3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold Design
2.2. Scaffold Fabrication
2.3. Examination of Blood Penetration and Hematoma Formation
2.4. Saos-2 Cell Cultivation
2.5. Scaffold Functionalization, Cell Adhesion, and Cell Seeding
2.6. Cell Viability
2.7. Osteogenic Differentiation
2.7.1. Alizarin Red Staining
2.7.2. Gene Expression Analysis
2.8. Evaluation of Compressive Strength
2.9. Statistics
3. Results
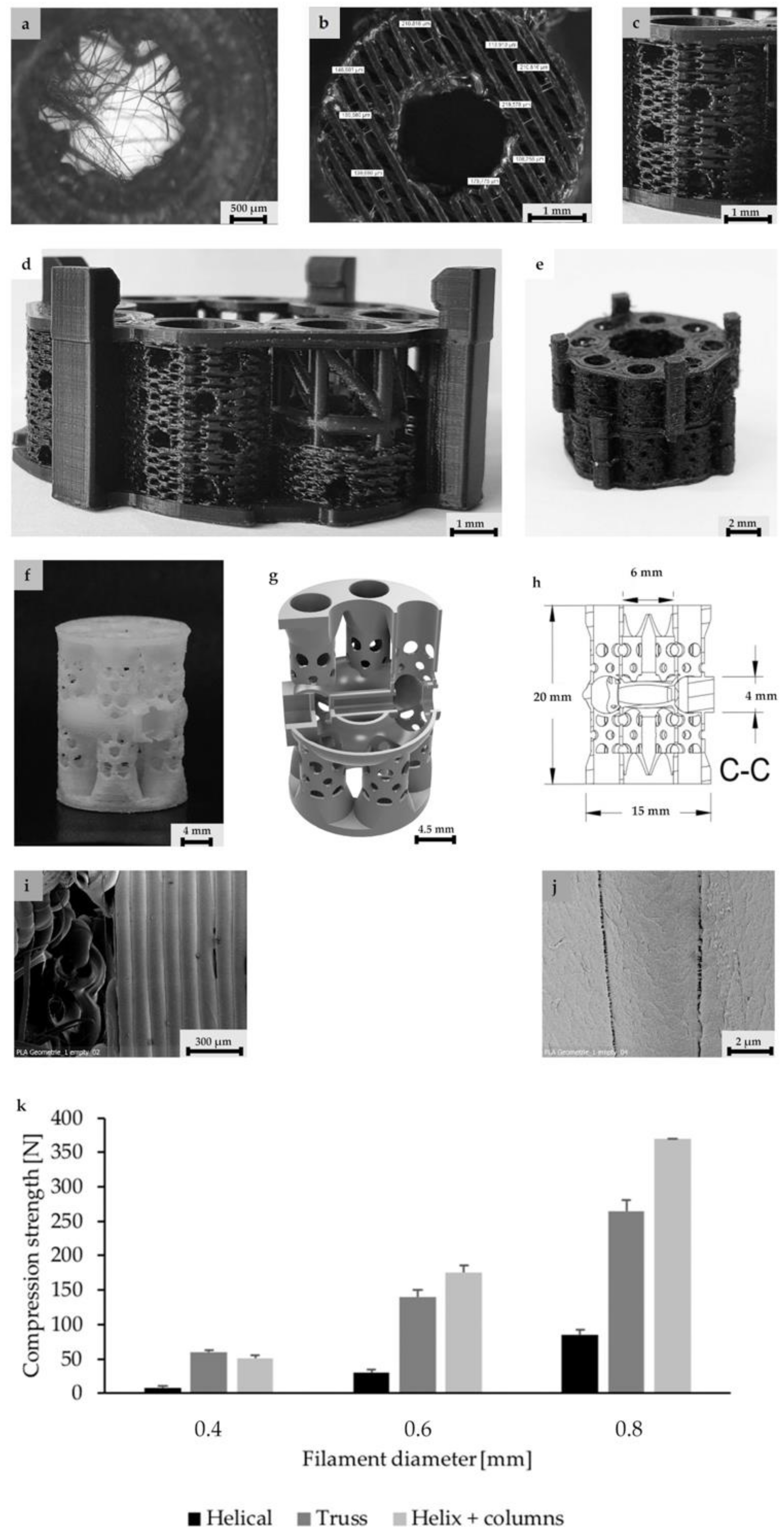
3.1. Design and Fabrication of a Hierarchical Bone TE Scaffold
3.2. Scaffold Architecture, Porosity, and Mechanical Properties
3.3. Examination of Blood Penetration and Hematoma Formation
3.4. Biological Characterization Hierarchically Designed Bone TE Scaffolds
4. Discussion
4.1. Process Development
4.2. Scaffold Design, Mechanics, and Biological Aspects
5. Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nauth, A.; McKee, M.D.; Einhorn, T.; Watson, J.T.; Li, R.; Schemitsch, E.H. Managing Bone Defects. J. Orthop. Trauma 2011, 25, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M.L.V. Autogenous Iliac Crest Bone Graft: Complications and Functional Assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fretwurst, T.; Gad, L.M.; Steinberg, T.; Schmal, H.; Zeiser, R.; Amler, A.-K.; Nelson, K.; Altmann, B. Detection of major histocompatibility complex molecules in processed allogeneic bone blocks for use in alveolar ridge reconstruction. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, B.M.; Rudert, M.; Hutmacher, D.W. Gerüstträgerbasiertes Knochen-Tissue-Engineering. Orthopäde 2017, 46, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Henkel, J.; Woodruff, M.A.; Epari, D.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions — A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- Shrivats, A.R.; McDermott, M.C.; Hollinger, J.O. Bone tissue engineering: State of the union. Drug Discov. Today 2014, 19, 781–786. [Google Scholar] [CrossRef]
- Hollister, S.; Murphy, W.L. Scaffold Translation: Barriers Between Concept and Clinic. Tissue Eng. Part. B: Rev. 2011, 17, 459–474. [Google Scholar] [CrossRef] [Green Version]
- Tayton, E.; Purcell, M.; Smith, J.O.; Lanham, S.; Howdle, S.M.; Shakesheff, K.M.; Goodship, A.; Blunn, G.; Fowler, D.; Dunlop, D.G.; et al. The scale-up of a tissue engineered porous hydroxyapatite polymer composite scaffold for use in bone repair: An ovine femoral condyle defect study. J. Biomed. Mater. Res. Part. A 2014, 103, 1346–1356. [Google Scholar] [CrossRef]
- Smith, J.O.; Tayton, E.R.; Khan, F.; Aarvold, A.; Cook, R.B.; Goodship, A.; Bradley, M.; Oreffo, R.O.C. Large animal in vivo evaluation of a binary blend polymer scaffold for skeletal tissue-engineering strategies; translational issues. J. Tissue Eng. Regen. Med. 2017, 11, 1065–1076. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Roohani, I.; Dunstan, C.; Quach, T.; Steck, R.; Saifzadeh, S.; Pivonka, P.; Zreiqat, H. Efficacy of novel synthetic bone substitutes in the reconstruction of large segmental bone defects in sheep tibiae. Biomed. Mater. 2016, 11, 15016. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.; Chai, Y.; Papantoniou, I.; Geris, L.; Schrooten, J.; Luyten, F.P. Cell based advanced therapeutic medicinal products for bone repair: Keep it simple? Adv. Drug Deliv. Rev. 2015, 84, 30–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bland, E.; Dréau, D.; Burg, K. Overcoming hypoxia to improve tissue-engineering approaches to regenerative medicine. J. Tissue Eng. Regen. Med. 2012, 7, 505–514. [Google Scholar] [CrossRef]
- Malda, J.; Klein, T.; Upton, Z. The Roles of Hypoxia in the In Vitro Engineering of Tissues. Tissue Eng. 2007, 13, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Volkmer, E.; Drosse, I.; Otto, S.; Stangelmayer, A.; Stengele, M.; Kallukalam, B.C.; Mutschler, W.; Schieker, M. Hypoxia in Static and Dynamic 3D Culture Systems for Tissue Engineering of Bone. Tissue Eng. Part. A 2008, 14, 1331–1340. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Chhaya, M.; Melchels, F.P.; Holzapfel, N.P.; Prodinger, P.M.; Von Eisenhart-Rothe, R.; Van Griensven, M.; Schantz, J.-T.; Rudert, M.; Hutmacher, D.W. Can Bone Tissue Engineering Contribute to Therapy Concepts after Resection of Musculoskeletal Sarcoma? Sarcoma 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chow, D.C.; Wenning, L.A.; Miller, W.M.; Papoutsakis, E.T. Modeling pO2 Distributions in the Bone Marrow Hematopoietic Compartment. I. Krogh’s Model. Biophys. J. 2001, 81, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.R.; Nukavarapu, S.P. Oxygen-tension controlled matrices for enhanced osteogenic cell survival and performance. Ann. Biomed. Eng. 2014, 42, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Kalfas, I.H. Principles of bone healing. Neurosurg. Focus 2001, 10, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kellner, K.; Liebsch, G.; Klimant, I.; Wolfbeis, O.S.; Blunk, T.; Schulz-Siegmund, M.; Goepferich, A. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol. Bioeng. 2002, 80, 73–83. [Google Scholar] [CrossRef]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.W.; Xiao, Y. Formation of Blood Clot on Biomaterial Implants Influences Bone Healing. Tissue Eng. Part. B: Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.-S.; Lee, C.-K.; Hong, K.-S.; Youn, H.-J.; Ryu, H.-S.; Chung, S.-S.; Park, K.-W. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials. 2000, 21, 1291–1298. [Google Scholar] [CrossRef]
- Habibovic, P.; Yuan, H.; Van Der Valk, C.M.; Meijer, G.; Van Blitterswijk, C.; De Groot, K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomater. 2005, 26, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, J.; Takita, H.; Ojima, Y.; Kobayashi, M.; Kohgo, T.; Kuboki, Y. Geometric effect of matrix upon cell differentiation: BMP-induced osteogenesis using a new bioglass with a feasible structure. J. Biochem. 2001, 129, 163–171. [Google Scholar] [CrossRef]
- Chang, W.; Mu, X.; Zhu, X.; Ma, G.; Li, C.; Xu, F.; Nie, J. Biomimetic composite scaffolds based mineralization of hydroxyapatite on electrospun calcium-containing poly(vinyl alcohol) nanofibers. Mater. Sci. Eng. C 2013, 33, 4369–4376. [Google Scholar] [CrossRef]
- Basha, R.Y.; Sampath Kumar, T.S.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef]
- Poh, P.S.; Hutmacher, D.W.; Holzapfel, B.M.; Solanki, A.K.; Stevens, M.M.; Woodruff, M.A. In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater. 2016, 30, 319–333. [Google Scholar] [CrossRef]
- Hernandez, I.; Kumar, A.; Joddar, B. A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Tajbakhsh, S.; Hajiali, F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mater. Sci. Eng. 2017, 70, 897–912. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.E.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Schantz, J.T.; Hutmacher, D.W.; Lam, C.X.F.; Brinkmann, M.; Wong, K.M.; Lim, T.C.; Chou, N.; Guldberg, R.E.; Teoh, S.H. Repair of Calvarial Defects with Customised Tissue-Engineered Bone Grafts II. Evaluation of Cellular Efficiency and Efficacyin Vivo. Tissue Eng. 2003, 9, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2014, 11, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Klenke, F.M.; Liu, Y.; Yuan, H.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W.; Hofstetter, W. Impact of pore size on the vascularization and osseointegration of ceramic bone substitutesin vivo. J. Biomed. Mater. Res. Part. A 2008, 85, 777–786. [Google Scholar] [CrossRef]
- Chan, B.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine, J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.C.; Arns, C.H.; Hutmacher, D.W.; Milthorpe, B.K.; Sheppard, A.P.; Knackstedt, M.A. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomater. 2009, 30, 1440–1451. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.-T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real- Time Quantitative PCR and the 2 -ΔΔCT Method. Methods 2001, 408, 402–408. [Google Scholar] [CrossRef]
- Nienhaus, V.; Spiehl, D.; Dörsam, E. Influence of slicer software on transformation of virtual data into porous microstructures. J. 3D Print. Med. 2017, 1, 231–238. [Google Scholar] [CrossRef]
- Siebert, H.; Spiehl, D.; Nienhaus, V.; Krauße, M.; Dörsam, E. Development of a Process for the Production of fine Grid Structures by the use of Fused Filament Fabrication. In Proceedings of the 16th Rapid.Tech Conference, Erfurt, Germany, 25–27 June 2019; pp. 148–161. [Google Scholar]
- Verboket, R.D.; Leiblein, M.; Seebach, C.; Nau, C.; Janko, M.; Bellen, M.; Bonig, H.; Henrich, D.; Marzi, I. Autologous cell-based therapy for treatment of large bone defects: From bench to bedside. Eur. J. Trauma Emerg. Surg. 2018, 44, 649–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrich, D.; Seebach, C.; Verboket, R.D.; Schaible, A.; Marz, I.; Bonig, H. The osteo-inductive activity of bone-marrow-derived mononuclear cells resides within the CD14+ population and is independent of the CD34+ population. Eur. Cells Mater. 2018, 35, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schröder, K.; Seebach, C.; Marzi, I.; Henrich, D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2017, 12, 653–666. [Google Scholar] [CrossRef]
- Nau, C.; Seebach, C.; Trumm, A.; Schaible, A.; Kontradowitz, K.; Meier, S.; Buechner, H.; Marzi, I.; Henrich, D. Alteration of Masquelet’s induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat’s femur. Injury 2016, 47, 325–334. [Google Scholar] [CrossRef]
- Lauer, A.; Wolf, P.; Mehler, D.; Götz, H.; Rüzgar, M.; Baranowski, A.; Henrich, D.; Rommens, P.M.; Ritz, U. Biofabrication of SDF-1 Functionalized 3D-Printed Cell-Free Scaffolds for Bone Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 2175. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, M.A.; Lange, C.; Reichert, J.; Berner, A.; Chen, F.; Fratzl, P.; Schantz, J.-T.; Hutmacher, D.W. Bone tissue engineering: From bench to bedside. Mater. Today 2012, 15, 430–435. [Google Scholar] [CrossRef]
- Habibovic, P.; Gbureck, U.; Doillon, C.J.; Bassett, D.; Van Blitterswijk, C.E.; Barralet, J. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials. 2008, 29, 944–953. [Google Scholar] [CrossRef]
- Xiong, Z. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scr. Mater. 2002, 46, 771–776. [Google Scholar] [CrossRef]
- Hart, N.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet. neuronal interactions 2017, 17, 114–139. [Google Scholar]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts: What they can offer and what they cannot. J. Bone Jt. Surg. 2007, 89, 574–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Eldesoqi, K.; Seebach, C.; Ngoc, C.N.; Meier, S.; Nau, C.; Schaible, A.; Marzi, I.; Henrich, D. High Calcium Bioglass Enhances Differentiation and Survival of Endothelial Progenitor Cells, Inducing Early Vascularization in Critical Size Bone Defects. PLoS ONE 2013, 8, e79058. [Google Scholar] [CrossRef]
- Eldesoqi, K.; Henrich, D.; El-Kady, A.; Arbid, M.; El Hady, B.A.; Marzi, I.; Seebach, C. Safety Evaluation of a Bioglass–Polylactic Acid Composite Scaffold Seeded with Progenitor Cells in a Rat Skull Critical-Size Bone Defect. PLoS ONE 2014, 9, e87642. [Google Scholar] [CrossRef]
- Verboket, R.D.; Anbar, B.; Söhling, N.; Kontradowitz, K.; Marzi, I.; Ghanaati, S.; Henrich, D. Changes in platelet-rich fibrin composition after trauma and surgical intervention. Platelets 2020, 1–11. [Google Scholar] [CrossRef]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’Heureux, N.; Fricain, J.-C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part. A 2017, 106, 887–894. [Google Scholar] [CrossRef]
- Danoux, C.B.; Barbieri, D.; Yuan, H.; De Bruijn, J.D.A.; Van Blitterswijk, C.; Habibovic, P. In vitro and in vivo bioactivity assessment of a polylactic acid/hydroxyapatite composite for bone regeneration. Biomatter 2014, 4, e27664. [Google Scholar] [CrossRef] [Green Version]
- Petersen, A.; Princ, A.; Korus, G.; Ellinghaus, A.; Leemhuis, H.; Herrera, A.; Klaumünzer, A.; Schreivogel, S.; Woloszyk, A.; Schmidt-Bleek, K.; et al. A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects. Nat. Commun. 2018, 9, 4430. [Google Scholar] [CrossRef] [Green Version]
- Fiume, E.; Barberi, J.; Vernè, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef] [Green Version]



| Level | Description | Pore Size | Visualization | Properties |
|---|---|---|---|---|
| 1 | PLA and internal porosity | 1–10 µm |  | Osteoconductive Resorbable Available disinfectable |
| 2 | Microfilamentary mesh | ≤150 µm |  | Osteoinductive Early resorption |
| 3 | Frame with interconnected pores | 0.15–5 mm |  | Supplementation Access Nutrition supply exchange Gas exchange |
| 4 | Tube assembly with central cavity | 15–20 mm |  | Mechanical stability Hematoma formation |
| 5 | Clamp for connectivity | – |  | Modular extendable Customizable |
| Temperature (°C) | Speed (mm/s) | Flow (%) | Infill Line Distance (mm) | Infill Pattern | |
|---|---|---|---|---|---|
| Walls | 195 | 10 | 160 | 0.1 | zig zag |
| Mesh | 195 | 10 | 30 | 0.3 | zig zag |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Söhling, N.; Neijhoft, J.; Nienhaus, V.; Acker, V.; Harbig, J.; Menz, F.; Ochs, J.; Verboket, R.D.; Ritz, U.; Blaeser, A.; et al. 3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds. Materials 2020, 13, 1836. https://doi.org/10.3390/ma13081836
Söhling N, Neijhoft J, Nienhaus V, Acker V, Harbig J, Menz F, Ochs J, Verboket RD, Ritz U, Blaeser A, et al. 3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds. Materials. 2020; 13(8):1836. https://doi.org/10.3390/ma13081836
Chicago/Turabian StyleSöhling, Nicolas, Jonas Neijhoft, Vinzenz Nienhaus, Valentin Acker, Jana Harbig, Fabian Menz, Joachim Ochs, René D. Verboket, Ulrike Ritz, Andreas Blaeser, and et al. 2020. "3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds" Materials 13, no. 8: 1836. https://doi.org/10.3390/ma13081836






