Effects of Temperature on Fluidity and Early Expansion Characteristics of Cement Asphalt Mortar
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Testing Methods
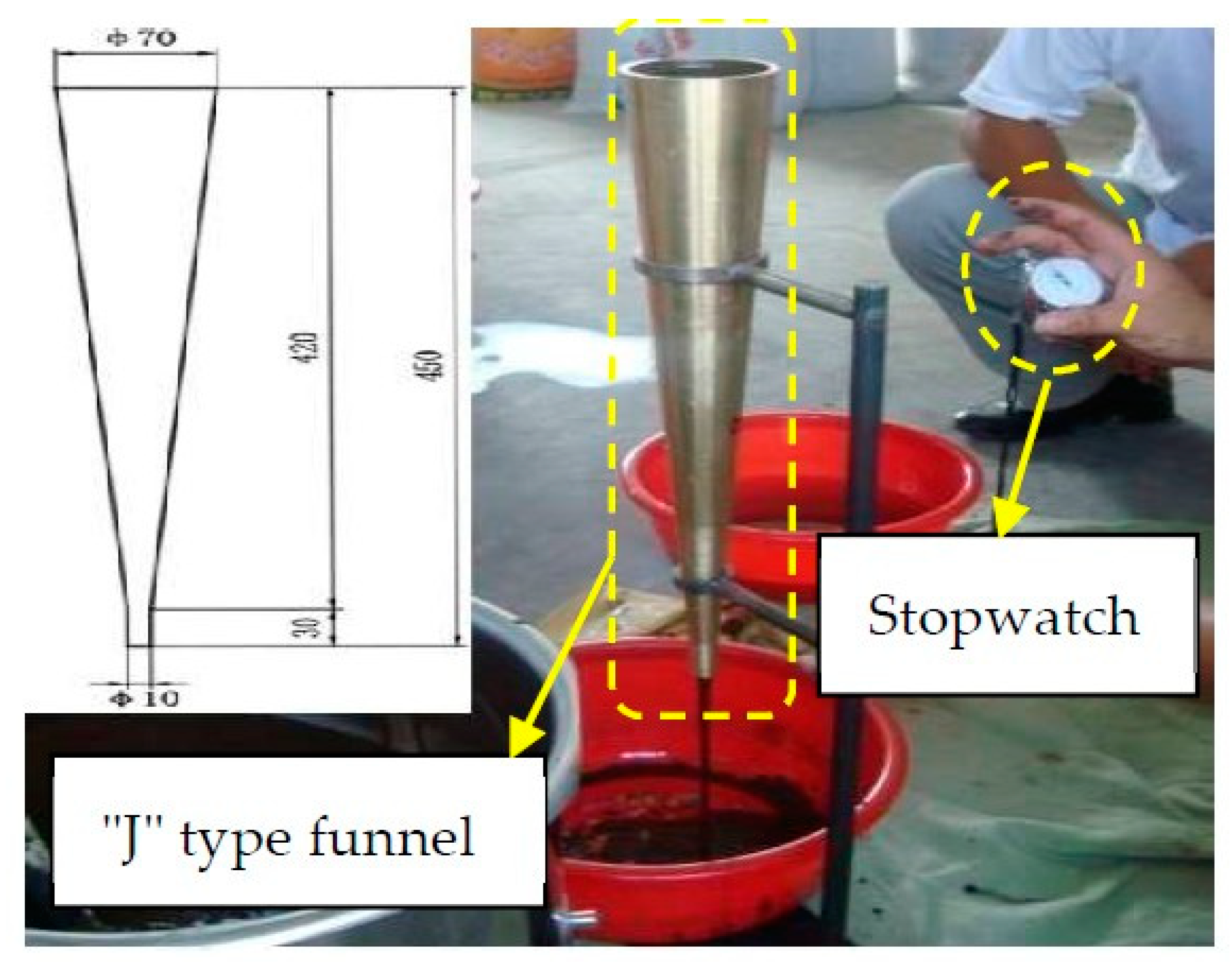
2.2.1. Test of Fluidity
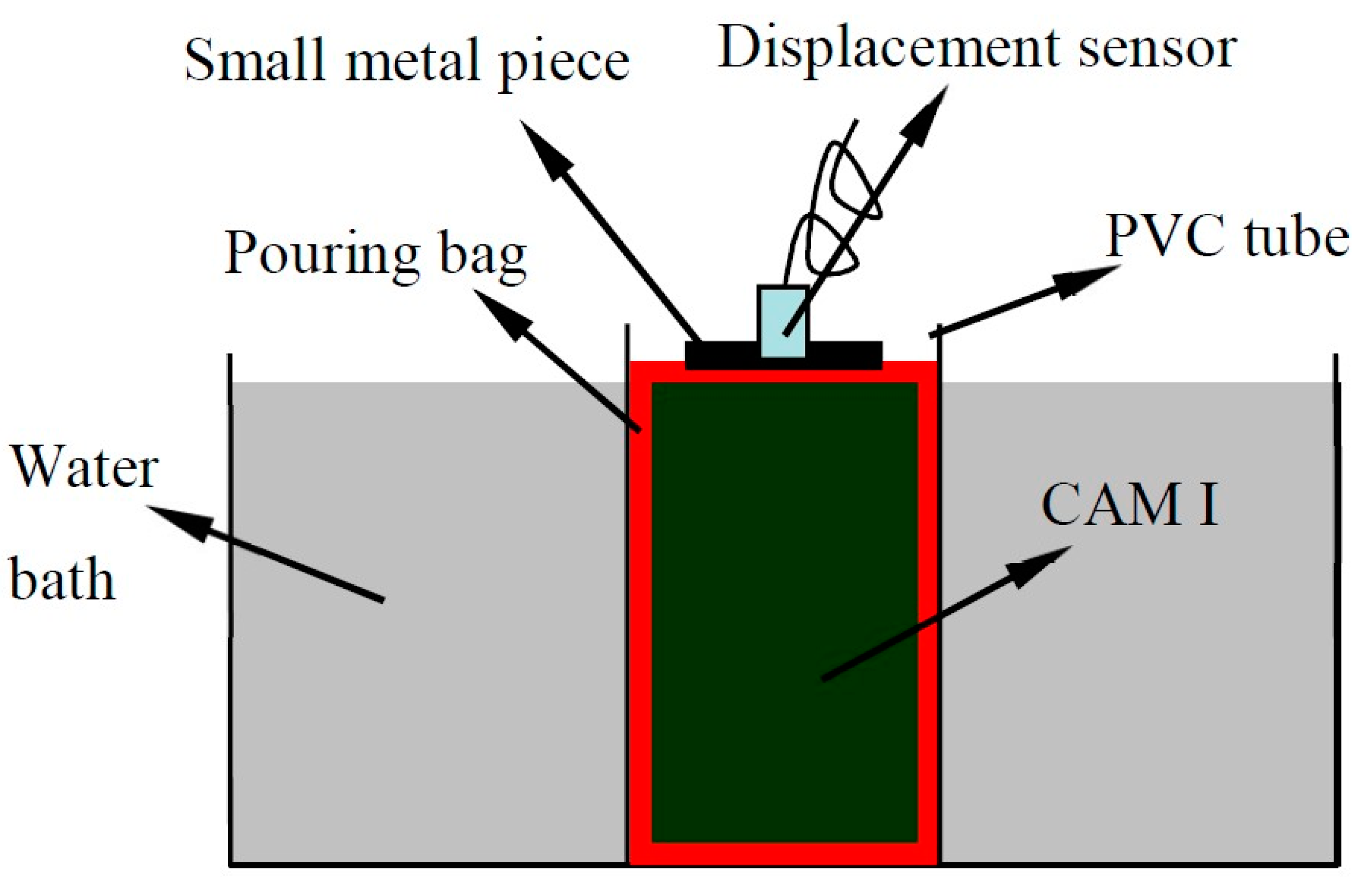
2.2.2. Test of the Early Expansion Ratio
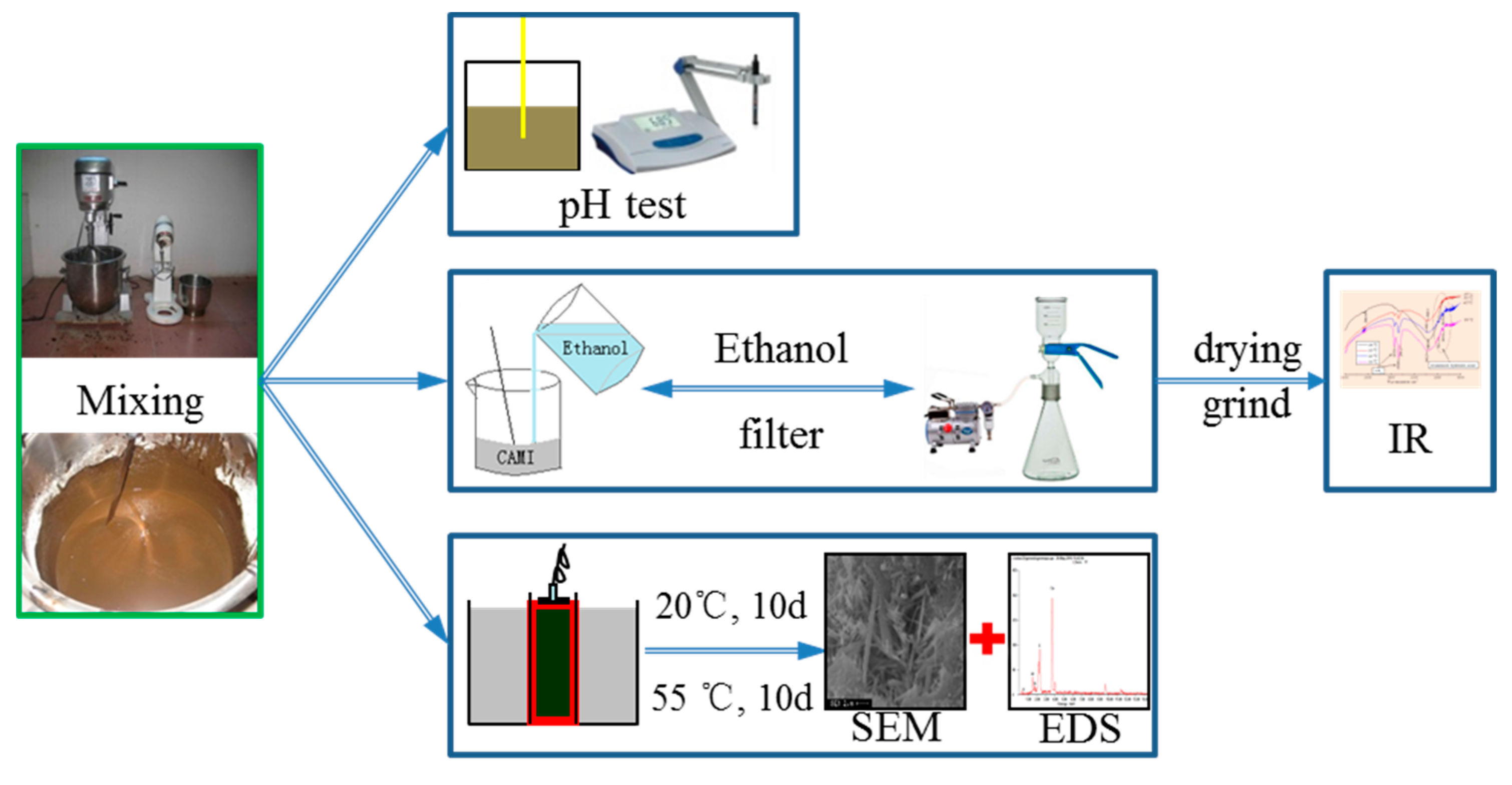
2.2.3. Test of pH Value
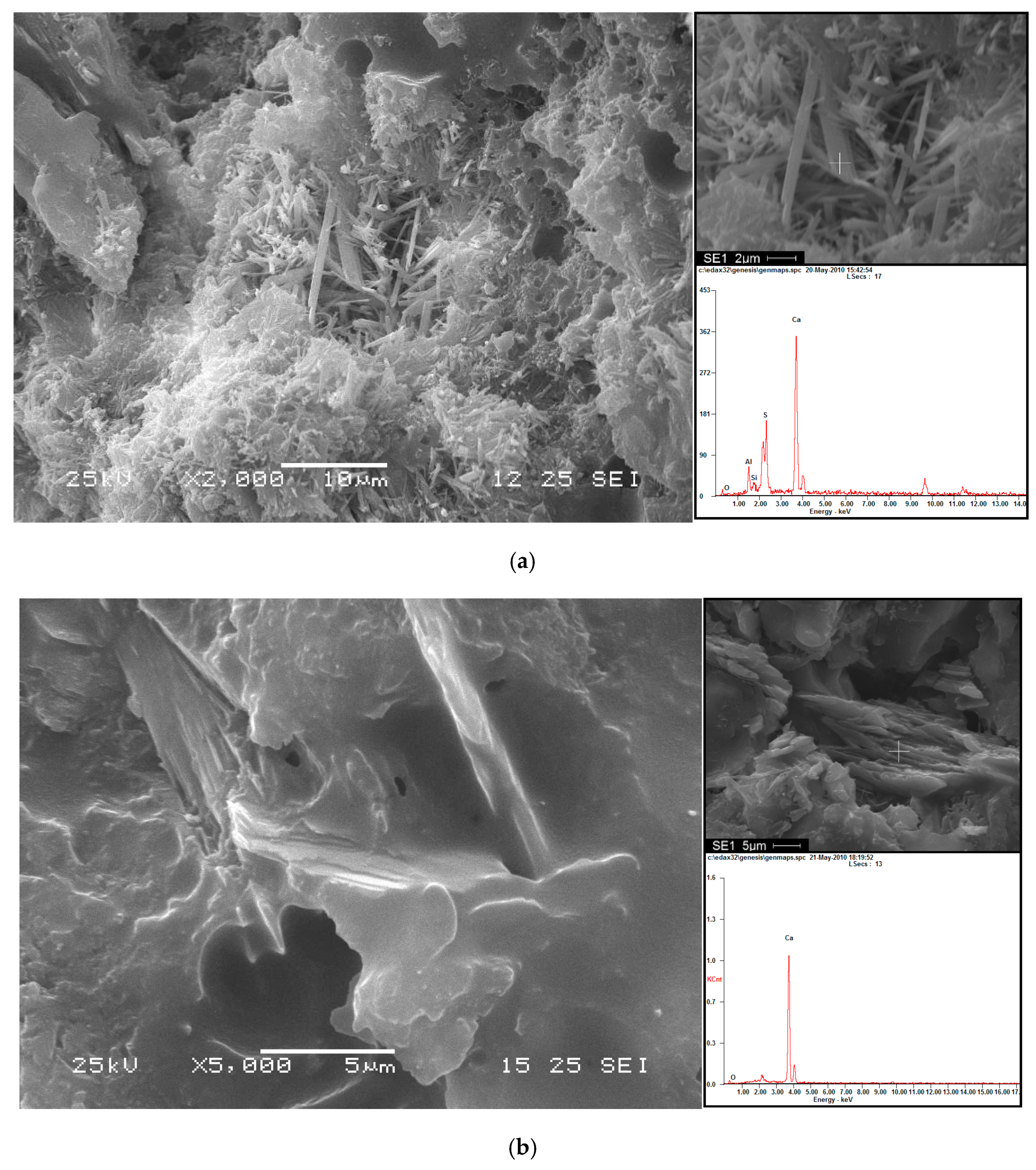
2.2.4. Microstructural Analysis
3. Results and Discussion
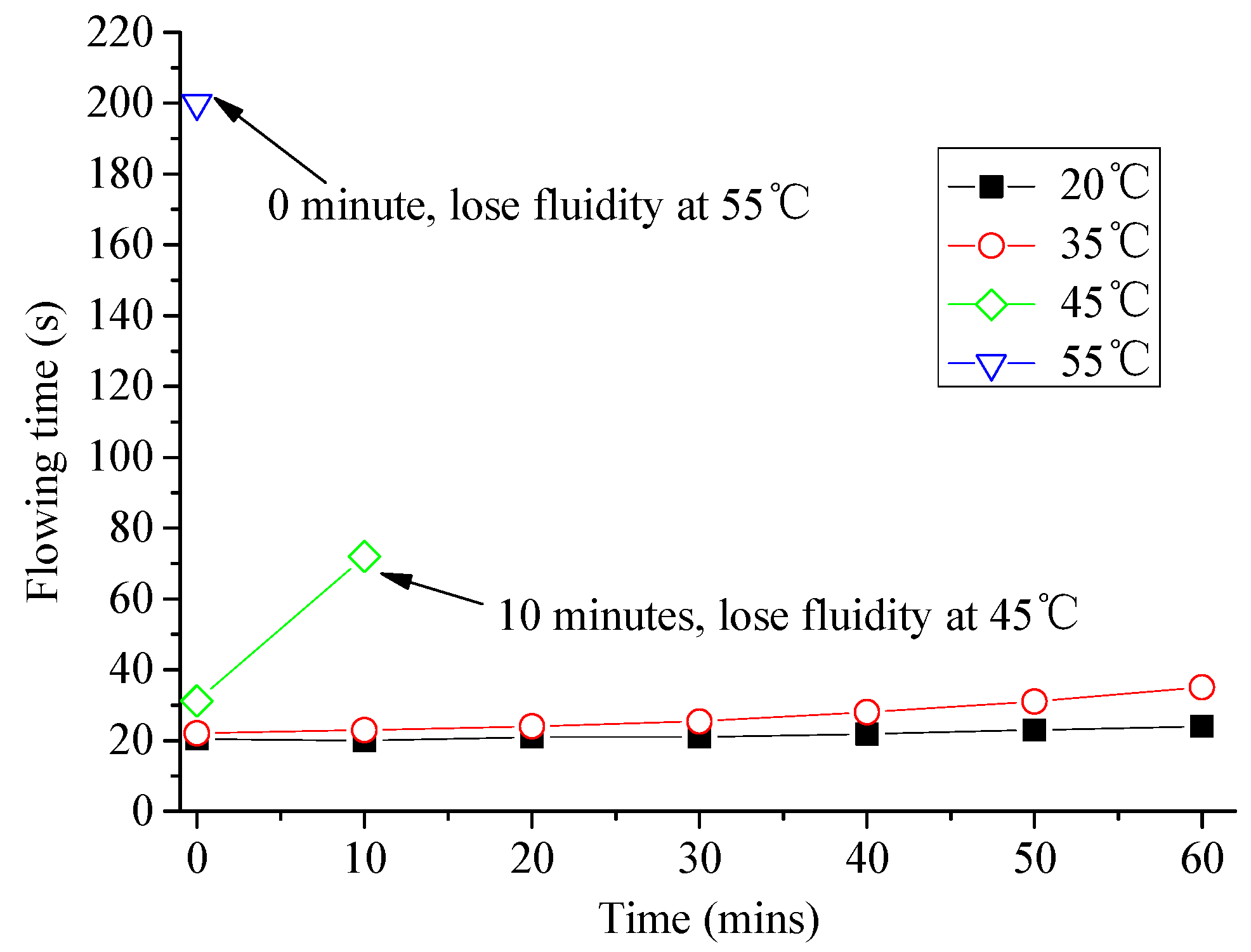
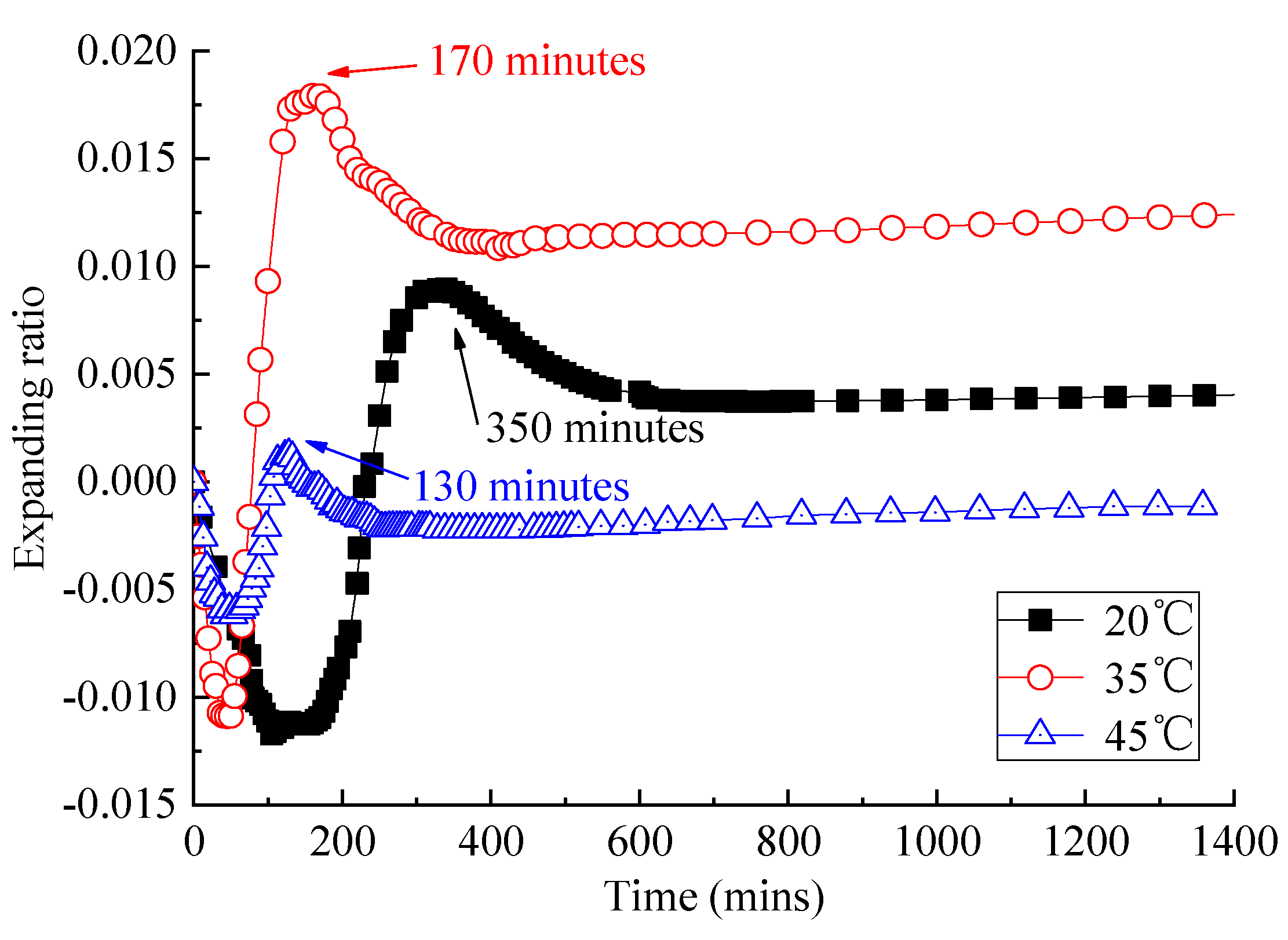
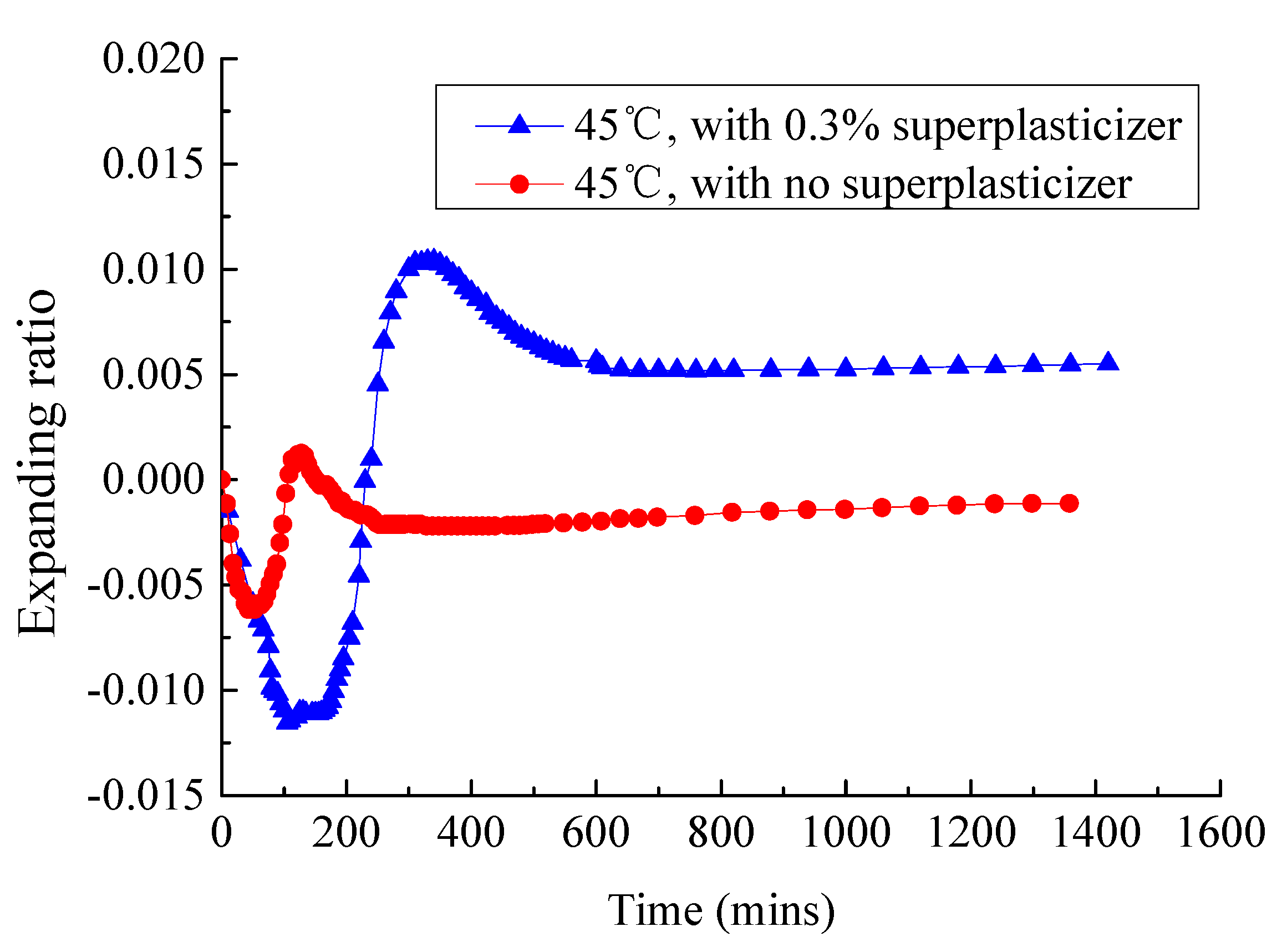
3.1. Analysis of Fluidity and Expansion Ratio of CAM I
3.2. Analysis of pH Value on Different Systems of CAM-I Paste
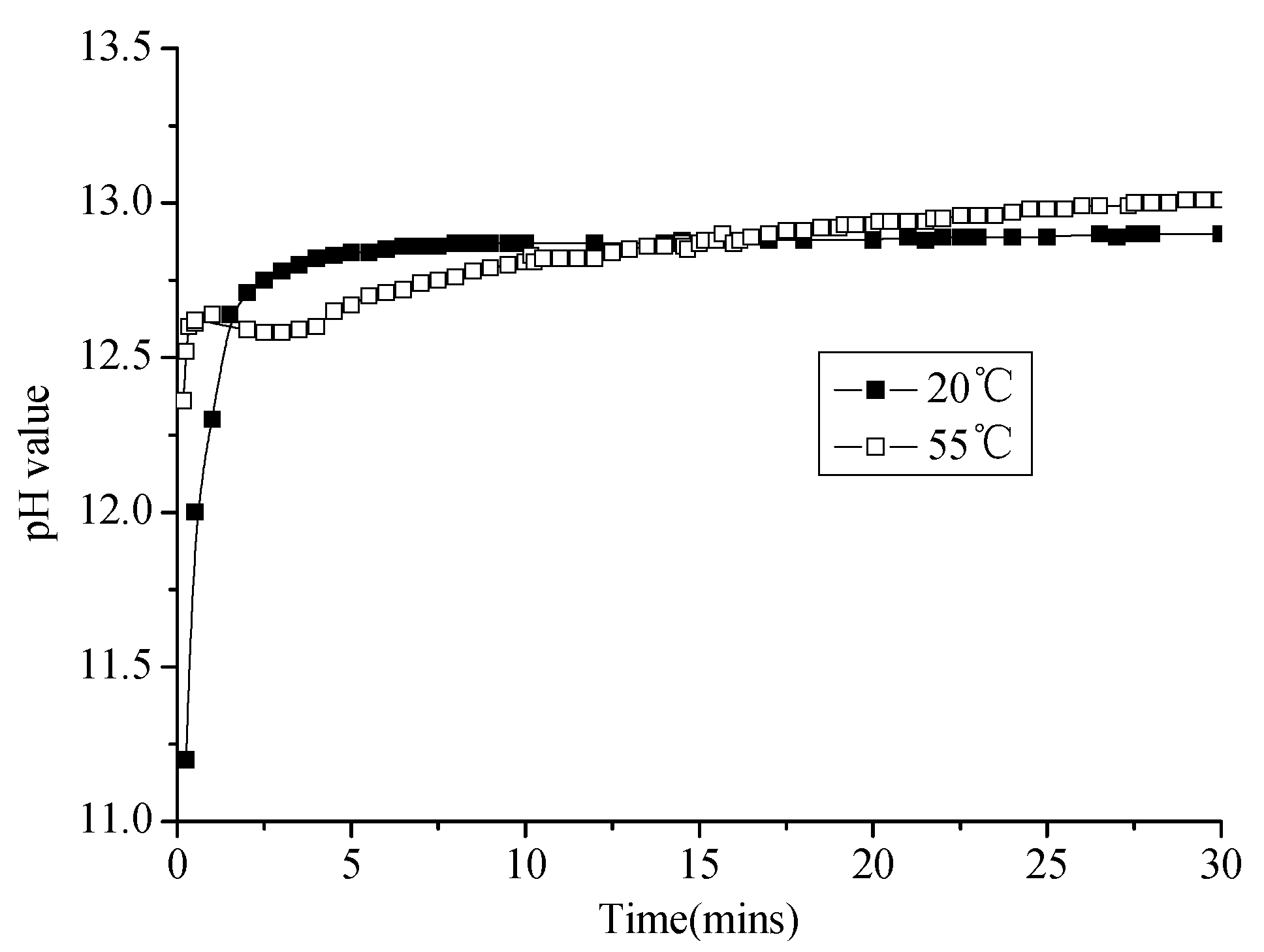
3.2.1. Change of pH Value of Emulsified Asphalt–Sand System
3.2.2. Change of pH Value of Emulsified Asphalt–Water System
3.2.3. Change of pH Value of Emulsified Asphalt–Cement System
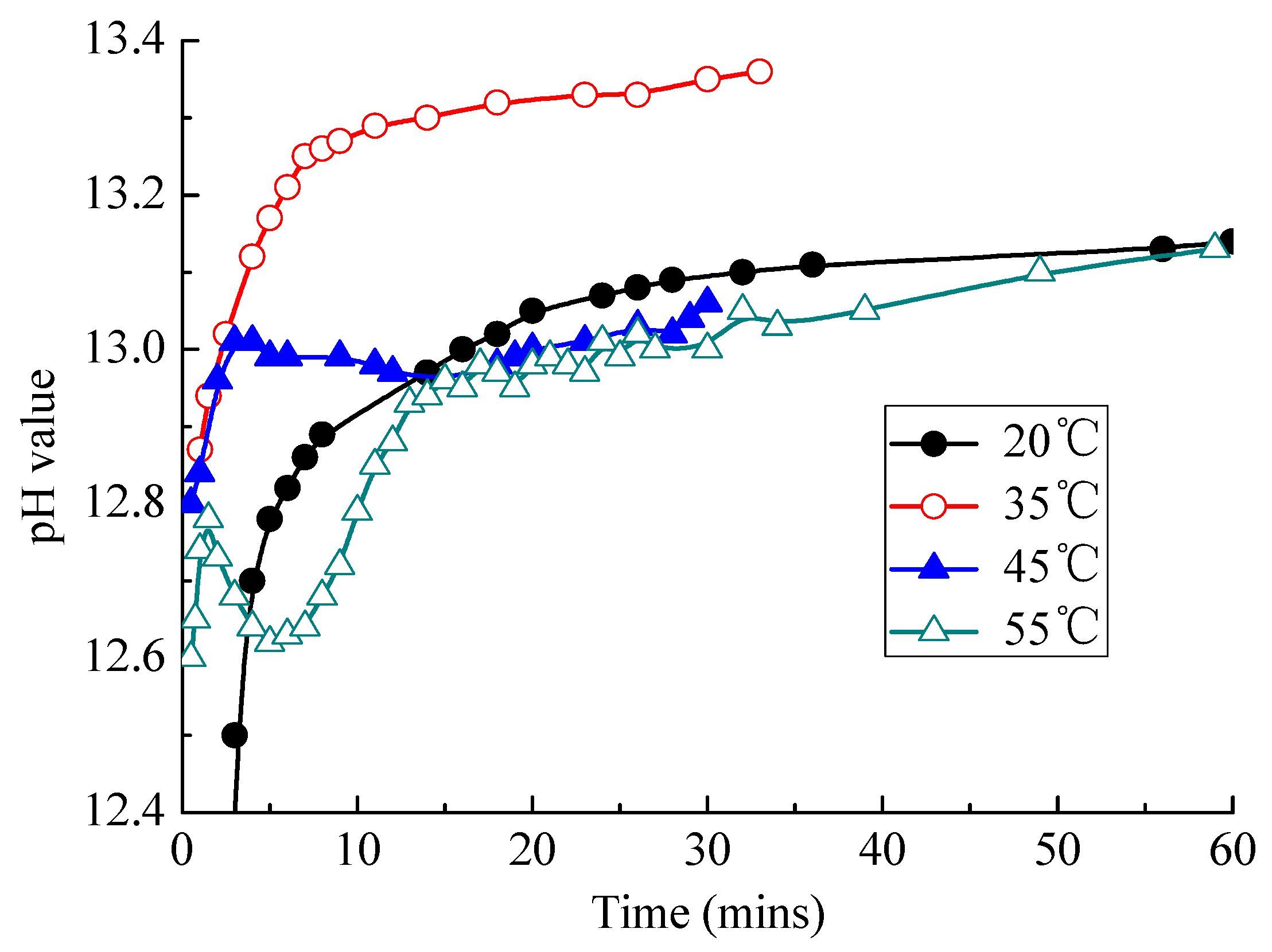
3.2.4. Change of pH Value of CAM I Paste
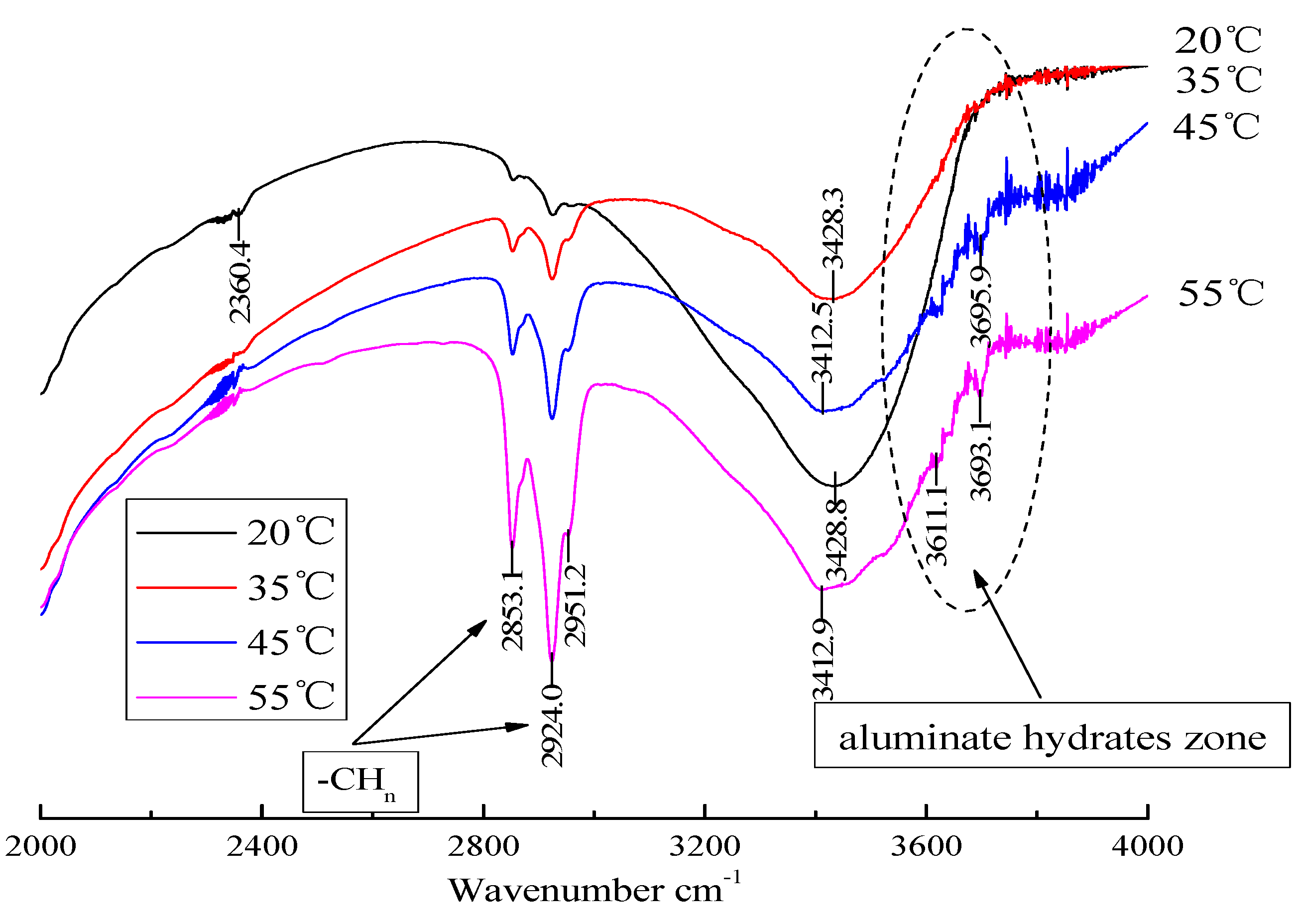
3.3. Analysis of Hydration Phase in CAM-I
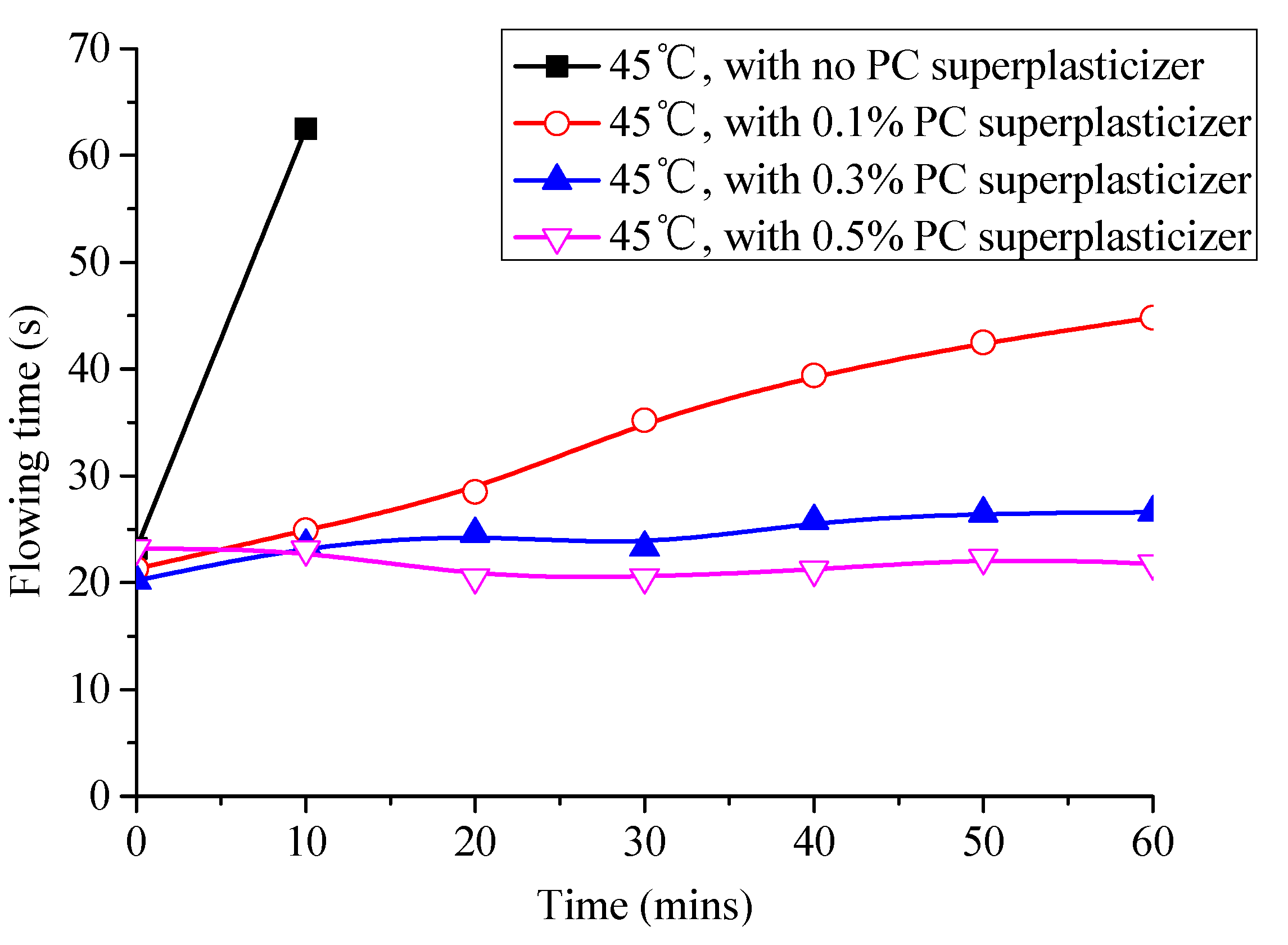
3.4. Effect of Superplasticizer on the Fluidity and Expansion Rate of CAM-I
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coenraad, E. Recent development in slab track. Eur. Railw. Rev. 2003, 9, 81–85. [Google Scholar]
- Miura, S.; Takai, H.; Uchida, M.; Fukada, Y. The mechanism of railway tracks. Jpn. Railw. Transp. Rev. 1998, 15, 38–45. [Google Scholar]
- Katsuoshi, A. Development of slab tracks for Hokuriku Shinkansen line. Q. Rep. RITI 2001, 42, 35–41. [Google Scholar]
- Wang, Y.; Yuan, Q.; Deng, D.; Ye, T.; Fang, L. Measuring the pore structure of cement asphalt mortar by nuclear magnetic resonance. Constr. Build. Mater. 2017, 137, 450–458. [Google Scholar] [CrossRef]
- Xie, Y.J.; Fu, Q.; Zheng, K.R.; Yuan, Q. Dynamic mechanical properties of cement and asphalt mortar based on SHPB test. Constr. Build. Mater. 2014, 70, 217–225. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, F.Z. Effect of Emulsified Asphalt on Temperature Susceptibility of Cement Asphalt Mortar. Adv. Mater. Res. 2011, 335–336, 124–127. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Zeng, X.H.; Chen, R. CA Mortar. Adv. Mater. Res. 2012, 557–559, 860–864. [Google Scholar]
- Wang, F.; Liu, Z.; Wang, T.; Hu, S. Temperature stability of compressive strength of cement asphalt mortar. ACI Mater. J. 2010, 107, 27–30. [Google Scholar]
- Wang, T. Research and Application on CAM I in Ballastless Slab Track of High Speed Railway. Master’s Thesis, Wuhan University of Science and Technology, Wuhan, China, 2008. (In Chinese). [Google Scholar]
- Wang, J.F.; Wu, X.; Fan, X.L.; Chen, Y.R. Stress–strain model of cement asphalt mortar subjected to temperature and loading rate. Constr. Build. Mater. 2016, 111, 164–174. [Google Scholar] [CrossRef]
- Kong, X.M.; Liu, Y.L.; Zhang, Y.R.; Zhang, Z.L.; Yan, P.Y.; Bai, Y. Influences of temperature on mechanical properties of cement asphalt mortars. Mater. Struct. 2014, 47, 285–292. [Google Scholar] [CrossRef]
- Shuguang, H.; Yunhua, Z.; Fazhou, W. Effect of temperature and pressure on the degradation of cement asphalt mortar exposed to water. Constr. Build. Mater. 2012, 34, 570–574. [Google Scholar] [CrossRef]
- Fu, Q.; Xie, Y.J.; Long, G.C.; Meng, F.; Song, H. Temperature sensitivity and model of stress relaxation properties of cement and asphalt mortar. Constr. Build. Mater. 2015, 84, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.; Hou, S.; Liu, Y.; Han, S. Study on the rheological properties of fresh cement asphalt paste. Constr. Build. Mater. 2012, 27, 534–544. [Google Scholar] [CrossRef]
- Liu, Y.P.; Wang, F.Z. Influence of Temperature on the Absorption behaviour between Cement and Asphalt Emulsion in CAM I. Adv. Mater. Res. 2011, 295–297, 939–944. [Google Scholar] [CrossRef]
- Ministry of Railways Science and Technology Department. Provisional Technical Conditions for Cement Asphalt Mortar and Resin Pours for CRTS I Slab Ballastless Track of Passenger Dedicated Railway; Ministry of Railways Science and Technology Department: Beijing, China, 2008.
- Wang, F.; Liu, Z.; Hu, S. Early age volume change of cement asphalt mortar in the presence of aluminum powder. Mater. Struct. 2010, 43, 493–498. [Google Scholar] [CrossRef]
- Cosgrove, T. Surfaces, Interfaces and Colloids: Principles and Applications; VCH Publishers: New York, NY, USA, 1991; pp. 190–210. [Google Scholar]
- Nanru, Y.; Wenhai, Y. Chromatogram Manual of Inorganic Nonmetallic Materials; Wuhan University of Technology: Wuhan, China, 2000. (In Chinese) [Google Scholar]
- Barnes, P. Structure and Performance of Cement, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 45–80. [Google Scholar]
- Tian, Q.; Yuan, Q.; Fang, L.; Wang, Y.; Liu, Z.; Deng, D. Estimation of elastic modulus of cement asphalt binder with high content of asphalt. Constr. Build. Mater. 2017, 133, 98–106. [Google Scholar] [CrossRef]
- Rutherford, T.; Wang, Z.; Shu, X.; Huang, B.; Clarke, D. Laboratory investigation into mechanical properties of cement emulsified asphalt mortar. Constr. Build. Mater. 2014, 65, 76–83. [Google Scholar] [CrossRef]
- Corstanje, W.A.; Stein, H.N.; Stevels, J.M. Hydration reactions in pastes C3S + C3A + CaSO4.2aq + water at 25°C. II. Cem. Concr. Res. 1974, 4, 193–202. [Google Scholar] [CrossRef]
- Skalny, J.; Tadros, M.E. Retardation of calcium aluminate hydration by sulphate. J. Am. Ceram. Soc. 1977, 60, 174–175. [Google Scholar] [CrossRef]
- Lu, C.T.; Kuo, M.F.; Shen, D.H. Composition and reaction mechanism of cement-asphalt mastic. Constr. Build. Mater. 2009, 23, 3580–3585. [Google Scholar] [CrossRef]
- Plank, J.; Winter, C. Competitive adsorption between superplasticizer and retarder molecules on mineral binder surface. Cem. Concr. Res. 2008, 38, 599–605. [Google Scholar] [CrossRef]
- Yamada, K.; Shoichi, O.; Hanehara, S. Controlling of the adsorption and dispersing force of polycarboxylate-type superplasticizer by sulfate ion concentration in aqueous phase. Cem. Concr. Res. 2001, 31, 375–383. [Google Scholar] [CrossRef]
- Peng, J.; Deng, D.; Huang, H.; Yuan, Q.; Peng, J. Influence of superplasticizer on the rheology of fresh cement asphalt paste. Case Stud. Constr. Mater. 2015, 3, 9–18. [Google Scholar] [CrossRef]
- Shian, L.; Donghong, L. Solution to the Problem of CRTSII Type Slab Ballastless Track Cement Emulsified Asphalt Mortar for Passenger Dedicated Railway; China Railway Publishing House: Beijing, China, 2009. [Google Scholar]
- Zhang, Y.R.; Kong, X.M.; Lu, Z.B.; Lu, Z.C.; Hou, S.S. Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retardation in cement pastes. Cem. Concr. Res. 2015, 67, 184–196. [Google Scholar] [CrossRef]





| Aluminum Powder | Portland Cement | Sand | 24 h Volume Expansion Rate | 7 Days Line Expansion Rate | 1 Day Compressive Strength |
|---|---|---|---|---|---|
| 10~50 g/m3 | 300~400 kg/m3 | 600~700 kg/m3 | 2.5% | 0.1% | 6.8 MPa |
| Type of Emulsified Asphalt | Solid Content | Viscosity (25 °C) | Storage Stability (1 Day, 25 °C) | Penetration (25 °C, 100 g) | Softening Point/°C |
|---|---|---|---|---|---|
| cationic | 58~63% | 6 | 0.6 | 68 | 56 |
| Emulsified Asphalt (kg/m3) | Dry Materials (kg/m3) | Water (kg/m3) |
|---|---|---|
| 550 | 1000 | 50 |
| NO. | Samples | 20 °C | 35 °C | 45 °C | 55 °C |
|---|---|---|---|---|---|
| 1 | fluidity of CAM I without superplasticizer | Y | Y | Y | Y |
| 2 | expansion ratio of CAM I without superplasticizer | Y | Y | Y | |
| 3 | pH value of Emulsified Asphalt-Sand system | Y | Y | ||
| 4 | pH value of Emulsified Asphalt-Water system | Y | Y | ||
| 5 | pH value of Emulsified Asphalt-Cement system | Y | Y | ||
| 6 | pH value of CAM I | Y | Y | Y | Y |
| 7 | fluidity of CAM I with superplasticizer | Y | |||
| 8 | expansion ratio of CAM I with superplasticizer | Y | |||
| 9 | IR | Y | Y | Y | Y |
| 10 | SEM | Y | Y |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Zhu, H.; Lan, X.; Liu, H.; Umar, H.A.; Xie, Y.; Long, G.; Ma, C. Effects of Temperature on Fluidity and Early Expansion Characteristics of Cement Asphalt Mortar. Materials 2020, 13, 1655. https://doi.org/10.3390/ma13071655
Zeng X, Zhu H, Lan X, Liu H, Umar HA, Xie Y, Long G, Ma C. Effects of Temperature on Fluidity and Early Expansion Characteristics of Cement Asphalt Mortar. Materials. 2020; 13(7):1655. https://doi.org/10.3390/ma13071655
Chicago/Turabian StyleZeng, Xiaohui, Huasheng Zhu, Xuli Lan, Haichuan Liu, H.A Umar, Youjun Xie, Guangcheng Long, and Cong Ma. 2020. "Effects of Temperature on Fluidity and Early Expansion Characteristics of Cement Asphalt Mortar" Materials 13, no. 7: 1655. https://doi.org/10.3390/ma13071655
APA StyleZeng, X., Zhu, H., Lan, X., Liu, H., Umar, H. A., Xie, Y., Long, G., & Ma, C. (2020). Effects of Temperature on Fluidity and Early Expansion Characteristics of Cement Asphalt Mortar. Materials, 13(7), 1655. https://doi.org/10.3390/ma13071655







