Use of Slag from the Combustion of Solid Municipal Waste as A Partial Replacement of Cement in Mortar and Concrete
Abstract
:1. Introduction
- The total content of organic carbon in slags and furnace ash should not exceed 3%.
- The percentage of combustible elements in slags and furnace ash should be less than 5%.
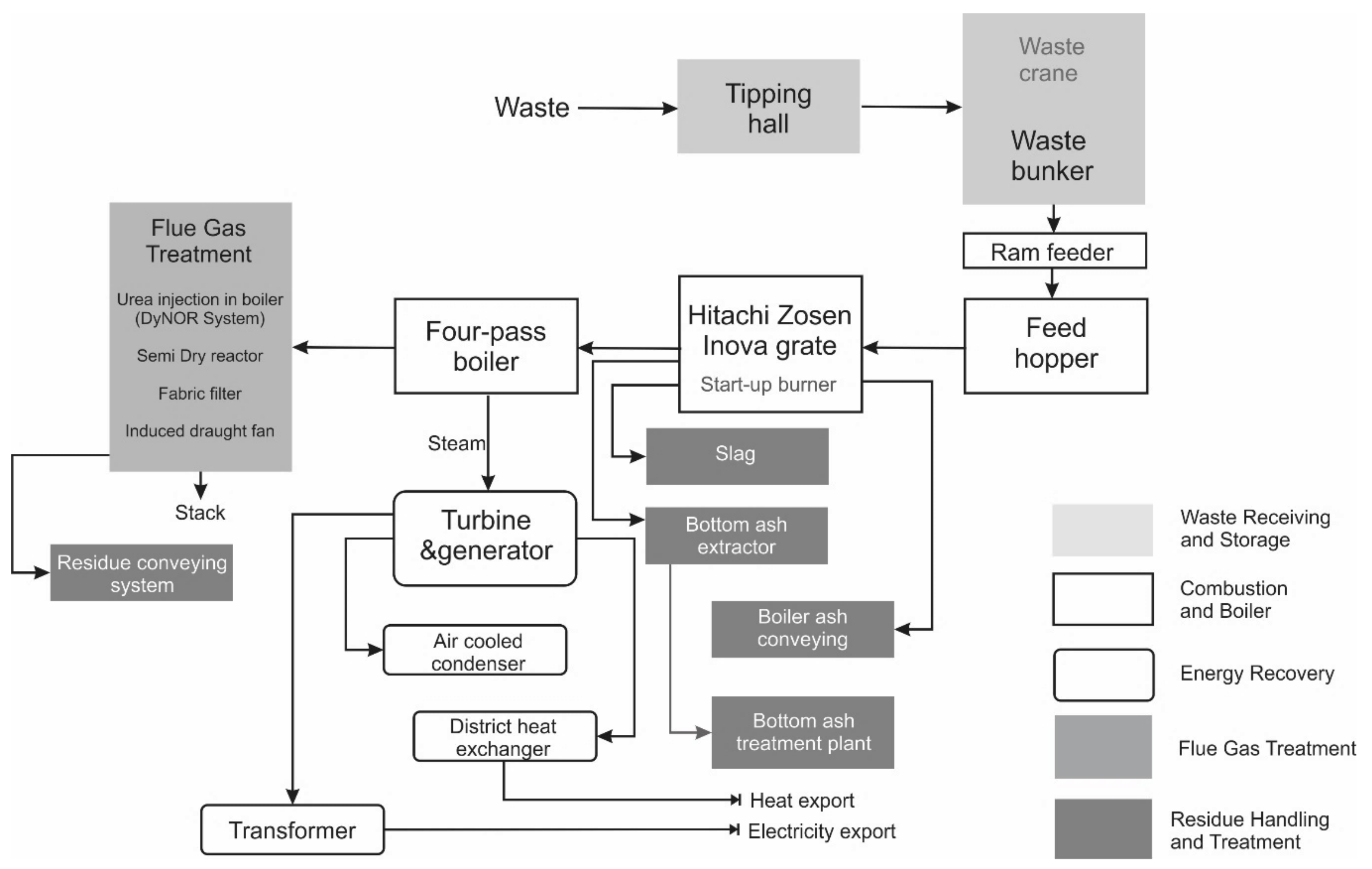
2. The Analyzed Municipal Solid Waste Incineration Plant
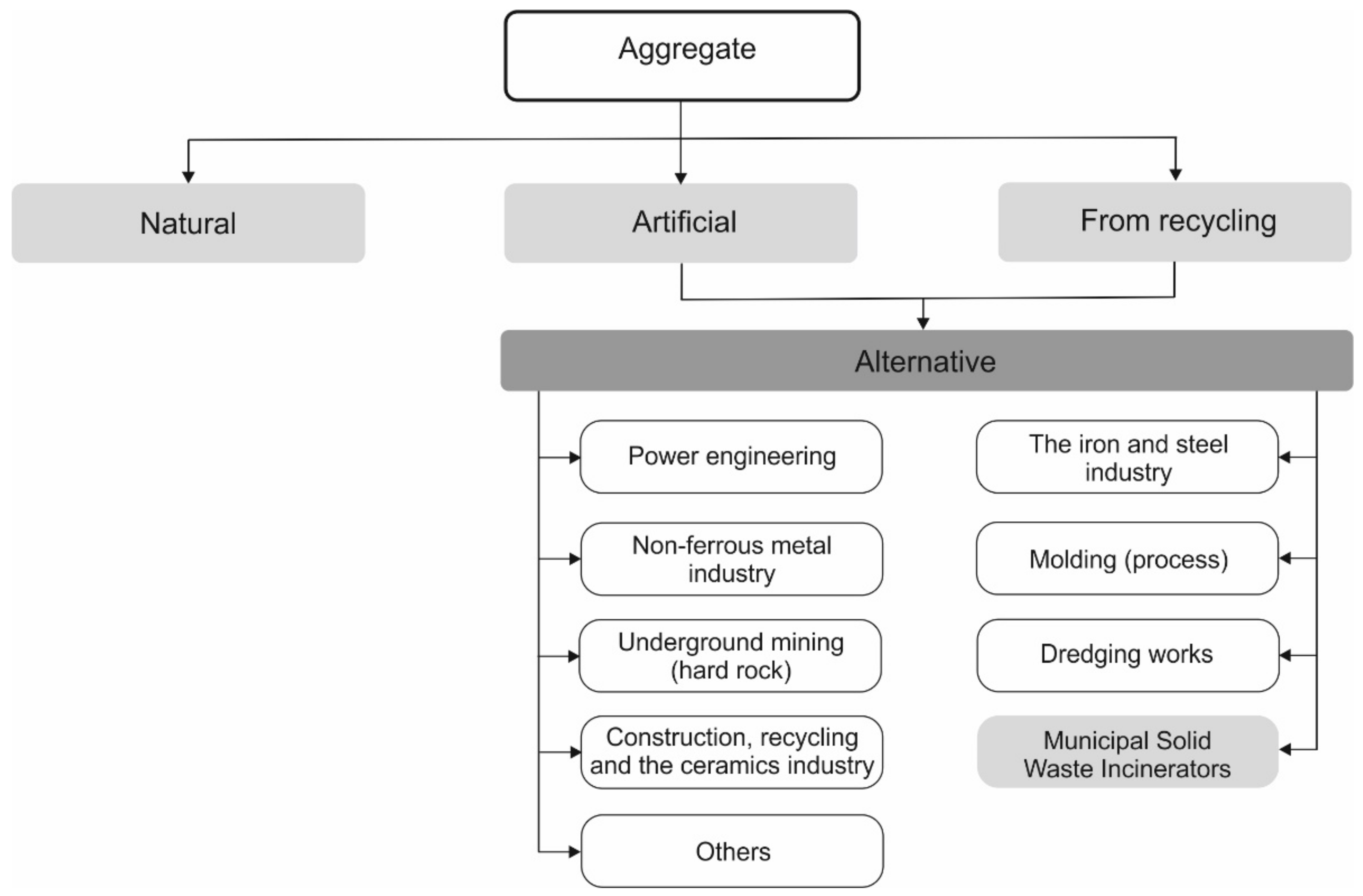
3. MSWI Slag as A Potential Component of Cement for Mortar and Concrete
4. Materials and Methods
4.1. Materials
4.2. Methods
5. Results and Discussion
5.1. Physical and Chemical Properties of MSWI Slag
5.2. The Research Results of Properties of Fresh and Hardened Mortar with MSWI Slag
5.2.1. Properties of Fresh Mortar in the Aspect of Requirements of Slag as An Addition to Mortars
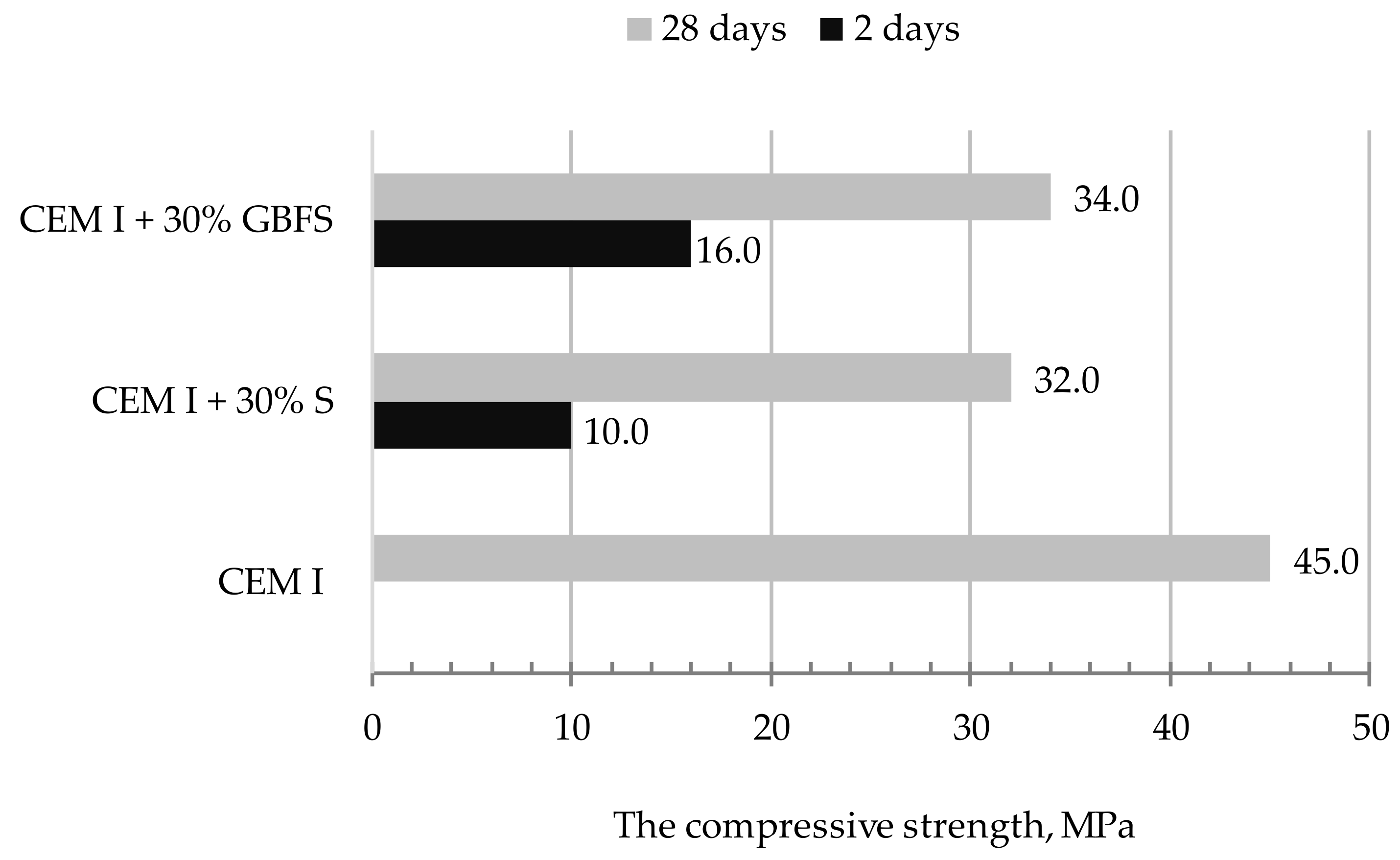
5.2.2. Properties of Hardened Mortar in the Aspect of Requirements of Slag as An Addition to Mortars
5.2.3. The Influence of Mortar with MSWI on Environment Research Results
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eurostat Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Main_Page (accessed on 19 February 2020).
- Czop, M.; Łaźniewska-Piekarczyk, B. Evaluation of the leachability of contaminations of fly ash and bottom ash from the combustion of solid municipal waste before and after stabilization process. Sustainability 2019, 11, 5384. [Google Scholar] [CrossRef] [Green Version]
- EUR-LEX. Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2018.150.01.0109.01.ENG (accessed on 1 March 2020).
- Czop, M.; Łaźniewska-Piekarczyk, B.; Bistuła, D.; Górniak, A.; Nowak, S.Z. Mechanical Properties of Cement Concrete Executed with the Dust from the Purification of Exhausts Following the Thermal Transformation of the Municipal Wastes. In Proceedings of the19th SGEM International Multidisciplinary Scientific GeoConference Green Buildings Technologies and Materials, Albena, Bulgaria, 28 June–7 July 2019. [Google Scholar]
- Polski Rynek Węgla. Minerały Antropogeniczne a Gospodarka o Obiegu Zamkniętym. Available online: https://polskirynekwegla.pl/sites/default/files/elfinder/GOZ/mineraly-antropogeniczne.pdf (accessed on 21 February 2020).
- European Commission. Best Available Techniques (BAT) Reference Document on Waste Incineration. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/best-available-techniques-bat-reference-document-waste-treatment-industrial-emissions (accessed on 21 February 2020).
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burke, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef] [Green Version]
- Robbie, M.A. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195. [Google Scholar]
- Materials provided by an industrial partner—SUEZ Poland.
- European Commission. Commission Decision of 18 December 2014 Amending Decision 2000/532/EC on the List of Waste Pursuant to Directive 2008/98/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014D0955 (accessed on 1 March 2020).
- Sorlini, S.; Abba, A.; Collivignarelli, C. Recovery of MSWI and soil washing residues of concrete aggregates. Waste Manag. 2011, 31, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.M. Properties of Concrete; Longman: Harlow, UK, 1995. [Google Scholar]
- Ferraris, M.; Salvo, M.; Ventrella, A.; Buzzi, L.; Veglia, M. Use of vitrified MSWI bottom ashes for concrete production. Waste Manag. 2009, 29, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Rübner, K. The microstructure of concrete made with municipal waste incinerator bottom ash as an aggregate component. Cem. Concr. Res. 2006, 36, 1434–1443. [Google Scholar] [CrossRef]
- Pera, J.; Coutaz, L.; Ambroise, J.; Chababbet, M. Use of incinerator bottom ash in concrete. Cem. Concr. Res. 1997, 27, 1–5. [Google Scholar] [CrossRef]
- BS EN 15167–1:2007: Ground Granulated Blast Furnace Slag for Use in Concrete, Mortar and Grout—Part 1: Definitions, Specifications and Conformity Criteria; British Standards Institution (BSI): London, UK, 2007.
- EN 196-1—Methods of Testing Cement—Determination of Strength; European Committee for Standardization (CEN): Brussels, Belgium, 2016.
- EN 197-1:2002, Cement. Część 1: Skład, Wymagania i Kryteria Zgodności Dotyczące Cementów Powszechnego Użytku; Polish Committee for Standardization: Warsaw, Poland, 2002. [Google Scholar]
- Król, A. Release of Heavy Metals From Mineral Composites Considering Environmental Impact; Opole University of Technology: Opole, Poland, 2012. [Google Scholar]
- Król, A. The effect of different exposure conditions on the characteristics of the mineral matrices stabilizing hazardous waste. Ecol. Eng. 2016, 47, 143–150. [Google Scholar] [CrossRef] [Green Version]
- PN Z-15008-02:1993—Determination of Moisture Content; Polish Committee for Standardization: Warsaw, Poland, 1993.
- EN 1097-3:2000—Determination of Bulk Density; European Committee for Standardization (CEN): Brussels, Belgium, 2000.
- EN 196-6:2019-01—Cement Testing Methods—Part 6: Determination of Grinding Degree; European Committee for Standardization (CEN): Brussels, Belgium, 2019.
- EN 15935:2013-02—Determination of Loss on Ignition; European Committee for Standardization (CEN): Brussels, Belgium, 2013.
- EN 196-2:2013-11—Determination of Loss on Ignition; European Committee for Standardization (CEN): Brussels, Belgium, 2013.
- EN 15407:2011—Methods for the Determination of Carbon (C), Hydrogen (H) and Nitrogen (N) Content; European Committee for Standardization (CEN): Brussels, Belgium, 2011.
- EN-Z-15011-3:2001—Municipal Solid Waste Compost—Determination of pH, Content of Organic Substance, Organic Carbon, Nitrogen, Phosphorus and Potassium; European Committee for Standardization (CEN): Brussels, Belgium, 2001.
- EN-ISO 334:1997—Determination of Sulfur with the Eschka Method; International Standardisation Organisation (ISO): Geneva, Switzerland, 1997.
- EN-ISO 587:2000—Determination of Chloride Using the Eschka Mixture; International Standardisation Organisation (ISO): Geneva, Switzerland, 2000.
- EN-ISO 9964-3:1994—Determination of Sodium, Potassium, Calcium, Lithium and Bar by Flame Photometry; International Standardisation Organisation (ISO): Geneva, Switzerland, 1994.
- EN ISO 15586:2005—Determination of Trace Elements by Atomic Absorption Spectrometry; International Standardisation Organisation (ISO): Geneva, Switzerland, 2005.
- PN-EN 12457-2:2006—Characterization of Waste-Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size Below 4 mm (Without or with Size Reduction); Polish Committee for Standardization: Warsaw, Poland, 2002.
- EN ISO 10523:2012—Determination of pH; International Standardisation Organisation (ISO): Geneva, Switzerland, 2012.
- EN 27888:1999—Determination of Electrical Conductivity; European Committee for Standardization (CEN): Brussels, Belgium, 1999.
- EN 1484:1999—Guidelines for the Determination of Total Organic Carbon (TOC); European Committee for Standardization (CEN): Brussels, Belgium, 1999.
- PN-ISO 9297:1994—Determination of Chloride Ion Concentration by Titration (Mohr’s Method); Polish Committee for Standardization: Warsaw, Poland, 1994.
- PN-ISO 9280:2002—Determination of Sulphates (VI)—Gravimetric Method with Barium Chloride; Polish Committee for Standardization: Warsaw, Poland, 2002.
- EN 1015-3—Methods of Test for Mortar for Masonry—Determination of Consistence of Fresh Mortar (by Flow Table); European Committee for Standardization (CEN): Brussels, Belgium, 2007.
- EN 1015-7:1999—Methods of Test for Mortar for Masonry—Determination of Air Content of Fresh Mortar; European Committee for Standardization (CEN): Brussels, Belgium, 1999.
- EN 196-3:2016—Methods of Testing Cement—Determination of Setting Times and Soundness; European Committee for Standardization (CEN): Brussels, Belgium, 2016.
- The Council of the European Union. Council Decision of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:011:0027:0049:EN:PDF (accessed on 1 March 2020).
- Cornelis, G.; Johnson, C.A.; Van Gerven, T.; Vandecasteele, C. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: A review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
- Bertolini, L.; Carsana, M.; Cassago, D.; Curzio, A.Q.; Collepardi, M. MSWI ashes as mineral additions in concrete. Cem. Concr. Res. 2004, 34, 1899–1906. [Google Scholar] [CrossRef]
- Forteza, R.; Far, M.; Segul, C.; Cerda, V. Characterization of bottom ash in municipal solid waste incinerators for its use in road base. Waste Manag. 2004, 24, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Ginés, O.; Chimenos, J.M.; Vizcarro, A.; Formosa, J.; Rosell, J.R. Combined use of MSWI bottom ash and fly ash as aggregate in concrete formulation: Enviromental and mechanical considerations. J. Hazard. Mater. 2009, 169, 643–650. [Google Scholar] [CrossRef] [PubMed]








| Parameter | Symbol | Unit | Standard Requirement [16] |
|---|---|---|---|
| Specific surface area | − | cm2/g | ≥ 2750.0 |
| Magnesium oxide | MgO | % | 18.0 |
| Sulfide | S2− | ≤ 2.0 | |
| Vitreous phase | − | ≥ 67.0 | |
| Sulfates | SO3 | ≤ 2.5 | |
| Loss on ignition | LOI | ≤ 3.0 | |
| Chloride | Cl− | ≤ 0.1 | |
| Moisture | MT | ≤ 1.0 |
| Properties | Symbol | CEM I | Slag-MSWI |
|---|---|---|---|
| Silicon dioxide | SiO2 | 14.00 | 57.90 |
| Iron(III) oxide | Fe2O3 | 3.03 | 4.97 |
| Aluminum oxide | Al2O3 | 7.47 | 10.80 |
| Manganese(II,III) oxide | Mn3O4 | 0.12 | 0.12 |
| Titanium dioxide | TiO2 | 1.09 | 0.50 |
| Calcium oxide | CaO | 51.20 | 12.50 |
| Magnesium oxide | MgO | 1.61 | 1.73 |
| Sulfur trioxide | SO3 | 9.59 | 0.74 |
| Phosphorus pentoxide | P2O5 | 1.01 | 0.74 |
| Sodium oxide | Na2O | 3.05 | 6.61 |
| Potassium oxide | K2O | 3.77 | 0.95 |
| Barium oxide | BaO | 0.14 | 0.14 |
| Strontium oxide | SrO | 0.05 | 0.06 |
| Type of Waste | Symbol of Mortar | CEM I, g | Water, g | Standard Sand, g [17] |
|---|---|---|---|---|
| Reference sample from Portland cement 42.5 R | CEM I | 450 | 225 | 1350 |
| Cement + 30% slag | CEM I + 30% S | 315 | 135 | 1350 |
| Cement + 30% Granulated Blast Furnace Slag | CEM I + 30% GBFS | 315 | 135 | 1350 |
| Properties | Symbol | CEM I | Slag-MSWI |
|---|---|---|---|
| Zinc | Zn | 617.00 | 1621.00 |
| Copper | Cu | 94.70 | 1918.00 |
| Lead | Pb | 87.80 | 687.00 |
| Nickel | Ni | 20.80 | 81.00 |
| Chrome | Cr | 113.00 | 342.00 |
| Cadmium | Cd | 3.30 | 3.35 |
| Arsenic | As | 6.11 | 16.50 |
| Vanadium | V | 34.40 | 30.00 |
| Thallium | Tl | < 1.00 | < 1.00 |
| Mercury | Hg | 0.07 | 0.24 |
| Properties | Symbol | Unit | Slag |
|---|---|---|---|
| Moisture | M | % | 4.48 |
| Bulk density | ρb | kg/m3 | 1700.0 |
| Specific surface area | S | cm2/g | 3200.0 |
| Total carbon | C | % | 2.26 |
| Total organic carbon | TOC | % | 0.52 |
| Sulfur | S | % | 0.78 |
| Chlorine | Cl | % | 0.12 |
| Properties | Symbol | Slag-MSWI | Criteria for Landfills for [41] | |
|---|---|---|---|---|
| Inert Waste | Non-Hazardous Waste | |||
| pH | pH | 7.9 | − | minimum 6 |
| Total Carbon | TC | 118.00 | − | − |
| Total Organic Carbon | TOC | BLQ* | 30000 | − |
| Total Inorganic Carbon | TIC | BLQ* | − | − |
| Chloride | Cl− | 780.00 | 800 | 15000 |
| Sulphate | SO42− | 1157.41 | 1000 | 20000 |
| Phosphate trianion | PO4− | 100.00 | − | − |
| Potassium | K | 354.60 | − | − |
| Calcium | Ca | 878.40 | − | − |
| Lithium | Li | 2.70 | − | − |
| Sodium | Na | 1104.00 | − | − |
| The sum of chloride and sulphate | TDS | 1937.41 | 4000 | 60000 |
| Properties | Symbol | CEM I | Slag-MSWI | Criteria for Landfills for [41] | |
|---|---|---|---|---|---|
| Inert Waste | Non-Hazardous Waste | ||||
| Bar | Ba | BLQ* | BLQ* | 20 | 100 |
| Zinc | Zn | BLQ* | 0.14 | 4 | 50 |
| Copper | Cu | 0.64 | BLQ* | 2 | 50 |
| Lead | Pb | 0.15 | 0.60 | 0.5 | 10 |
| Cadmium | Cd | BLQ* | 0.04 | 0.04 | 1 |
| Chrome | Cr | 50.1 | BLQ* | 0.5 | 10 |
| Cobalt | Co | BLQ* | BLQ* | − | − |
| Iron | Fe | 1.80 | BLQ* | − | − |
| Manganese | Mn | BLQ* | BLQ* | − | − |
| Nickel | Ni | 2.08 | 0.22 | 0.4 | 10 |
| Ingredient Content | The Standard Specification for Content (% by Weight) in Case of GBFS |
|---|---|
| Magnesium oxide (MgO) | ≤ 18.0 |
| Sulfides (S2−) | ≤ 2.0 |
| Vitreous phase | ≥ 67.4 |
| Sulfates (SO3) | ≤ 2.5 |
| Roasting loss | ≤ 3.0 |
| Chlorides (Cl−) | ≤ 1.0 |
| Humidity | ≤ 1.0 |
| Requirements of EN 197-1 | Value |
|---|---|
| content of the vitreous phase | > 95.0% |
| CaO + MgO + SiO2 | ≥ 2/3 |
| Ca + MgO/ SiO2 | ≥ 1.0% |
| Properties | Unit | CEM I | CEM I + 30% S-MSWI | CEM I + 30% GBFS |
|---|---|---|---|---|
| Flow value (initial) | mm | 150.0 | 160.0 | 160.0 |
| Flow value (after 60 min) | mm | 140.0 | 150.0 | 150.0 |
| Air content | % | 2.5 | 3.1 | 2.9 |
| Properties | Symbol | CEM I | CEM I + 30% S | Criteria for Landfills for [41] | |
|---|---|---|---|---|---|
| Inert Waste | Non-Hazardous Waste | ||||
| pH | pH | 8.9 | 11.1 | − | min. 6 |
| Total Carbon | TC | 89.20 | 89.20 | − | − |
| Total Organic Carbon | TOC | 55.80 | 55.80 | 30,000 | − |
| Total Inorganic Carbon | TIC | 33.40 | 33.40 | − | − |
| Chloride | Cl− | 161.28 | 46.08 | 800 | 15,000 |
| Sulphate | SO42− | 633.56 | 292.09 | 1000 | 20,000 |
| Phosphate trianion | PO4− | 20.33 | 24.00 | − | − |
| Potassium | K | 3.56 | 3.00 | − | − |
| Calcium | Ca | 4.97 | 2.54 | − | − |
| Lithium | Li | BLQ* | BLQ* | − | − |
| Sodium | Na | 3.08 | 2.08 | − | - |
| The sum of chlorides and sulphates | TDS | 794.84 | 338.17 | 4000 | 60,000 |
| Properties | Symbol | CEM I | CEM I + 30% S | Criteria for Landfills for [41] | |
|---|---|---|---|---|---|
| Inert Waste | Non-Hazardous Waste | ||||
| Bar | Ba | BLQ* | BLQ* | 20 | 100 |
| Zinc | Zn | BLQ* | BLQ* | 4 | 50 |
| Copper | Cu | BLQ* | BLQ* | 2 | 50 |
| Lead | Pb | 0.11 | 0.22 | 0.5 | 10 |
| Cadmium | Cd | 0.01 | 0.04 | 0.04 | 1 |
| Chrome | Cr | BLQ* | BLQ* | 0.5 | 10 |
| Cobalt | Co | BLQ* | BLQ* | − | − |
| Iron | Fe | BLQ* | BLQ* | − | − |
| Manganese | Mn | 0.04 | BLQ* | − | − |
| Nickel | Ni | BLQ* | 0.84 | 0.4 | 10 |
| Properties | Symbol | CEM I | CEM I + 30% Slag-MSWI | Criteria for Landfills for [41] | |
|---|---|---|---|---|---|
| Inert Waste | Non-Hazardous Waste | ||||
| pH | pH | 12.4 | 12.8 | − | min. 6 |
| Total Carbon | TC | 68.20 | 106.20 | − | − |
| Total Organic Carbon | TOC | 54.90 | 66.40 | 30,000 | − |
| Total Inorganic Carbon | TIC | 13.30 | 39.80 | − | − |
| Chlorides | Cl− | 1198.08 | 944.64 | 800 | 15,000 |
| Sulfate | SO42− | 283.00 | 373.00 | 1000 | 20,000 |
| Phosphate trianion | PO4− | 25.33 | 12.33 | − | − |
| Potassium | K | 299.13 | 479.67 | − | − |
| Calcium | Ca | 590.43 | 1335.33 | − | − |
| Lithium | Li | 2.77 | 4.90 | − | − |
| Sodium | Na | 94.43 | 182.40 | − | − |
| The sum of chlorides and sulphates | TDS | 1200.91 | 948.37 | 4000 | 60,000 |
| Properties | Symbol | CEM I | CEM I + 30% S | Criteria for Landfills for [41] | |
|---|---|---|---|---|---|
| Inert Waste | Non-Hazardous Waste | ||||
| Bar | Ba | BLQ* | BLQ* | 20 | 100 |
| Zinc | Zn | BLQ* | BLQ* | 4 | 50 |
| Copper | Cu | 0.07 | BLQ* | 2 | 50 |
| Lead | Pb | 0.55 | BLQ* | 0.5 | 10 |
| Cadmium | Cd | BLQ* | BLQ* | 0.04 | 1 |
| Chrome | Cr | BLQ* | BLQ* | 0.5 | 10 |
| Cobalt | Co | BLQ* | BLQ* | − | − |
| Iron | Fe | BLQ* | BLQ* | − | − |
| Manganese | Mn | BLQ* | BLQ* | − | − |
| Nickel | Ni | 0.13 | 1.87 | 0.4 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czop, M.; Łaźniewska-Piekarczyk, B. Use of Slag from the Combustion of Solid Municipal Waste as A Partial Replacement of Cement in Mortar and Concrete. Materials 2020, 13, 1593. https://doi.org/10.3390/ma13071593
Czop M, Łaźniewska-Piekarczyk B. Use of Slag from the Combustion of Solid Municipal Waste as A Partial Replacement of Cement in Mortar and Concrete. Materials. 2020; 13(7):1593. https://doi.org/10.3390/ma13071593
Chicago/Turabian StyleCzop, Monika, and Beata Łaźniewska-Piekarczyk. 2020. "Use of Slag from the Combustion of Solid Municipal Waste as A Partial Replacement of Cement in Mortar and Concrete" Materials 13, no. 7: 1593. https://doi.org/10.3390/ma13071593
APA StyleCzop, M., & Łaźniewska-Piekarczyk, B. (2020). Use of Slag from the Combustion of Solid Municipal Waste as A Partial Replacement of Cement in Mortar and Concrete. Materials, 13(7), 1593. https://doi.org/10.3390/ma13071593






