Nano/Micro Hierarchical Bioceramic Coatings for Bone Implant Surface Treatments
Abstract
1. Introduction
2. Materials and Methods
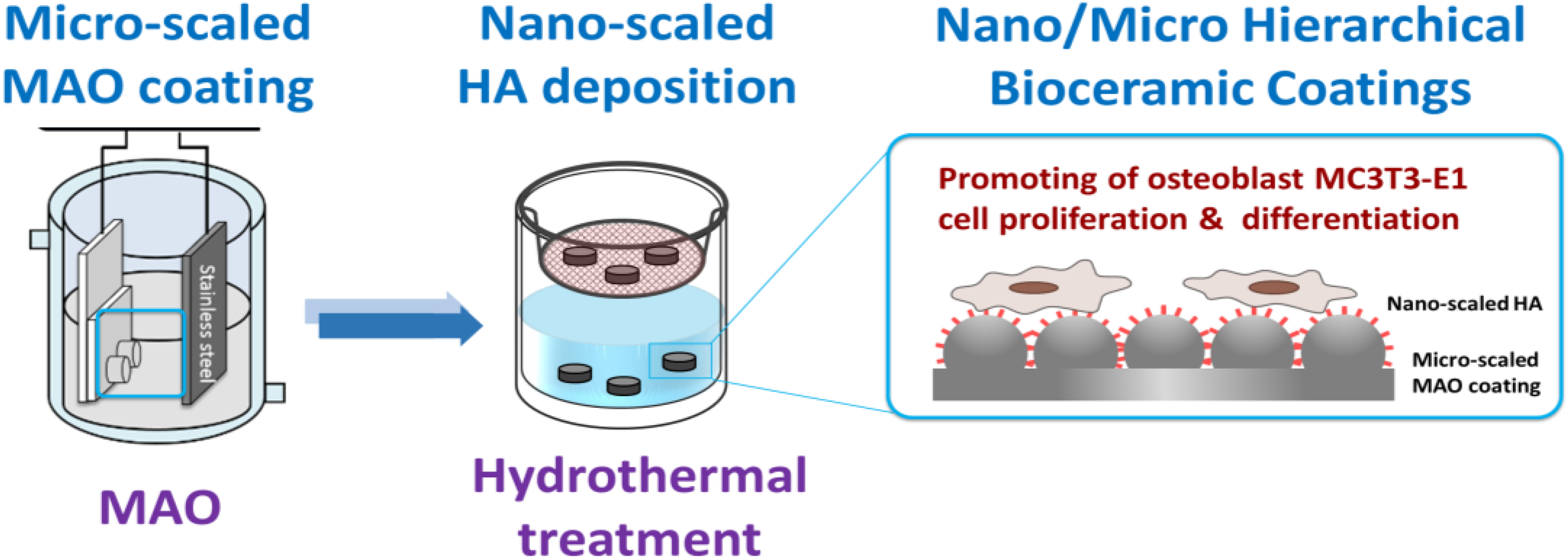
2.1. Sample Preparation
2.2. Characterization of Samples
2.3. Cell Test in Vitro
3. Results and Discussion
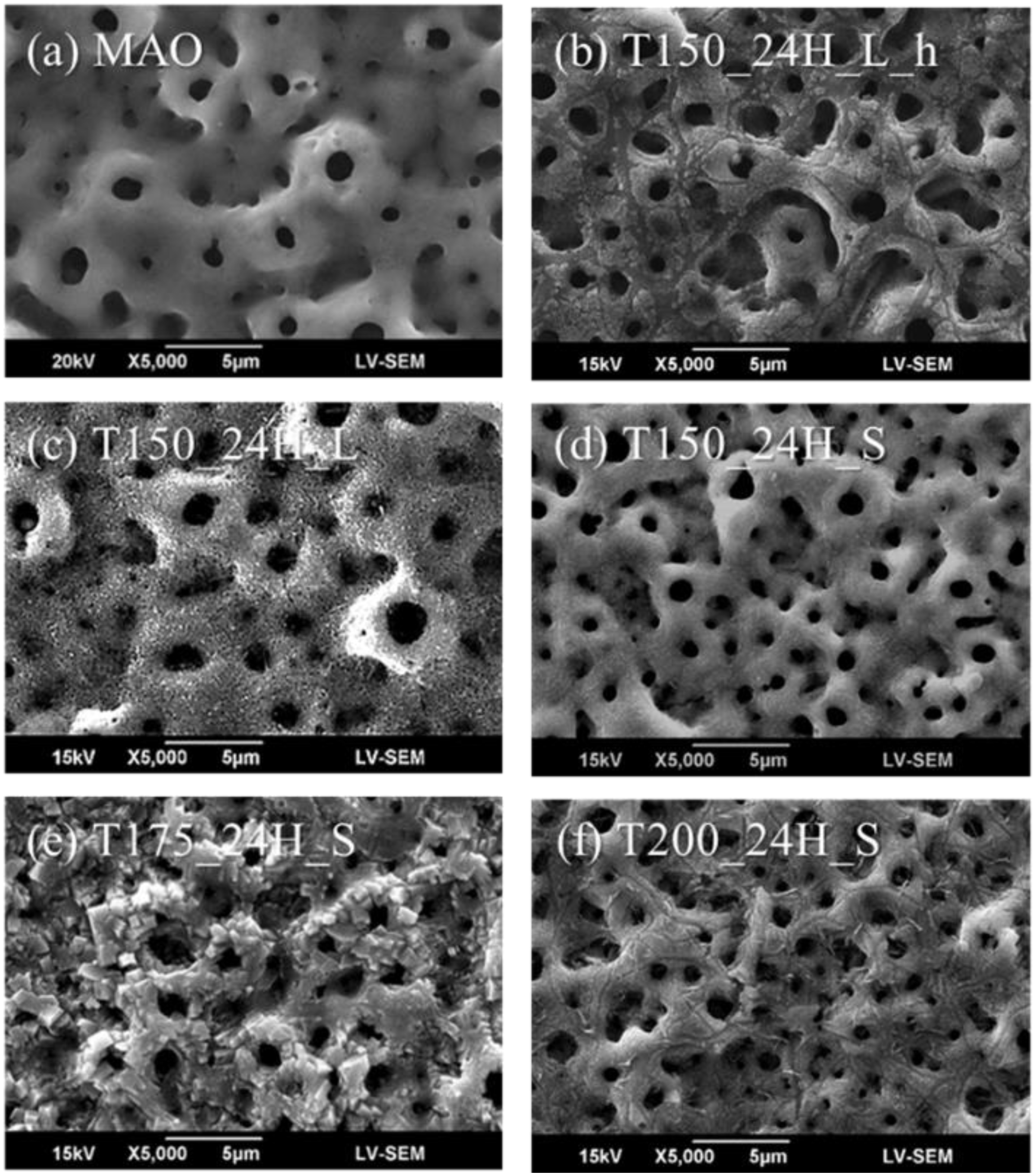
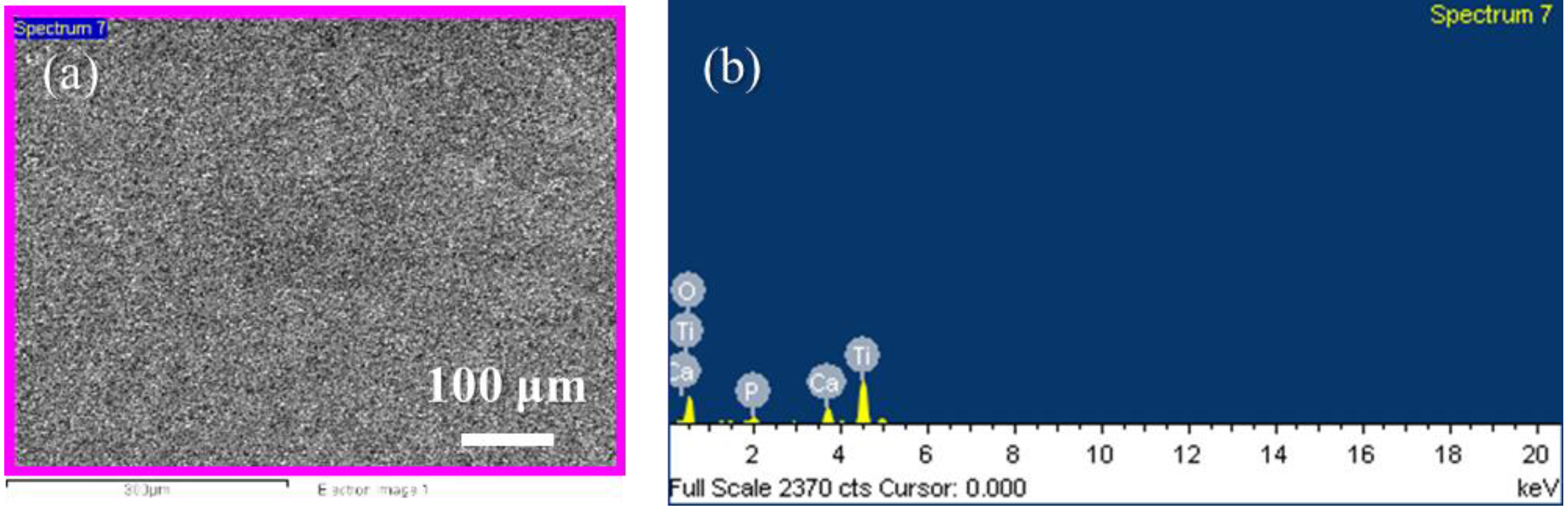
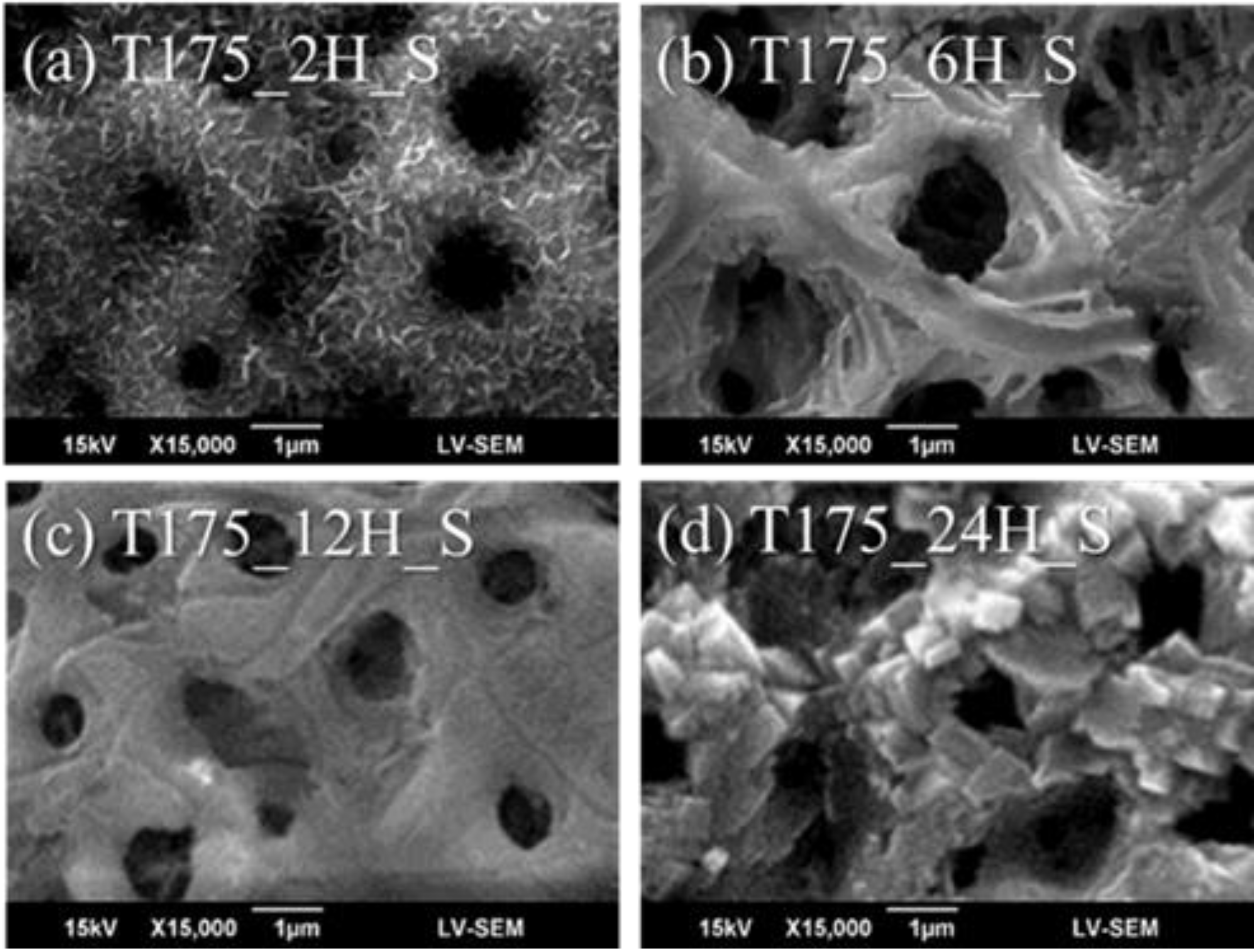
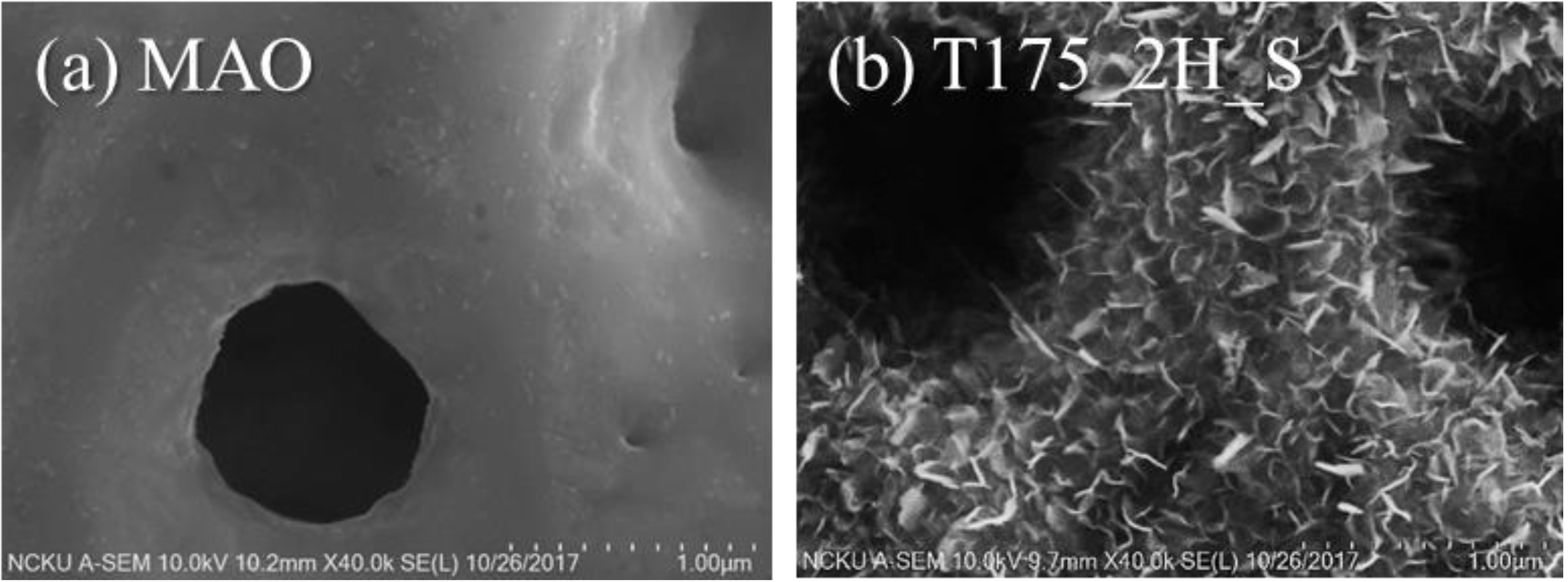
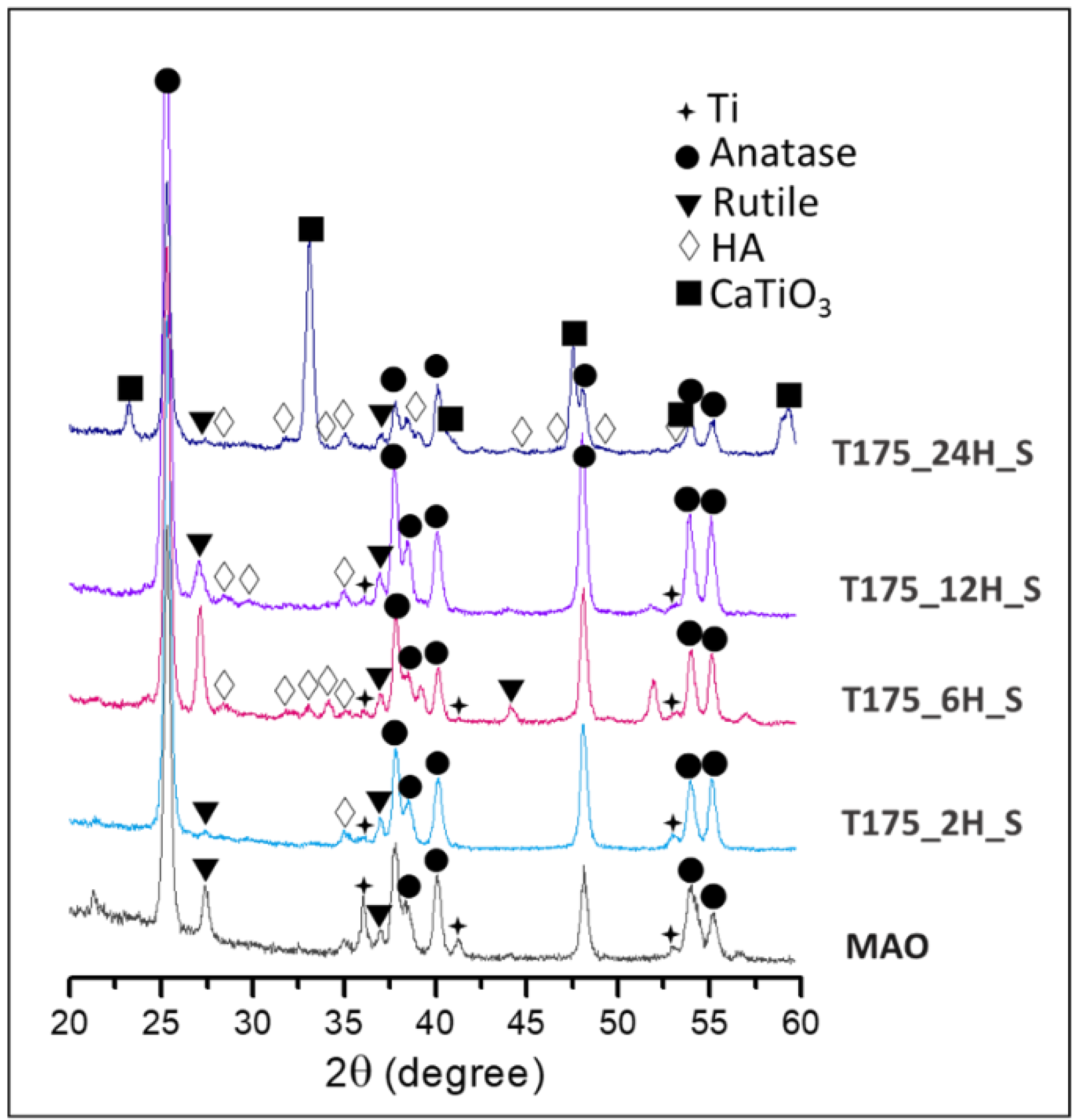
3.1. Characterization of the Hierarchical Bioceramics Coatings
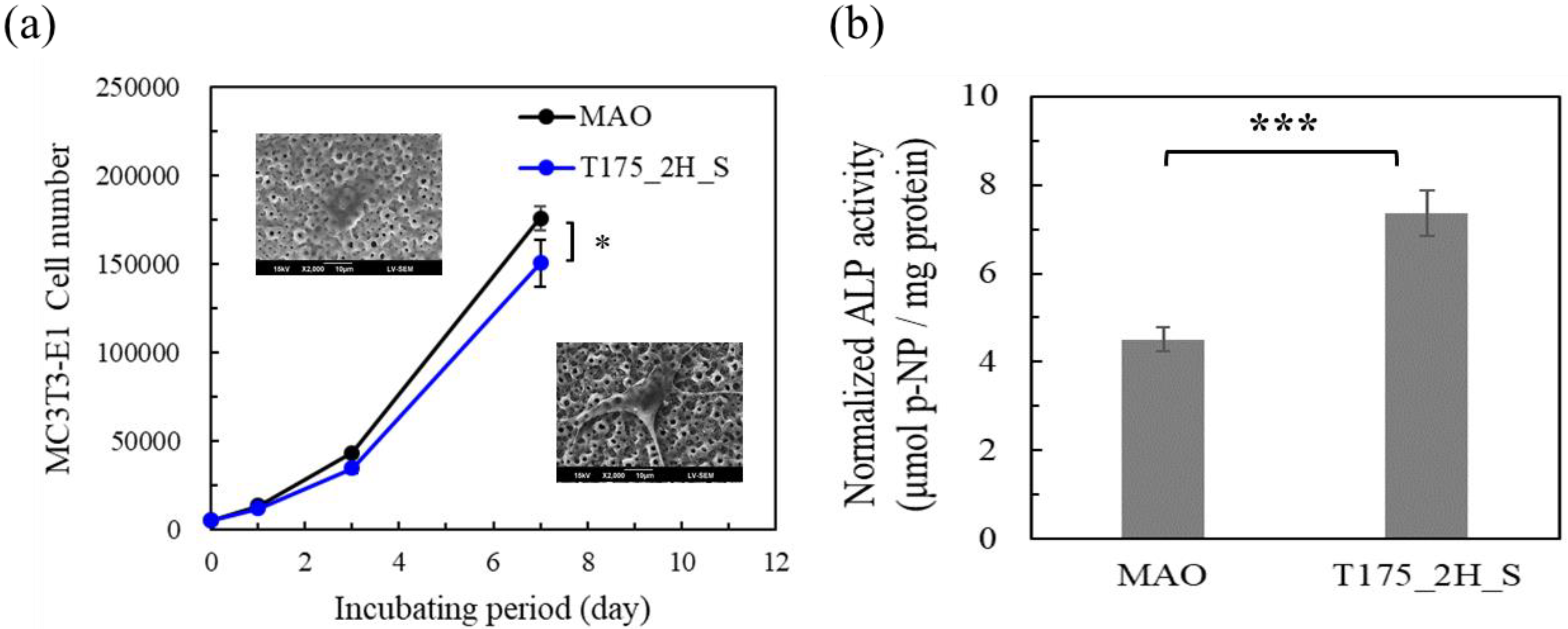
3.2. MC3T3-E1 Cell Test in Vitro
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao-hong, R.; Run-guang, L.; Gui-yong, J.; Chao-jie, C.; Zhi-gang, B. A solution to the vessel shortage during free vascularized fibular grafting for reconstructing infected bone defects of the femur: Bridging with vein transplantation. Injury 2017, 48, 486–494. [Google Scholar] [CrossRef]
- Olesen, U.K.; Eckardt, H.; Bosemark, P.; Paulsen, A.W.; Dahl, B.; Hede, A. The Masquelet technique of induced membrane for healing of bone defects. A review of 8 cases. Injury 2015, 46, S44–S47. [Google Scholar] [CrossRef]
- Textor, M.; Sittig, C.; Frauchiger, V.; Tosatti, S.; Brunette, D.M. Properties and Biological Significance of Natural Oxide Films on Titanium and Its Alloys. In Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springe: Berlin/Heidelberg, Germany, 2001; pp. 171–230. [Google Scholar]
- Xie, Y.; Li, J.; Yu, Z.M.; Wei, Q. Nano modified SLA process for titanium implants. Mater. Lett. 2017, 186, 38–41. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Zadeh, A.S.A.H.; Vaezi, M.R.; Mozafari, M. A new double-layer hydroxyapatite/alumina-silica coated titanium implants using plasma spray technique. Surf. Coat. Technol. 2018, 352, 474–482. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Y.; Xu, D.; Zhao, J.; Ke, P.; Wang, A. Bactericidal abilities and in vitro properties of diamond-like carbon films deposited onto MAO-treated titanium. Mater. Lett. 2019, 244, 155–158. [Google Scholar] [CrossRef]
- Kubo, K.; Tsukimura, N.; Iwasa, F.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.-A.; Ogawa, T. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials 2009, 30, 5319–5329. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Du, Z.; Weng, Z.; Yao, W.; Zhang, X.; Huang, X.; Yao, X.; Crawford, R.; Hang, R.; et al. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018, 76, 344–358. [Google Scholar] [CrossRef]
- Hegarty, P.; Walls, A.; O’Brien, S.; Gamble, B.; Cusick, L.; Beverland, D.E. A Prospective Randomized Study Comparing Postoperative Pain, Biological Fixation, and Clinical Outcomes Between Two Uncemented Rotating Platform Tibial Tray Designs. J. Arthroplast. 2019, 35, 429–437. [Google Scholar] [CrossRef]
- Mutsuzaki, H.; Kinugasa, T.; Sakane, M. Biological fixation between calcium phosphate-hybridized tendon graft and tibial bone tunnel in anatomic single-bundle ACL reconstruction—A case report. J. Orthop. 2019, 16, 419–421. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, X.; Yin, S.; Liu, L.; Liu, X.; Zhao, G.; Ma, W.; Qi, W.; Ren, Z.; Liao, H.; et al. Influence of the pore size and porosity of selective laser melted Ti6Al4V ELI porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater. Sci. Eng. C 2020, 106, 110289. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Chang, J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater. Today 2019, 24, 41–56. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2019, 45, 2852–2857. [Google Scholar] [CrossRef]
- Sun, J.; Cai, S.; Wei, J.; Shen, K.; Ling, R.; Sun, J.; Liu, J.; Xu, G. Long-term corrosion resistance and fast mineralization behavior of micro-nano hydroxyapatite coated magnesium alloy in vitro. Ceram. Int. 2020, 46, 824–832. [Google Scholar] [CrossRef]
- Li, B.; Han, Y.; Qi, K. Formation Mechanism, Degradation Behavior, and Cytocompatibility of a Nanorod-Shaped HA and Pore-Sealed MgO Bilayer Coating on Magnesium. ACS Appl. Mater. Interfaces 2014, 6, 18258–18274. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Yang, X.; Shen, Z.; Feng, Q. Effects of hierarchical micro/nano-topographies on the morphology, proliferation and differentiation of osteoblast-like cells. Colloids Surf. B Biointerfaces 2016, 145, 37–45. [Google Scholar] [CrossRef]
- Kung, K.-C.; Lee, T.-M.; Lui, T.-S. Bioactivity and corrosion properties of novel coatings containing strontium by micro-arc oxidation. J. Alloy. Compd. 2010, 508, 384–390. [Google Scholar] [CrossRef]
- Han, Y.; Xu, K.; Lu, J. Morphology and composition of hydroxyapatite coatings prepared by hydrothermal treatment on electrodeposited brushite coatings. J. Mater. Sci. Mater. Med. 1999, 10, 243–248. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Wang, Y.; Liu, Q.; Yang, J.; Zhang, L.; He, F. Fabrication of Al2O3 by anodic oxidation and hydrothermal synthesis of strong-bonding hydroxyapatite coatings on its surface. Appl. Surf. Sci. 2019, 470, 959–969. [Google Scholar] [CrossRef]
- Thiel, B.L.; Toth, M. Secondary electron contrast in low-vacuum/environmental scanning electron microscopy of dielectrics. J. Appl. Phys. 2005, 97, 051101. [Google Scholar] [CrossRef]
- Baker, J.L.; Jimison, L.H.; Mannsfeld, S.; Volkman, S.; Yin, S.; Subramanian, V.; Salleo, A.; Alivisatos, A.P.; Toney, M.F. Quantification of Thin Film Crystallographic Orientation Using X-ray Diffraction with an Area Detector. Langmuir 2010, 26, 9146–9151. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Kung, K.-C.; Yang, C.-Y.; Lee, T.-M.; Lui, T.-S. Engineering three-dimensional structures using bio-inspired dopamine and strontium on titanium for biomedical application. J. Mater. Chem. B 2014, 2, 7927–7935. [Google Scholar] [CrossRef]
- Yang, S.-P.; Wen, H.-S.; Lee, T.-M.; Lui, T.-S. Cell response on the biomimetic scaffold of silicon nano- and micro-topography. J. Mater. Chem. B 2016, 4, 1891–1897. [Google Scholar] [CrossRef]
- Qian, D.-Y.; Yan, G.-B.; Bai, B.; Chen, Y.; Zhang, S.-J.; Yao, Y.-C.; Xia, H. Differential circRNA expression profiles during the BMP2-induced osteogenic differentiation of MC3T3-E1 cells. Biomed. Pharmacother. 2017, 90, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Hu, L.; Li, L.; Jin, W.; Huo, K.; Chu, P.K. Hydrothermal synthesis of perovskite-type MTiO3 (M = Zn, Co, Ni)/TiO2 nanotube arrays from an amorphous TiO2 template. CrystEngComm 2014, 16, 10280–10285. [Google Scholar] [CrossRef]
- Sudo, H.; Kodama, H.A.; Amagai, Y.; Yamamoto, S.; Kasai, S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J. Cell Biol. 1983, 96, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.J.P.; Richards, R.G.; Dalby, M.J. Nanotopographical modification: A regulator of cellular function through focal adhesions. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; He, Y.; He, P.; Luo, X.; Guo, Z.; Li, H. Fabrication of two distinct hydroxyapatite coatings and their effects on MC3T3-E1 cell behavior. Colloids Surf. B Biointerfaces 2018, 171, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Huang, D.; Du, J.; Wei, Y.; Hu, Y.; Lian, X.; Xie, X.; Chen, W.; Zhang, Y.S. Nano-hydroxyapatite crystal formation based on calcified TiO2 nanotube arrays. Appl. Surf. Sci. 2019, 478, 237–246. [Google Scholar] [CrossRef]

| Samples | Temperature (°C) | Time (hour) | V/A Ratio |
|---|---|---|---|
| T150_24H_L_h * | 150 | 24 | 70 |
| T150_24H_L | 150 | 24 | 70 |
| T150_24H_S | 150 | 24 | 7 |
| T175_24H_S | 175 | 24 | 7 |
| T200_24H_S | 200 | 24 | 7 |
| T175_12H_S | 175 | 12 | 7 |
| T175_6H_S | 175 | 6 | 7 |
| T175_2H_S | 175 | 2 | 7 |
| Samples | Atomic Ratio (%) | Ca/P Ratio | |||
|---|---|---|---|---|---|
| O | P | Ca | Ti | ||
| MAO | 71.14 | 2.78 | 1.72 | 24.36 | 0.62 |
| T150_24H_L_h | 72.78 | 1.98 | 0.83 | 24.41 | 0.42 |
| T150_24H_L | 74.59 | 1.79 | 1.99 | 21.63 | 1.11 |
| T150_24H_S | 71.93 | 0.68 | 0.52 | 26.87 | 0.76 |
| T175_24H_S | 71.31 | 1.82 | 5.24 | 21.64 | 2.88 |
| T200_24H_S | 72.10 | 0.70 | 1.51 | 25.69 | 2.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.-C.; Lee, T.-M.; Kuo, N.-W.; Liu, C.; Huang, C.-L. Nano/Micro Hierarchical Bioceramic Coatings for Bone Implant Surface Treatments. Materials 2020, 13, 1548. https://doi.org/10.3390/ma13071548
Chen K-C, Lee T-M, Kuo N-W, Liu C, Huang C-L. Nano/Micro Hierarchical Bioceramic Coatings for Bone Implant Surface Treatments. Materials. 2020; 13(7):1548. https://doi.org/10.3390/ma13071548
Chicago/Turabian StyleChen, Ken-Chung, Tzer-Min Lee, Nai-Wei Kuo, Cheng Liu, and Chih-Ling Huang. 2020. "Nano/Micro Hierarchical Bioceramic Coatings for Bone Implant Surface Treatments" Materials 13, no. 7: 1548. https://doi.org/10.3390/ma13071548
APA StyleChen, K.-C., Lee, T.-M., Kuo, N.-W., Liu, C., & Huang, C.-L. (2020). Nano/Micro Hierarchical Bioceramic Coatings for Bone Implant Surface Treatments. Materials, 13(7), 1548. https://doi.org/10.3390/ma13071548







