Improved Microstructure and Hardness Properties of Low-Temperature Microwave-Sintered Y2O3 Stabilized ZrO2 Ceramics with Additions of Nano TiO2 Powders
Abstract
1. Introduction
2. Experiment
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
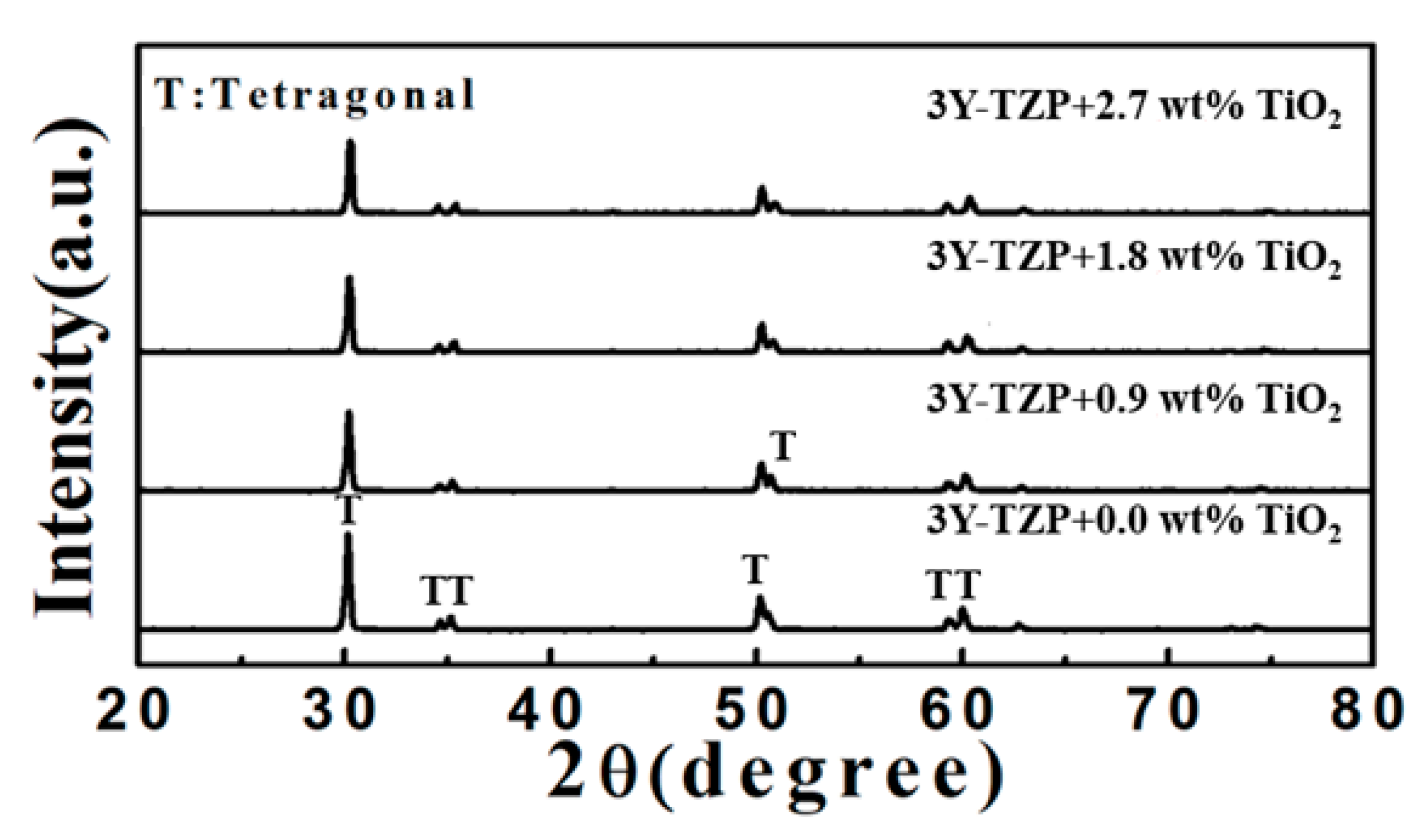
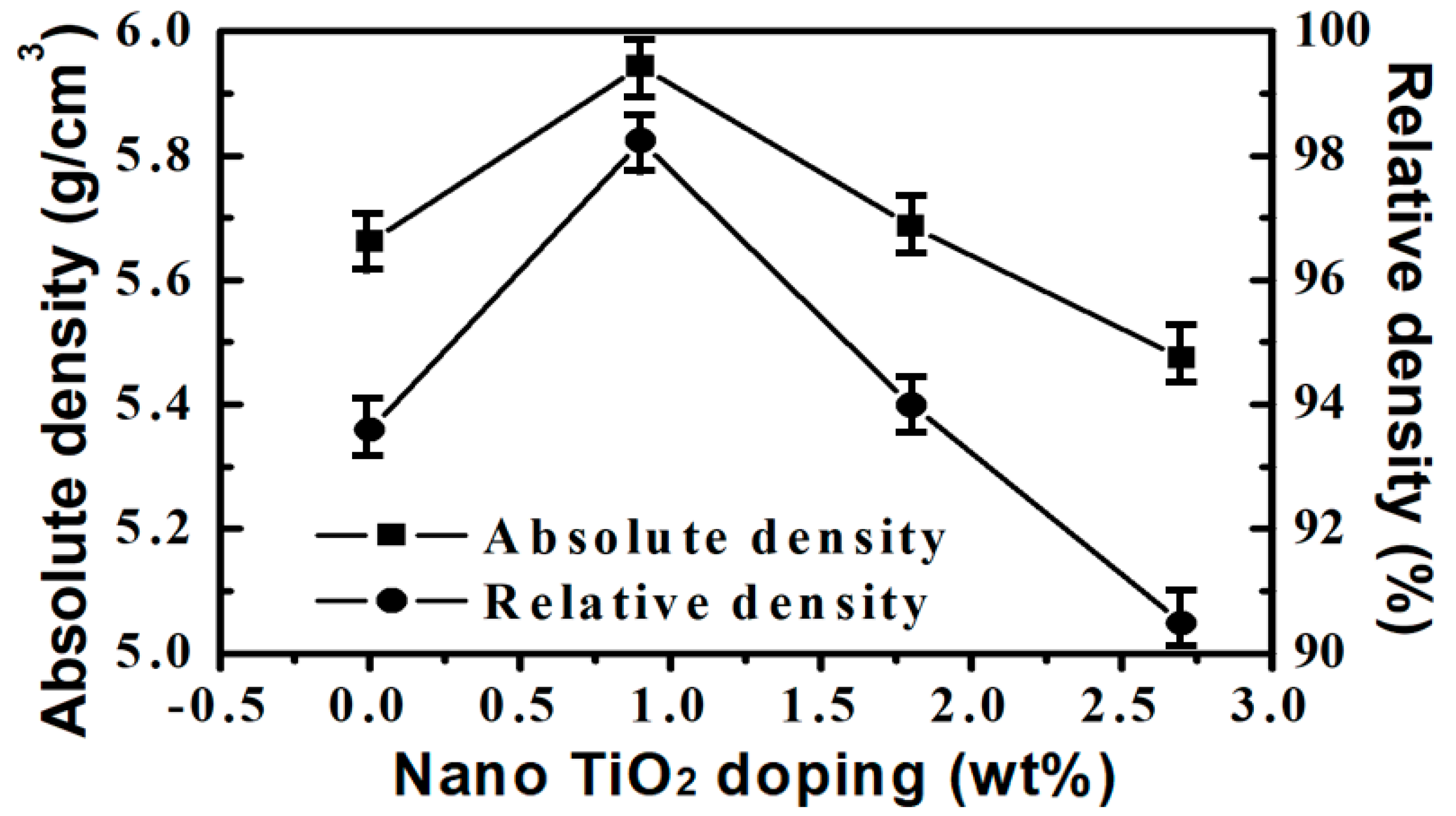
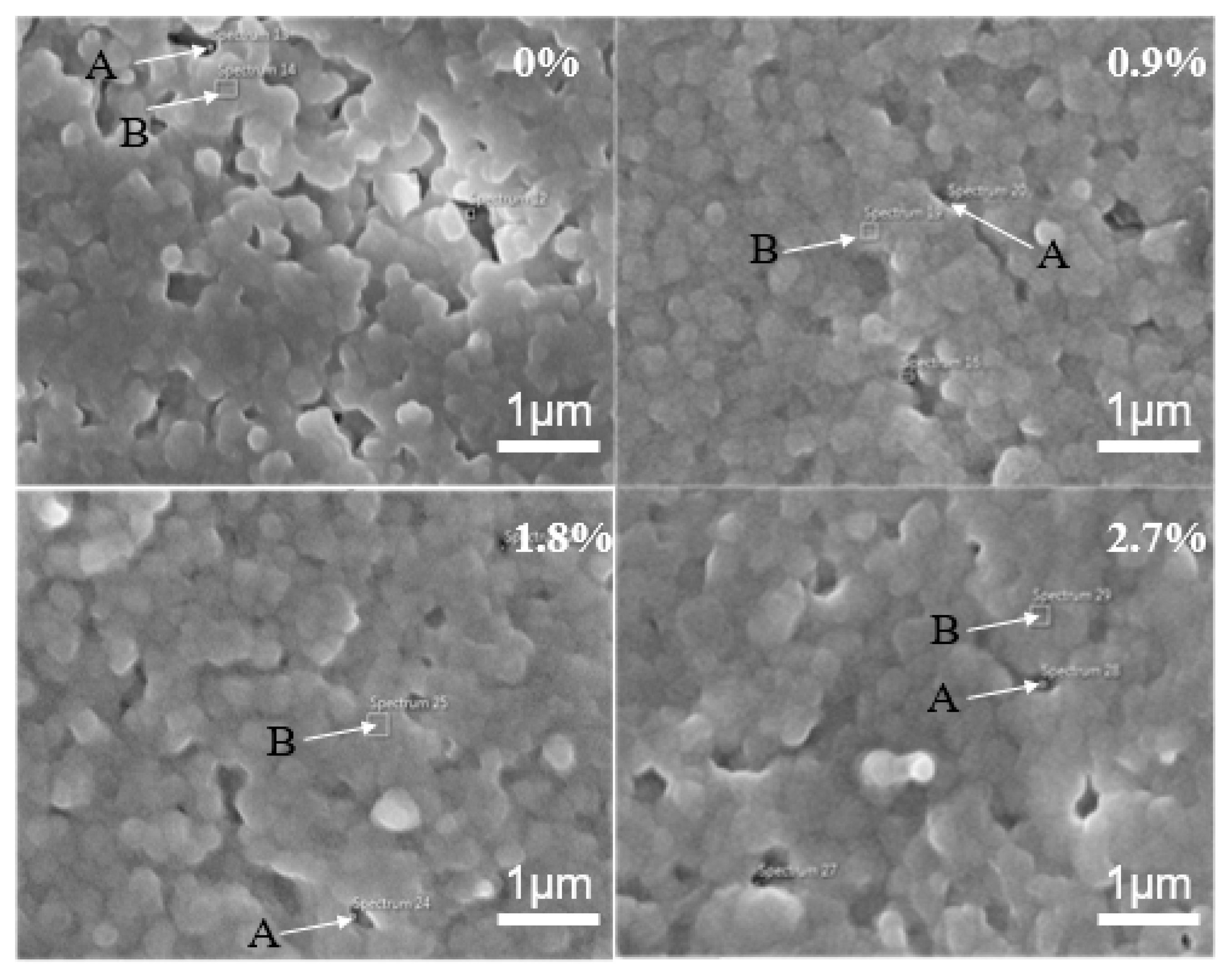
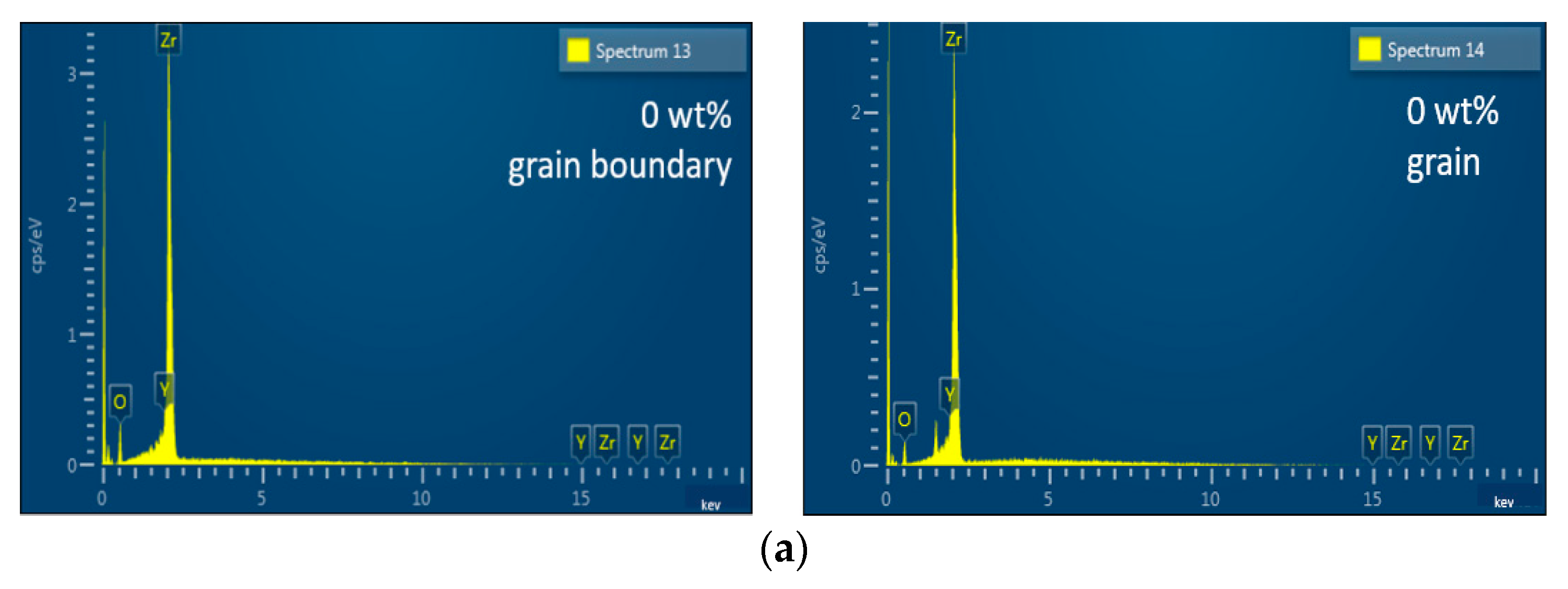
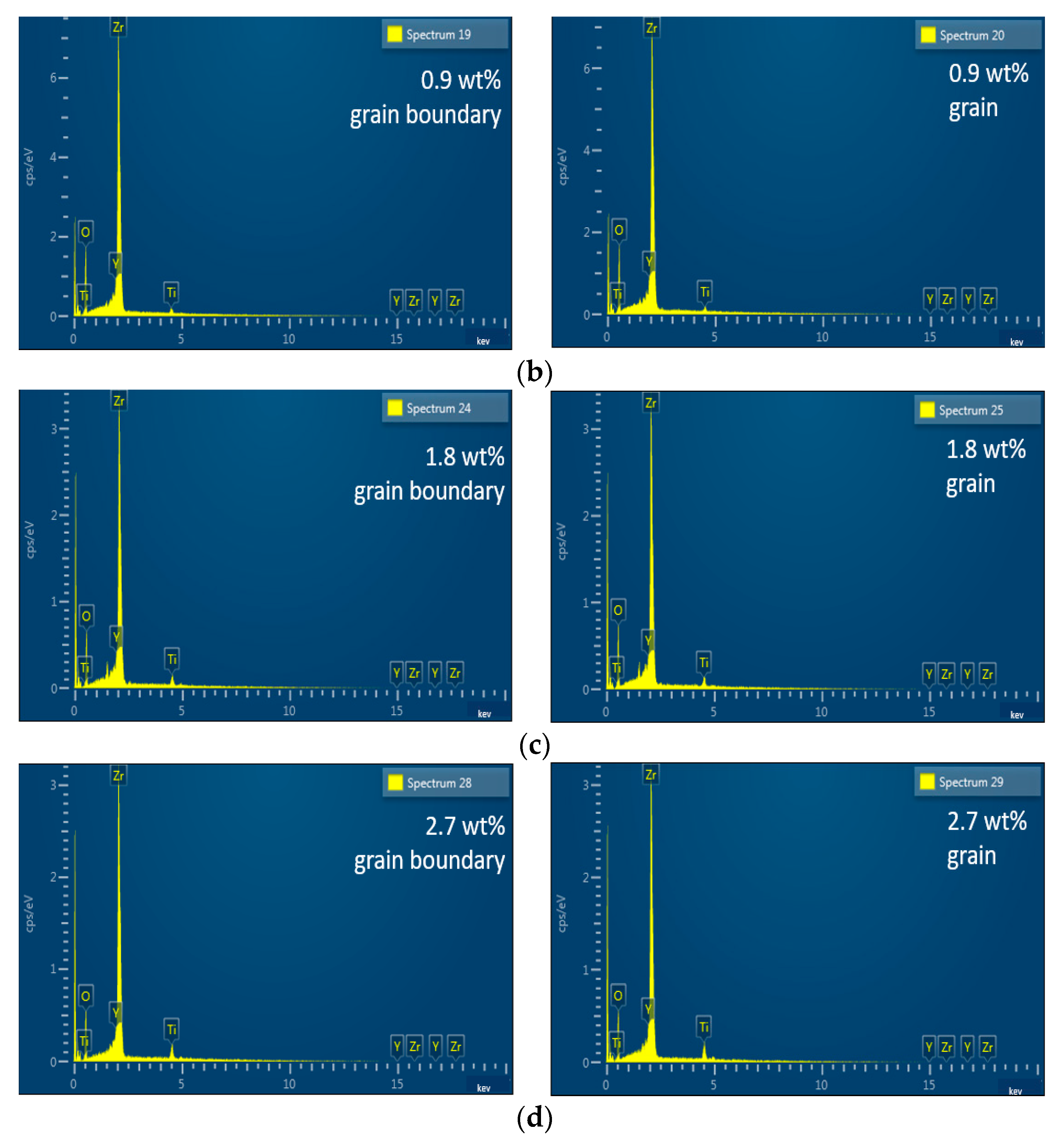
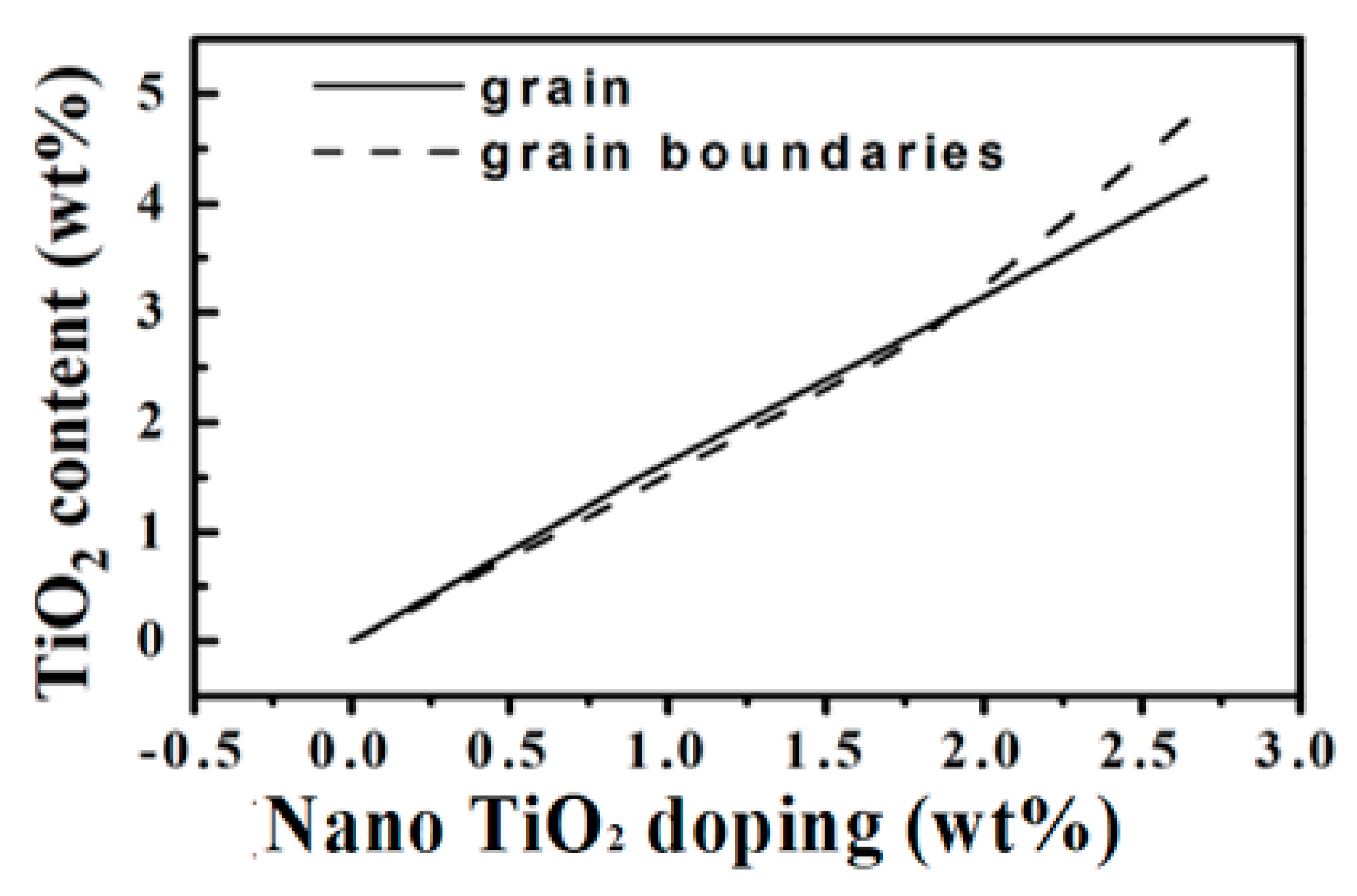
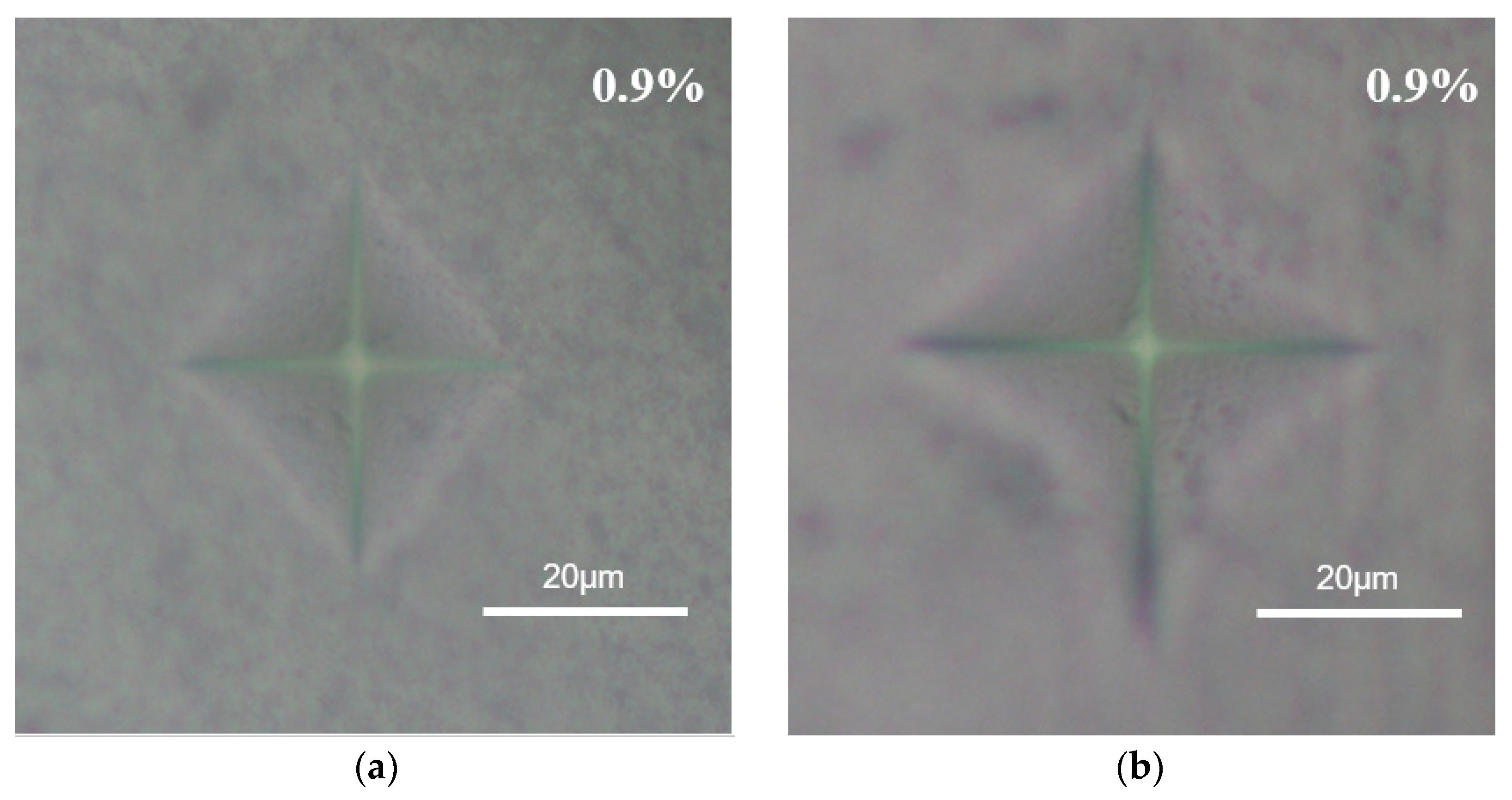
3.1. Microstructure
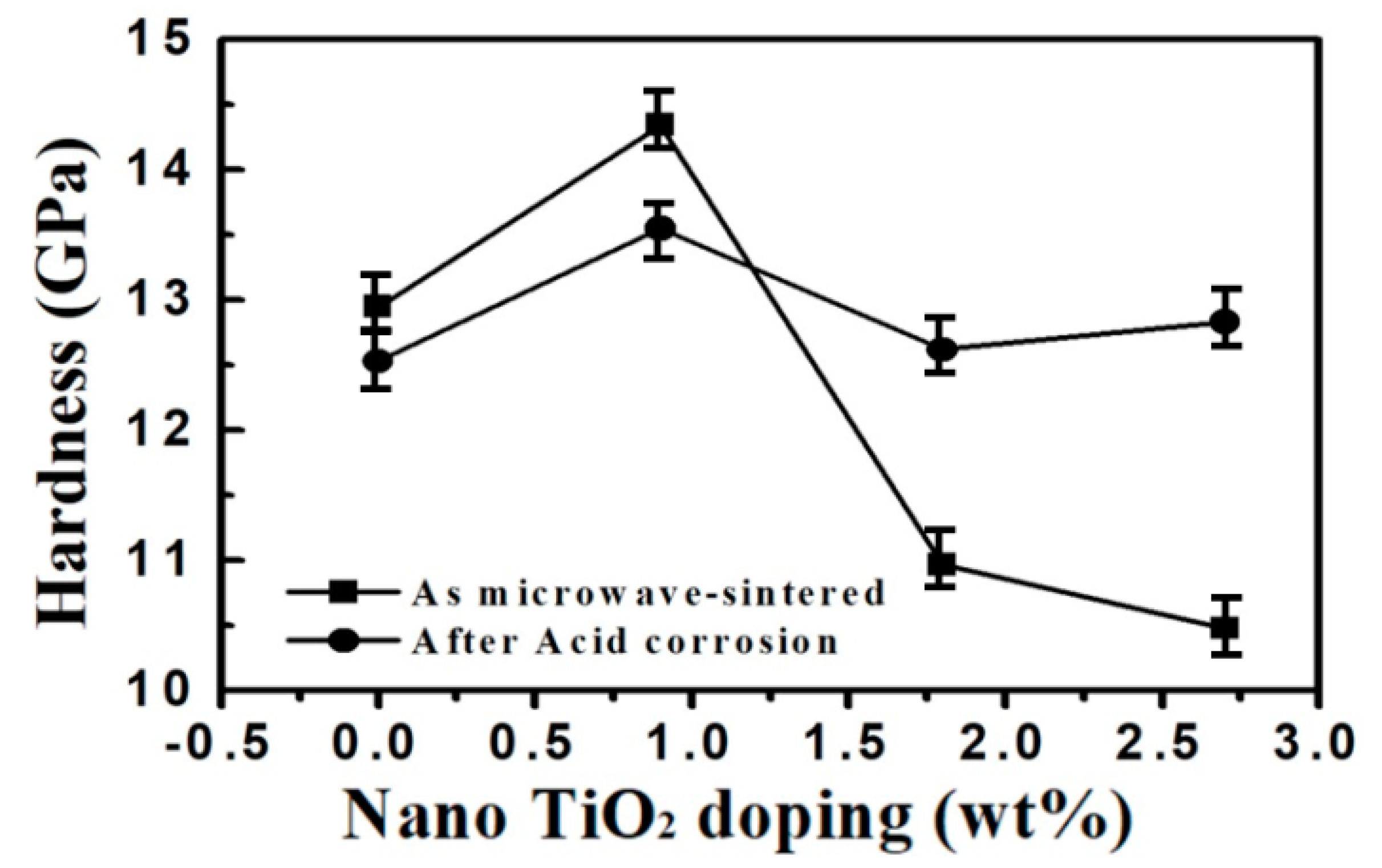
3.2. Hardness Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Özkurt, Z.; Kazazoğlu, E. Zirconia Dental Implants: A Literature Review. J. Oral Implant. 2011, 37, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Grech, J.; Antunes, E. Zirconia in dental prosthetics: A literature review. J. Mater. Res. Technol. 2019, 8, 4956–4964. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Expert Rev. Med Devices 2016, 13, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lawn, B. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2017, 97, 140–147. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Kanade, K.; Baeg, J.; Apte, S.; Prakash, T.; Kale, B.B. Synthesis and characterization of nanocrystallined zirconia by hydrothermal method. Mater. Res. Bull. 2008, 43, 723–729. [Google Scholar] [CrossRef]
- Abden, M.J.; Islam, M.K.; Afroze, J.D. Microstructure and mechanical properties of 3YSZ ceramics reinforced with Al2O3 particles. Int. J. Mater. Eng. 2014, 4, 129–135. [Google Scholar]
- Ban, S.; Sato, H.; Suehiro, Y.; Nakanishi, H.; Nawa, M. Biaxial flexure strength and low-temperature degradation of Ce-TZP/Al2O3 nanocomposite and Y-TZP as sental restoratives. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 492–498. [Google Scholar] [CrossRef]
- Llorca, J.; Pastor, J.Y.; Poza, P.; Peña, J.I.; Francisco, I.; Larrea, A.; Orera, V. Influence of the Y2O3Content and Temperature on the Mechanical Properties of Melt-Grown Al2O3-ZrO2Eutectics. J. Am. Ceram. Soc. 2004, 87, 633–639. [Google Scholar] [CrossRef]
- Mago, S.; Sharma, C.; Sharma, P.; Singh, K.L.; Singh, A.P. Comparative Analysis of Yttria Stabilized Zirconia (YSZ) and Titania Doped YSZ (YZT) Sintered by two Different Routes: Conventional and Microwave Processing. Orient. J. Chem. 2018, 34, 2539–2547. [Google Scholar] [CrossRef]
- Laberty-Robert, C.; Ansart, F.; Deloget, C.; Gaudon, M.; Rousset, A. Dense yttria stabilized zirconia: Sintering and microstructure. Ceram. Int. 2003, 29, 151–158. [Google Scholar] [CrossRef]
- Takigawa, Y.; Naka, Y.; Higashi, K. Effect of Titania Doping on Phase Stability of Zirconia Bioceramics in Hot Water. Mater. Trans. 2007, 48, 332–336. [Google Scholar] [CrossRef]
- Almazdi, A.A.; Khajah, H.M.; Monaco, E.A.; Kim, H. Applying microwave technology to sintering dental zirconia. J. Prosthet. Dent. 2012, 108, 304–309. [Google Scholar] [CrossRef]
- Upadhyaya, D.D.; Ghosh, A.; Dey, G.K.; Prasad, R.; Suri, A.K. Microwave sintering of zirconia ceramics. J. Mater. Sci. 2001, 36, 4707–4710. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Weng, M.-H.; Chang, S.-J.; Yang, R.-Y. Preparation of Sr2SiO4:Eu3+ phosphors by microwave-assisted sintering and their luminescent properties. Ceram. Int. 2012, 38, 125–130. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Weng, M.-H.; Chen, H.-Y.; Hsiung, C.-M.; Chen, S.-H. The influence of Eu3+ doping on photoluminescent properties of red emitting phosphor YInGe2O7:Eu3+ by microwave-assisted and conventional sintering method. J. Lumin 2012, 132, 478–483. [Google Scholar] [CrossRef]
- Kuo, H.-N.; Chou, J.-H.; Liu, T.-K. Microstructure and Mechanical Properties of Microwave Sintered ZrO2 Bioceramics with TiO2 Addition. Appl. Bionics Biomech. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Standard Test Methods for Determining Average Grain Size, ASTM E112-13; ASTM International: West Conshohocken, PA, USA, 1 October 2013.
- Presenda, A.; Salvador, M.D.; Penaranda-Foix, F.; Moreno, R.; Borrell, A. Effect of microwave sintering on microstructure and mechanical properties in Y-TZP materials used for dental applications. Ceram. Int. 2015, 41, 7125–7132. [Google Scholar] [CrossRef]
- Marinis, A.; Aquilino, S.A.; Lund, P.S.; Gratton, D.G.; Stanford, C.M.; Diaz-Arnold, A.M.; Qian, F. Fracture toughness of yttria-stabilized zirconia microwave-assisted sintered in conventional and microwave ovens. J. Prosthet Dent. 2013, 109, 165–171. [Google Scholar] [CrossRef]
- Borkhodoev, V. Estimation of limits of detection and determination in X-ray fluorescence analysis by the dependence of the relative standard deviation on analyte concentration. J. Anal. Chem. 2016, 71, 872–877. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Schneider, J.; Begand, S.; Kriegel, R.; Kaps, C.; Glien, W.; Oberbach, T. Low-Temperature Aging Behavior of Alumina-Toughened Zirconia. J. Am. Ceram. Soc. 2008, 91, 3613–3618. [Google Scholar] [CrossRef]
- Tredici, I.G.; Sebastiani, M.; Massimi, F.; Bemporad, E.; Resmini, A.; Merlati, G.; Anselmi-Tamburini, U. Low-temperature degradation resistant nanostructured yttria-stabilized zirconia for dental applications. Ceram. Int. 2016, 42, 8190–8197. [Google Scholar] [CrossRef]
- Ye, Y.; Li, J.; Zhou, H.; Chen, J. Microstructure and mechanical properties of yttria-stabilized ZrO2/Al2O3 nanocomposite ceramics. Ceram. Int. 2008, 34, 1797–1803. [Google Scholar] [CrossRef]
- Ai, Y.; Xie, X.; He, W.; Liang, B.; Fan, Y. Microstructure and properties of Al2O3(n)/ZrO2 Dental ceramics prepared by two-step microwave sintering. Mater. Des. 2015, 65, 1021–1027. [Google Scholar] [CrossRef]
- Ramesh, S.; Meenaloshini, S.; Tan, C.Y.; Sopyan, I.; Teng, W.D. Sintering and ageing properties of manganese doped Y-TZP Ceramics. In Proceedings of the 33rd International Conference & Exposition on Advanced Ceramics and Composites, Daytona Beach, FL, USA, 1–23 January 2009. [Google Scholar]
- Golieskardi, M.; Satgunam, M.; Ragurajan, D. Densification Behaviour and Mechanical Properties of Aluminium Oxide and Cerium Oxide-Doped Yttria Tetragonal Zirconia Polycrystal Ceramics Using Two-Step Sintering. J. Nanosci. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, M.-H.; Lin, C.-X.; Huang, C.-S.; Tsai, C.-Y.; Yang, R.-Y. Improved Microstructure and Hardness Properties of Low-Temperature Microwave-Sintered Y2O3 Stabilized ZrO2 Ceramics with Additions of Nano TiO2 Powders. Materials 2020, 13, 1546. https://doi.org/10.3390/ma13071546
Weng M-H, Lin C-X, Huang C-S, Tsai C-Y, Yang R-Y. Improved Microstructure and Hardness Properties of Low-Temperature Microwave-Sintered Y2O3 Stabilized ZrO2 Ceramics with Additions of Nano TiO2 Powders. Materials. 2020; 13(7):1546. https://doi.org/10.3390/ma13071546
Chicago/Turabian StyleWeng, Min-Hang, Cheng-Xun Lin, Cian-Song Huang, Chin-Yi Tsai, and Ru-Yuan Yang. 2020. "Improved Microstructure and Hardness Properties of Low-Temperature Microwave-Sintered Y2O3 Stabilized ZrO2 Ceramics with Additions of Nano TiO2 Powders" Materials 13, no. 7: 1546. https://doi.org/10.3390/ma13071546
APA StyleWeng, M.-H., Lin, C.-X., Huang, C.-S., Tsai, C.-Y., & Yang, R.-Y. (2020). Improved Microstructure and Hardness Properties of Low-Temperature Microwave-Sintered Y2O3 Stabilized ZrO2 Ceramics with Additions of Nano TiO2 Powders. Materials, 13(7), 1546. https://doi.org/10.3390/ma13071546





