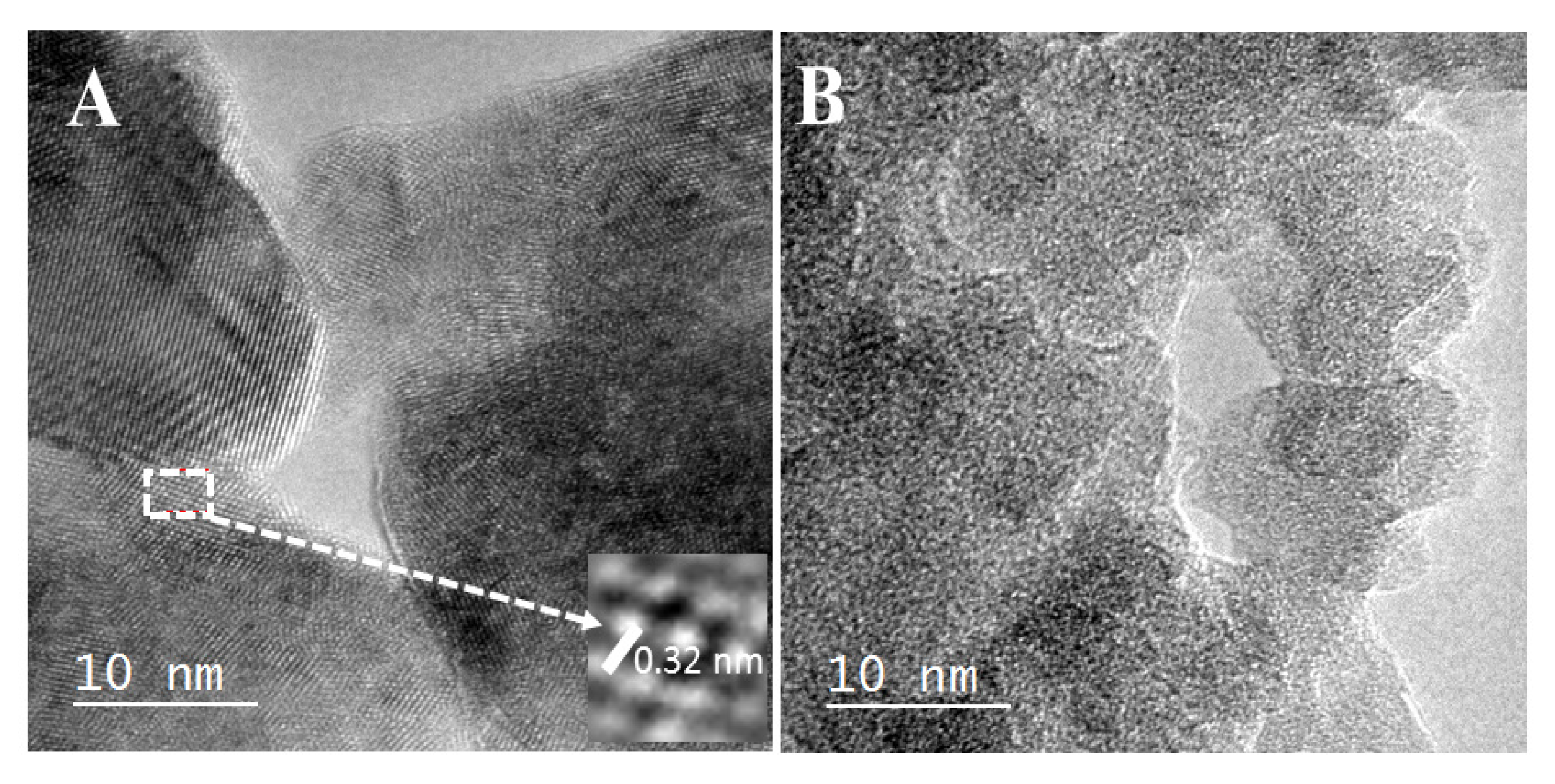
3.2. Crystalline Structure of Catalysts
3.3. Band Gap Energy of Al2O3-Nd2O3 Binary Oxides
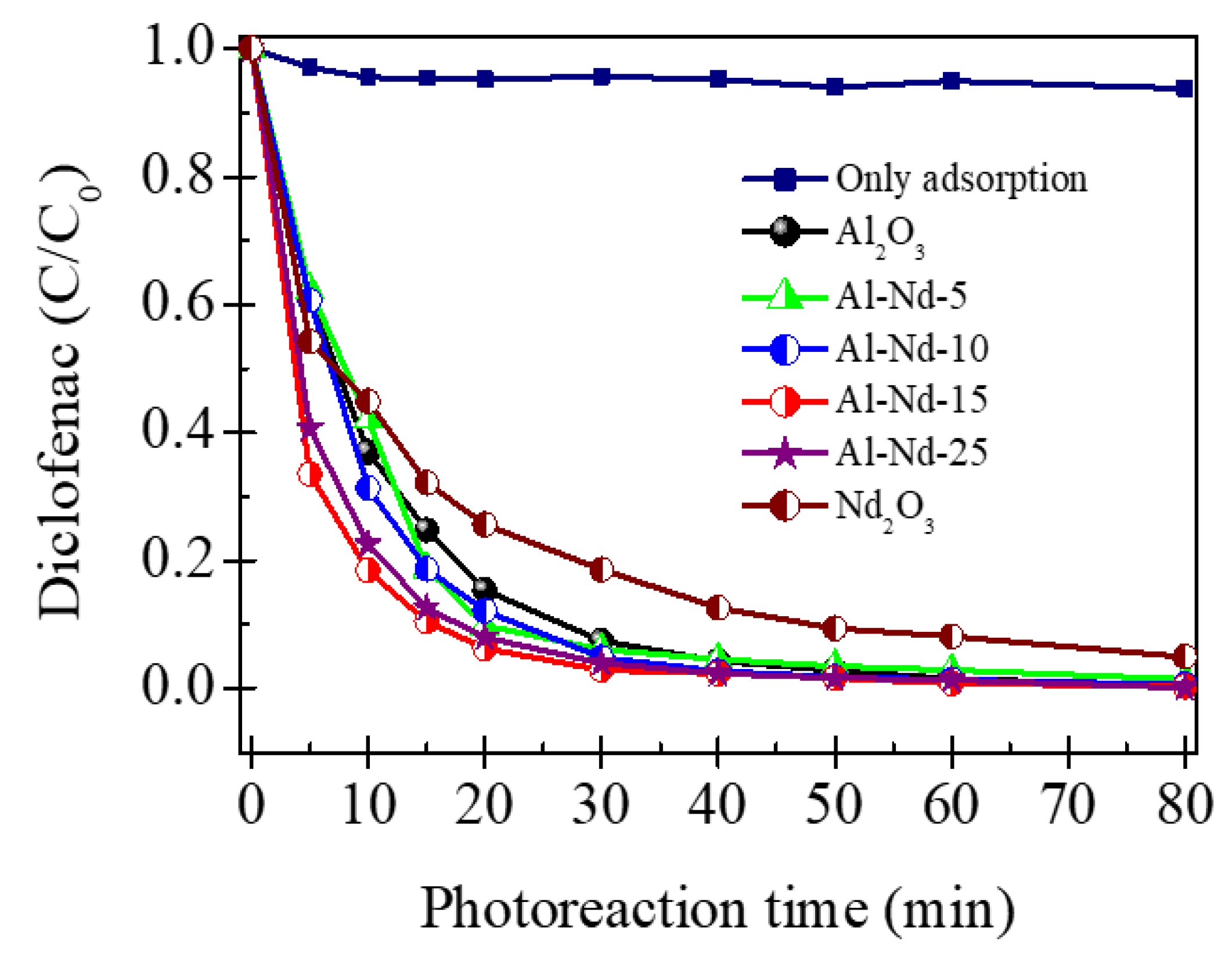
3.4. Photocatalytic Activity of Al2O3-Nd2O3 Binary Oxides in the Degradation of Diclofenac
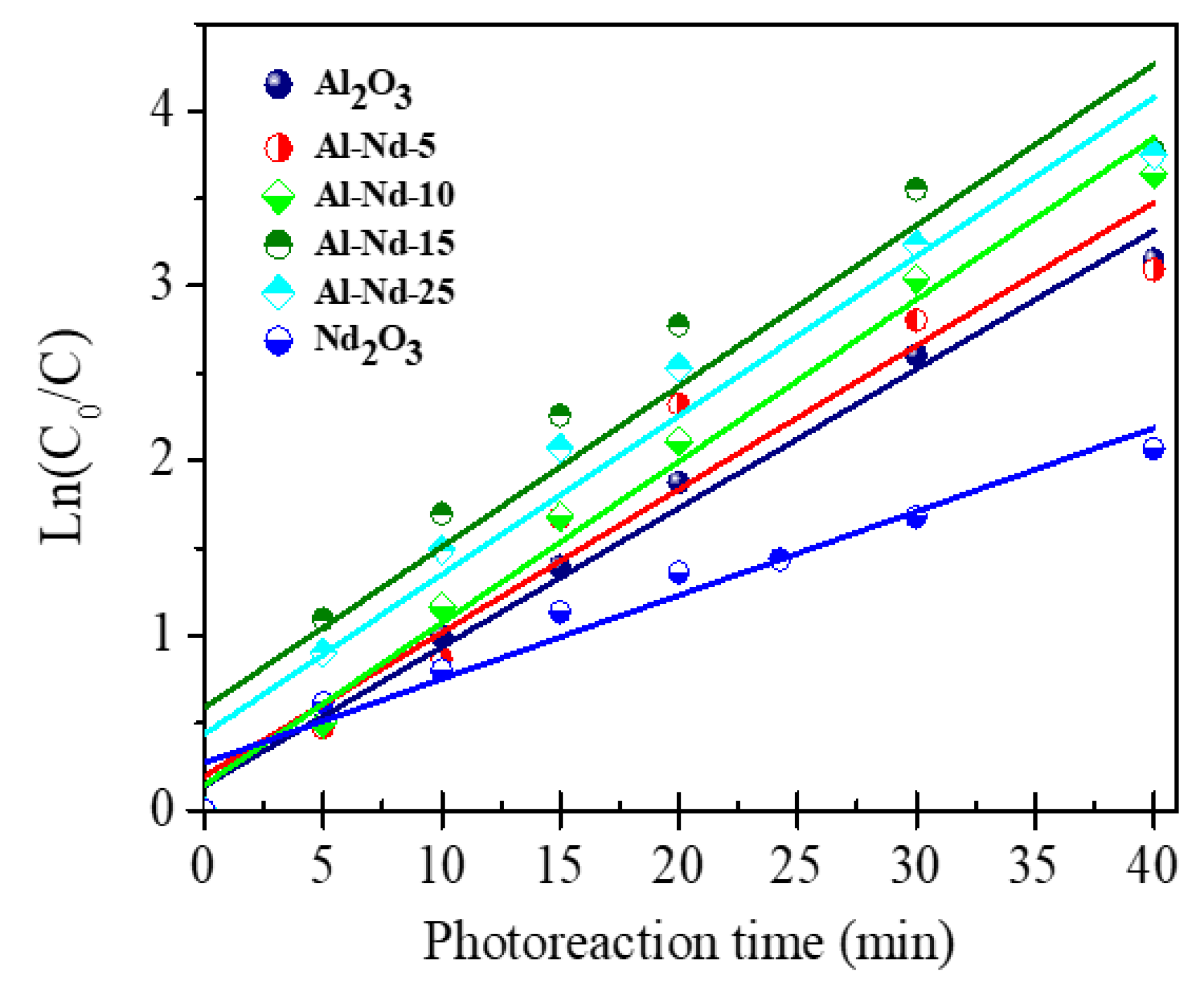
Kinetic Behavior of Al2O3-Nd2O3 Binary Oxides in the Photodegradation of Diclofenac
3.5. Recombination Rate of Electron–Hole Pairs in the Binary Oxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shin, B.; Weber, J.R.; Long, R.D.; Hurley, P.K.; Van de Walle, C.G.; MacIntyre, P.C. Origin and passivation of fixed charge in atomic layer deposited aluminum oxide gate insulators on chemically treated InGaAs substrates. Appl. Phys. Lett. 2010, 96, 152908. [Google Scholar] [CrossRef] [Green Version]
- Price, J.; Bersuker, G.; Lysaght, P.S. Identification of interfacial defects in high-k gate stack films by spectroscopic ellipsometry. J. Vac. Sci. Technol. B 2009, 27, 310–312. [Google Scholar] [CrossRef]
- French, R.A. Electronic band structure of Al2O3, with comparison to Alon and AIN. J. Am. Ceram. Soc. 1990, 73, 477–489. [Google Scholar] [CrossRef]
- Ealet, B.; Elyakhloufi, M.H.; Gillet, E.; Ricci, M. Electronic and crystallographic structure of γ-alumina thin films. Thin Solid Films 1994, 250, 92–100. [Google Scholar] [CrossRef]
- Mullins, W.M.; Averbach, B.L. The electronic structure of anodized and etched aluminum alloy surfaces. Surf. Sci. 1988, 206, 52–60. [Google Scholar] [CrossRef]
- Mullins, W.M. The effect of Fermi energy on reaction of water with oxide surfaces. Surf. Sci. 1989, 217, 459–467. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Piña, Y.; Mantilla, A.; Aguilar-Martinez, O.; Galindo-Hernández, F.; Bokhimi, X.; Barrera, A. Hydroxylated sol-gel Al2O3 as photocatalyst for the degradation of phenolic compounds in presence of UV light. Catal. Today 2014, 220–222, 49–55. [Google Scholar] [CrossRef]
- Barrera, A.; Tzompantzi, F.; Campa-Molina, J.; Casillas, J.E.; Pérez-Hernández, R.; Ulloa-Godinez, S.; Velásquez, C.; Arenas-Alatorre, J. Photocatalytic activity of Ag/Al2O3-Gd2O3 photocatalysts prepared by the sol-gel method in the degradation of 4-chlorophenol. RSC Adv. 2018, 8, 3108–3119. [Google Scholar] [CrossRef] [Green Version]
- Casillas, J.E.; Tzompantzi, F.; Castellanos, S.G.; Mendoza-Damián, G.; Pérez-Hernández, R.; López-Gaona, A.; Barrera, A. Promotion effect of ZnO on the photocatalytic activity of coupled Al2O3-Nd2O3-ZnO composites prepared by the sol-gel method in the degradation of phenol. Appl. Catal. B Environ. 2017, 208, 161–170. [Google Scholar] [CrossRef]
- Barrera, A.; Tzompantzi, F.; Padilla, J.M.; Casillas, J.E.; Jacome-Acatitla, G.; Cano, M.E.; Gomez, R. Reusable PdO/Al2O3-Nd2O3 photocatalysts in the UV photodegradation of phenol. Appl. Catal. B Environ. 2014, 144, 362–368. [Google Scholar] [CrossRef]
- Barrera, A.; Tzompantzi, F.; Lara, V.; Gómez, R. Photodegradation of 2,4-D over PdO/Al2O3–Nd2O3 photocatalysts prepared by the sol-gel method. J. Photochem. Photobiol. A Chem. 2012, 227, 45–50. [Google Scholar] [CrossRef]
- Cordero-García, A.; Turnes Palomino, G.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Maya-Teviño, L.; Hernández-Ramírez, A. Photocatalytic behaviour of WO3/TiO2-N for diclofenac degradation using simulated solar radiation as an activation source. Environ. Sci. Pollut. Res. 2017, 24, 4613–4624. [Google Scholar] [CrossRef] [PubMed]
- Mugunthan, M.; Saidutta, M.B.; Jagadeeshbabu, P.E. Photocatalytic degradation of diclofenac using TiO2-SnO2 mixed oxide catalysts. Environ. Technol. 2019, 40, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, L.; Shi, J.; Deng, H. Synthesis of Ag3PO4/G-C3N4 composite with enhanced photocatalytic performance for the photodegradation of diclofenac under visible light irradiation. Catalists 2018, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Bojanowska-Czajka, A.; Kciuk, G.; Gumiela, M.; Borowiecka, S.; Nałęcz-Jawecki, G.; Koc, A.; Garcia-Reyes, J.F.; Ozbay, D.S.; Trojanowicz, M. Analytical, toxicological and kinetic investigation of decomposition of the drug diclofenac in waters and wastes using gamma radiation. Environ. Sci. Pollut. Res. 2015, 22, 20255–20270. [Google Scholar] [CrossRef] [Green Version]
- Khatem, R.; Miguel, R.O.; Bakhti, A. Use of synthetic clay for removal of diclofenac anti-inflammatory. Eur. J. Soil Sci. 2015, 4, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhou, L.; Deng, H. Ag modified g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Mol. Catal. A Chem. 2016, 423, 270–276. [Google Scholar] [CrossRef]
- Valente, J.S.; Tzompantzi, F.; Prince, J.; Cortez, J.G.H.; Gomez, R. Adsorption and photocatalytic degradation of phenol and 2,4 dichlorophenoxiacetic acid by Mg-Zn-Al layered double hydroxides. Appl. Catal. B 2009, 90, 330–338. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy, 4th ed.; Cengage Learning: Washington, DC, USA, 2009. [Google Scholar]
- Padhye, P.; Poddar, P. Static and dynamic photoluminescence and photocatalytic properties of uniform, monodispersed up/down-converting, highly luminescent, lanthanide-ion-doped β-NaYF4 phosphor microcrystals with controlled multiform morphologies. J. Mater. Chem. A 2014, 45, 19189–19200. [Google Scholar] [CrossRef]
- Sobczynski, A.; Duczmal, L.; Zmudzinski, W. Phenol destruction by photocatalysis on TiO2: An attempt to solve the reaction mechanism. J. Mol. Catal. A Chem. 2004, 213, 225–230. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K. Comments on “Photocatalytic properties of TiO2 modified with platinum and silver nanoparticles in the degradation of oxalic acid in aqueous solution” Langmuir Hinshelwood kinetics—A theoretical study. Appl. Catal. B 2008, 79, 108–109. [Google Scholar] [CrossRef]
- Zhang, N.; Yi, R.; Zhou, L.; Gao, G.; Shi, R.; Qiu, G.; Liu, X. Lanthanide hydroxide nanorods and their thermal decomposition to lanthanide oxide nanorods. Mater. Chem. Phys. 2009, 114, 160–167. [Google Scholar] [CrossRef]
- Barrera, A.; Fuentes, S.; Viniegra, M.; Borja, M.A.; Bogdanchikova, N.; Campa-Molina, J. Structural properties of Al2O3-La2O3 binary oxides prepared by the sol-gel method. Mater. Res. Bull. 2007, 42, 640–648. [Google Scholar] [CrossRef]
- Pérez Osorio, G.; Fuentes Moyado, S.; Petranovskii, V.; Simakov, A. PdO/Al2O3-(Ce1-X Zr X )O2 catalysts: Effect of the sol-gel support composition. Catal. Lett. 2006, 110, 53–60. [Google Scholar] [CrossRef]
- Umesh, B.; Eraiah, B.; Nagabhushana, H.; Nagabhushana, B.M.; Nagaraja, G.; Shivakumara, C.; Chakradhar, R.P.S. Synthesis and characterization of spherical and and rod like nanocrystalline Nd2O3 phosphors. J. Alloy. Compd. 2011, 509, 1146–1151. [Google Scholar] [CrossRef]
- Caro, P. Structure Electronique des Elements de Transition, 1st ed.; Presses Universitaires de France: Paris, France, 1976. [Google Scholar]
- Yuvakkumar, R.; Hong, S.I. Nd2O3: Novel synthesis and characterization. J. Sol. Gel Sci. Technol. 2015, 73, 511–517. [Google Scholar] [CrossRef]
- Pan, L.K.; Chang, Q.S.; Li, C.M. Elucidating Si-Si dimmer vibration from the size-dependent Raman shift of nanosolid Si. J. Phys. Chem. B 2004, 108, 3404–3406. [Google Scholar] [CrossRef]
- Scarel, G.; Svane, A.; Fanciulli, M. Scientific and technological issues related to rare earth oxides. Rare Earth oxide thin films: Growth, characterization, and applications. In Topics in Applied Physics; Scarel, G., Fanciulli, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 106. [Google Scholar]
- Petit, L.; Svane, A.; Szotek, Z.; Temmerman, W.M. First principles study of rare-earth oxides. Phys. Rev. B 2005, 72. [Google Scholar] [CrossRef] [Green Version]


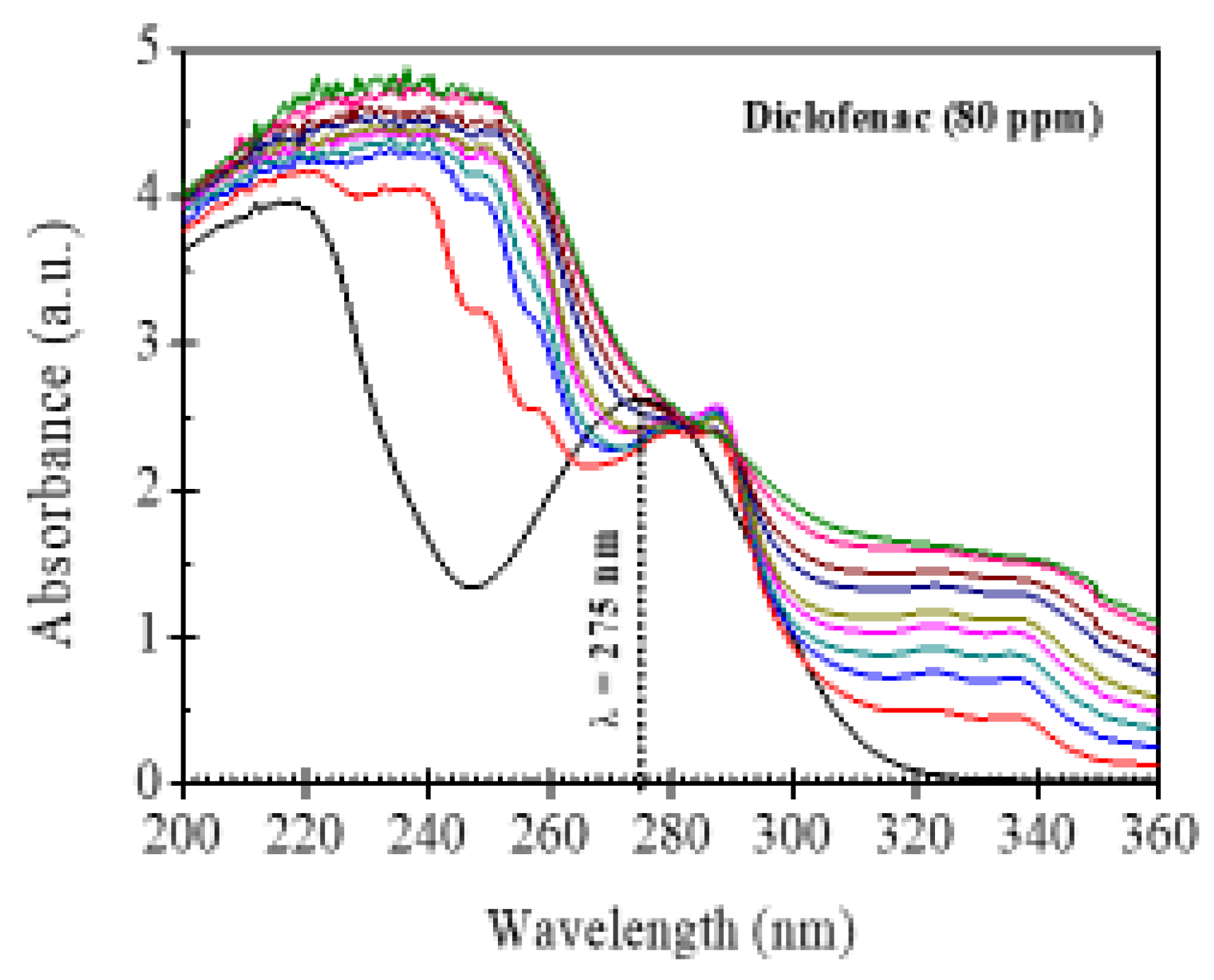


| Material | Band Gap Energy (eV) |
|---|---|
| γ-Al2O3 | 3.35 |
| Al-Nd-5 | 3.54 |
| Al-Nd-10 | 3.20 |
| Al-Nd-15 | 3.13 |
| Al-Nd-25 | 3.19 |
| Nd2O3 | 3.98 |
| Photocatalyst | k × 10−2 (min−1) | τ1/2 (min) |
|---|---|---|
| Al2O3 | 7.9 | 8.74 |
| Al-Nd-5 | 8.2 | 8.46 |
| Al-Nd-10 | 9.3 | 7.50 |
| Al-Nd-15 | 9.5 | 7.29 |
| Al-Nd-25 | 9.1 | 7.61 |
| Nd2O3 | 4.8 | 14.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casillas, J.E.; Campa-Molina, J.; Tzompantzi, F.; Carbajal Arízaga, G.G.; López-Gaona, A.; Ulloa-Godínez, S.; Cano, M.E.; Barrera, A. Photocatalytic Degradation of Diclofenac Using Al2O3-Nd2O3 Binary Oxides Prepared by the Sol-Gel Method. Materials 2020, 13, 1345. https://doi.org/10.3390/ma13061345
Casillas JE, Campa-Molina J, Tzompantzi F, Carbajal Arízaga GG, López-Gaona A, Ulloa-Godínez S, Cano ME, Barrera A. Photocatalytic Degradation of Diclofenac Using Al2O3-Nd2O3 Binary Oxides Prepared by the Sol-Gel Method. Materials. 2020; 13(6):1345. https://doi.org/10.3390/ma13061345
Chicago/Turabian StyleCasillas, José Eduardo, Jorge Campa-Molina, Francisco Tzompantzi, Gregorio Guadalupe Carbajal Arízaga, Alejandro López-Gaona, Sandra Ulloa-Godínez, Mario Eduardo Cano, and Arturo Barrera. 2020. "Photocatalytic Degradation of Diclofenac Using Al2O3-Nd2O3 Binary Oxides Prepared by the Sol-Gel Method" Materials 13, no. 6: 1345. https://doi.org/10.3390/ma13061345
APA StyleCasillas, J. E., Campa-Molina, J., Tzompantzi, F., Carbajal Arízaga, G. G., López-Gaona, A., Ulloa-Godínez, S., Cano, M. E., & Barrera, A. (2020). Photocatalytic Degradation of Diclofenac Using Al2O3-Nd2O3 Binary Oxides Prepared by the Sol-Gel Method. Materials, 13(6), 1345. https://doi.org/10.3390/ma13061345






