Influence of Oxidation of Copper on Shear Bond Strength to an Acrylic Resin Using an Organic Sulfur Compound
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
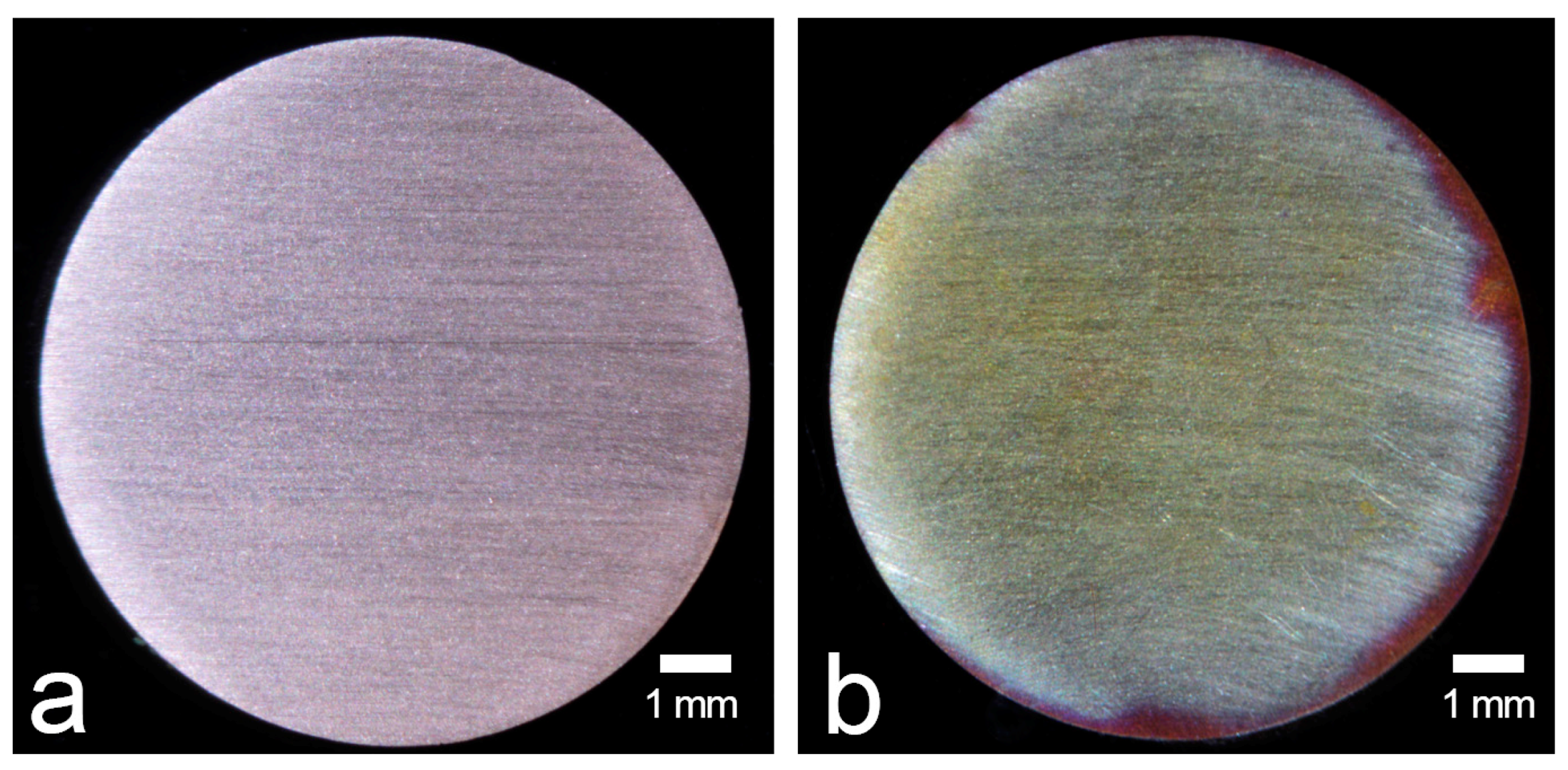
2.2. Preparation of Specimens
2.3. Preparation for Shear Bond Strength Testing
2.4. Statistical Analysis
2.5. X-Ray Photoelectron Spectroscopy (XPS) Analysis
3. Results
3.1. Shear Bond Strength Testing
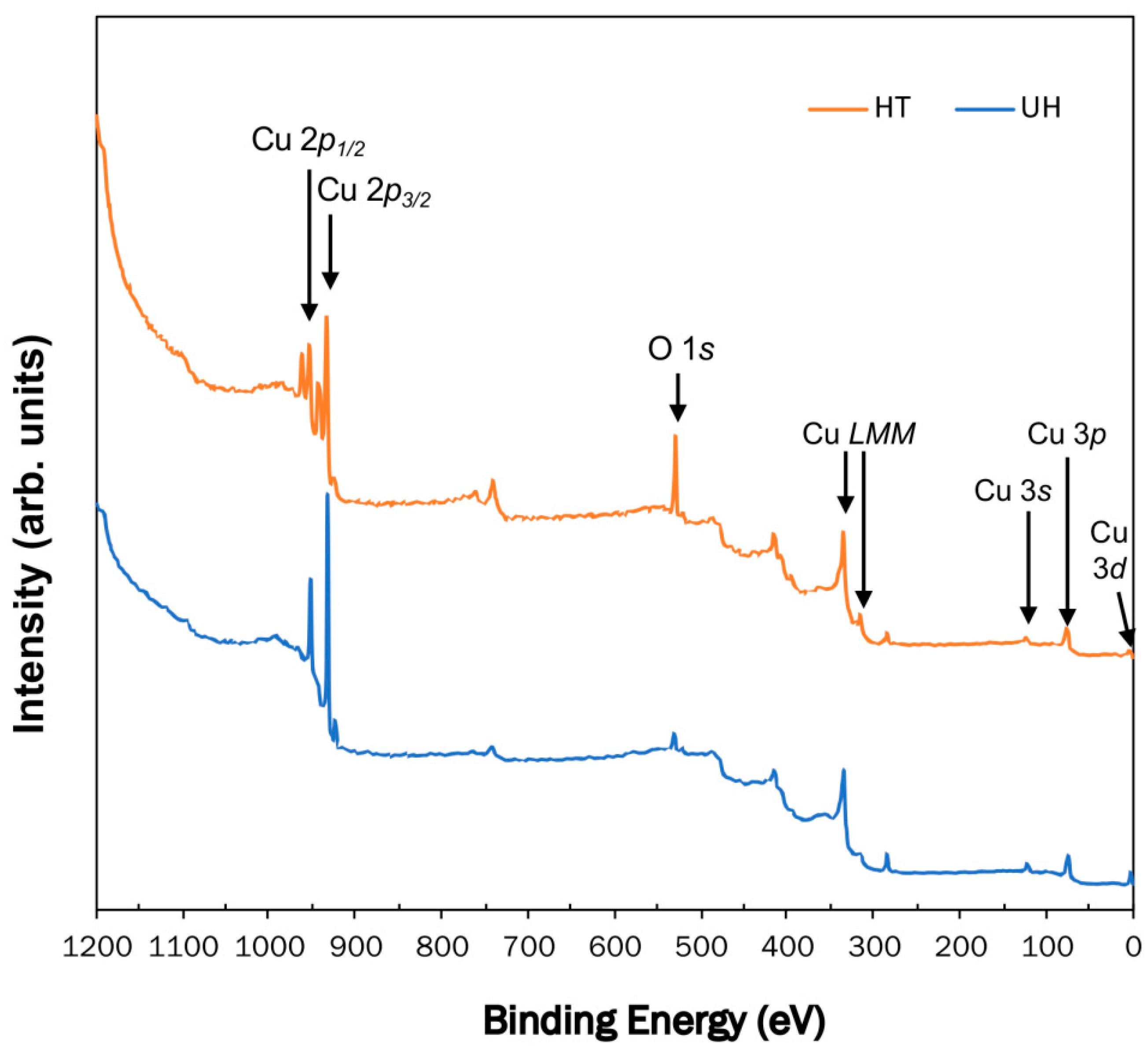
3.2. XPS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lupu, A. Thermogravimetry of copper and copper oxides (Cu2O-CuO). J. Therm. Anal. Calorim. 1970, 2, 445–458. [Google Scholar] [CrossRef]
- Wagner, C. Oxidation of alloys involving noble metals. J. Electrochem. Soc. 1956, 103, 571–580. [Google Scholar] [CrossRef]
- Meijering, J.L. Internal Oxidation and Related Phenomena in Alloys. Angew. Chem. Int. Ed. 1969, 8, 775. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamamoto, M.; Fujishima, A.; Miyazaki, T.; Hisamitsu, H.; Kojima, K.; Kadoma, Y. Raman and IR studies on adsorption behavior of adhesive monomers in a metal primer for Au, Ag, Cu, and Cr surfaces. J. Biomed. Mater. Res. 2002, 62, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Koizumi, H.; Ishii, T.; Nakayama, D.; Oba, Y.; Matsumura, H. Adhesive performance of silver-palladium-copper-gold alloy and component metals bonded with organic sulfur-based priming agents and a tri-n-butylborane initiated luting material. Acta Odontol. Scand. 2013, 71, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Koizumi, H.; Shimoe, S.; Hirata, I.; Matsumura, H.; Nikawa, H. Effect of thione primers on adhesive bonding between an indirect composite material and Ag-Pd-Cu-Au alloy. Dent. Mater. J. 2014, 33, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Hiraba, H.; Nogawa, H.; Koizumi, H.; Kodaira, A.; Akahane, S. Effect of multi-purpose primers on the bond durability between tri-n-butylborane initiated resin and gold alloy. J. Prosthodont. Res. 2019, 63, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Atsuta, M.; Nakahayashi, N.; Masuhara, E. Surface treatment of gold allnys for adhesion. J. Prosthet. Dent. 1988, 60, 271–279. [Google Scholar] [CrossRef]
- Matsumura, H.; Kawahara, M.; Tanaka, T.; Atsuta, M. Surface preparations for metal frameworks of composite resin veneered prostheses made with an adhesive opaque resin. J. Prosthet. Dent. 1991, 66, 10–15. [Google Scholar] [CrossRef]
- Mori, K.; Nakamura, Y. Study on triazine thiols V Polymerization of 6-(4-vinylbenzyl propyl)amino-1,3,5-triazine-2,4-dithiol on copper plates and their corrosion resistance. J. Polym. Sci. Polym. Lett. Ed. 1983, 21, 889–895. [Google Scholar] [CrossRef]
- Hiraba, H.; Koizumi, H.; Nogawa, H.; Kodaira, A.; Okamura, K.; Matsumura, H. Trace of organic sulfur compounds detected from debonded interface between transparent acrylic resin and gold alloy. J. Oral. Sci. 2017, 59, 511–517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taira, Y.; Kamada, K.; Atsuta, M. Effects of primers containing thiouracil and phosphate monomers on bonding of resin to Ag–Pd–Au alloy. Dent. Mater. J. 2008, 27, 69–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghijsen, J.; Tjeng, L.H.; van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic structure of Cu2O and CuO. Phys. Rev. B Condens. Matter. 1988, 38, 11322–11330. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Du, T.; Desai, V.; Tamboli, D.; Chathapuram, V.; Sundaram, K.B. Mechanisms of Copper Removal during Chemical Mechanical Polishing. In Copper Interconnects, New Contact Metallurgies/Structures, and Low-K Interlevel Dielectrics; Mathad, G.S., Ed.; PV 2002-22; The Electrochemical Society Inc.: Pennington, NJ, USA, 2003; pp. 235–245. [Google Scholar]
- Kusano, F.K.; Uchikoshi, M.; Mimura, K.; Isshiki, M. Low-Temperature Oxidation of Cu(100), Cu(110) and Cu(111). Oxid. Met. 2014, 82, 181–193. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Miyahara, H.; Ikeda, H.; Fujio, Y.; Yoshii, S.; Nagamatsu, Y.; Kitamura, C.; Shimizu, H. Chemical alteration of Ag-Pd-Cu-Au alloy surface by alumina air-abrasion and its effect on bonding to resin cement. Dent. Mater. J. 2019, 38, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Larson, P.E. X-ray induced photoelectron and auger spectra of Cu, CuO, Cu2O, and Cu2S thin films. J. Electron. Spectros. Relat. Phenom. 1974, 4, 213–218. [Google Scholar] [CrossRef]


| Material/Trade Name | Manufacturer | Lot | Composition |
|---|---|---|---|
| Element metal | |||
| Copper metal | Nilaco Corp., Tokyo, Japan | 44225602 | Cu 99.9 mass % |
| Primer | |||
| Metaltite | Tokuyama Dental Corp., Tokyo, Japan | 0382 | MTU-6, ethanol |
| Luting material | |||
| Super-Bond C&B Catalyst V | Sun Medical Co., Ltd., Moriyama, Japan | RG23F | TBB, TBB-O, hydrocarbon |
| Super-Bond C&B Opaque Ivory Powder | Sun Medical Co., Ltd., Moriyama, Japan | RM1 | PolyMMA, titanium oxide |
| Methyl methacrylate | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan | ZJ3WJIJ | MMA, 99.8% |
| Treatment–Primer | Median | IQR | CA | A |
|---|---|---|---|---|
| UH–MTU-6 | 28.3 a | 0.8 | 11 | 0 |
| HT–MTU-6 | 19.1 b | 8.2 | 6 | 5 |
| UH–unprimed | 2.3 c | 0.3 | 0 | 11 |
| HT–unprimed | 4.1 d | 0.9 | 1 | 10 |
| Element | Peak Energy (eV) | Peak Assignment (Compound) | References |
|---|---|---|---|
| Cu 2p3/2 | 932.4 | Cu2O or Cu | [13,14] |
| Cu 2p1/2 | 952.2 | Cu2O or Cu | [13,14] |
| Cu 2p3/2 | 933.7 | CuO | [13,14] |
| Cu 2p3/2 | 943.4 | satellite peaks of CuO | [13,14] |
| Cu 2p1/2 | 953.3 | CuO | [13,14] |
| Cu 2p1/2 | 962.0 | satellite peaks of CuO | [13,14] |
| Cu LMM | 335 | Cu | [15,16] |
| Cu LMM | 336 | CuO | [15,16] |
| Cu LMM | 337 | Cu2O | [15,16] |
| O 1s | 529.7 | CuO | [13,14] |
| O 1s | 530 | Cu2O | [13,14] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiraba, H.; Koizumi, H.; Kodaira, A.; Nogawa, H.; Yoneyama, T.; Matsumura, H. Influence of Oxidation of Copper on Shear Bond Strength to an Acrylic Resin Using an Organic Sulfur Compound. Materials 2020, 13, 2092. https://doi.org/10.3390/ma13092092
Hiraba H, Koizumi H, Kodaira A, Nogawa H, Yoneyama T, Matsumura H. Influence of Oxidation of Copper on Shear Bond Strength to an Acrylic Resin Using an Organic Sulfur Compound. Materials. 2020; 13(9):2092. https://doi.org/10.3390/ma13092092
Chicago/Turabian StyleHiraba, Haruto, Hiroyasu Koizumi, Akihisa Kodaira, Hiroshi Nogawa, Takayuki Yoneyama, and Hideo Matsumura. 2020. "Influence of Oxidation of Copper on Shear Bond Strength to an Acrylic Resin Using an Organic Sulfur Compound" Materials 13, no. 9: 2092. https://doi.org/10.3390/ma13092092
APA StyleHiraba, H., Koizumi, H., Kodaira, A., Nogawa, H., Yoneyama, T., & Matsumura, H. (2020). Influence of Oxidation of Copper on Shear Bond Strength to an Acrylic Resin Using an Organic Sulfur Compound. Materials, 13(9), 2092. https://doi.org/10.3390/ma13092092





