Effect of Different Compatibilizers on the Properties of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate) Blends Prepared under Intense Shear Flow Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
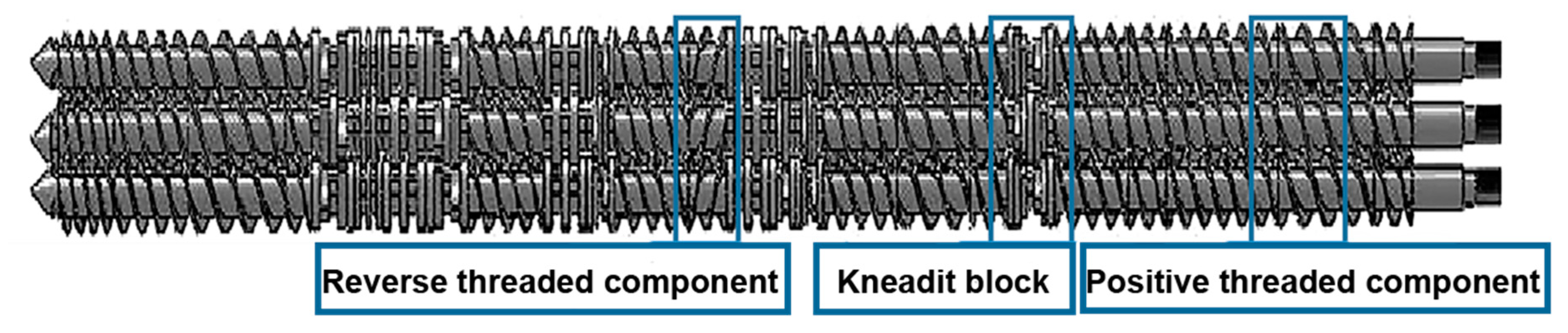
2.2. Sample Preparation
2.3. Scanning Electron Microscopy (SEM)
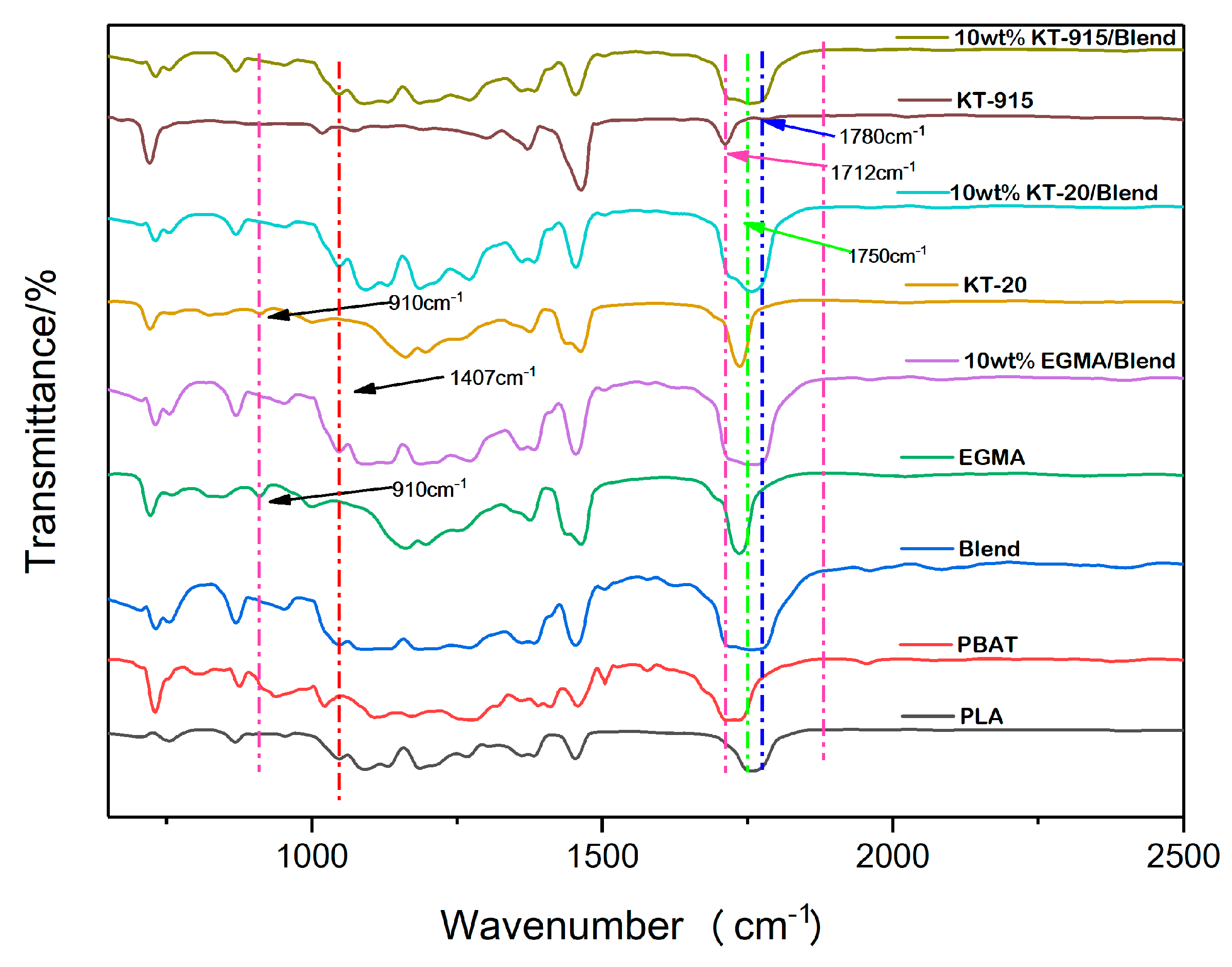
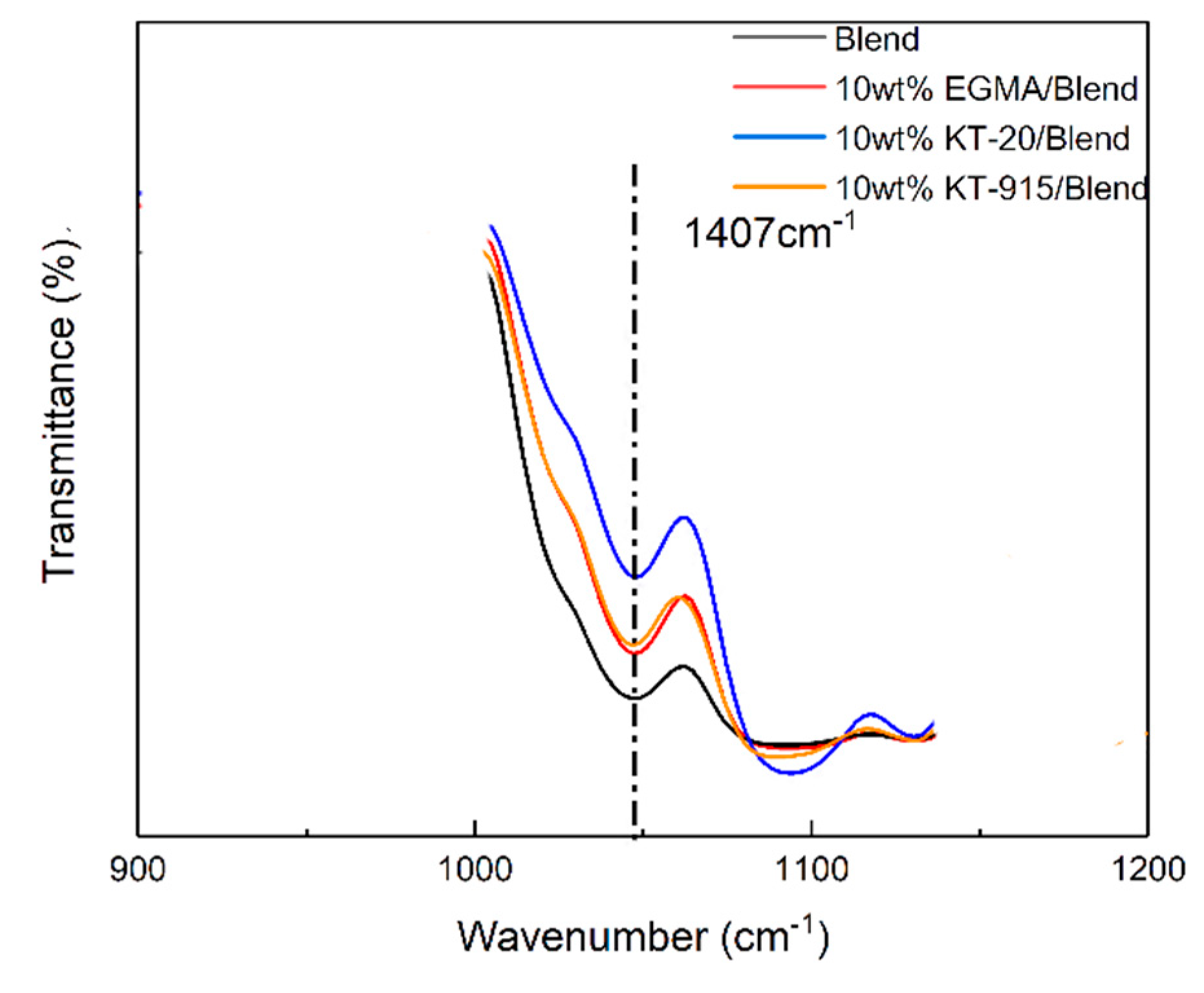
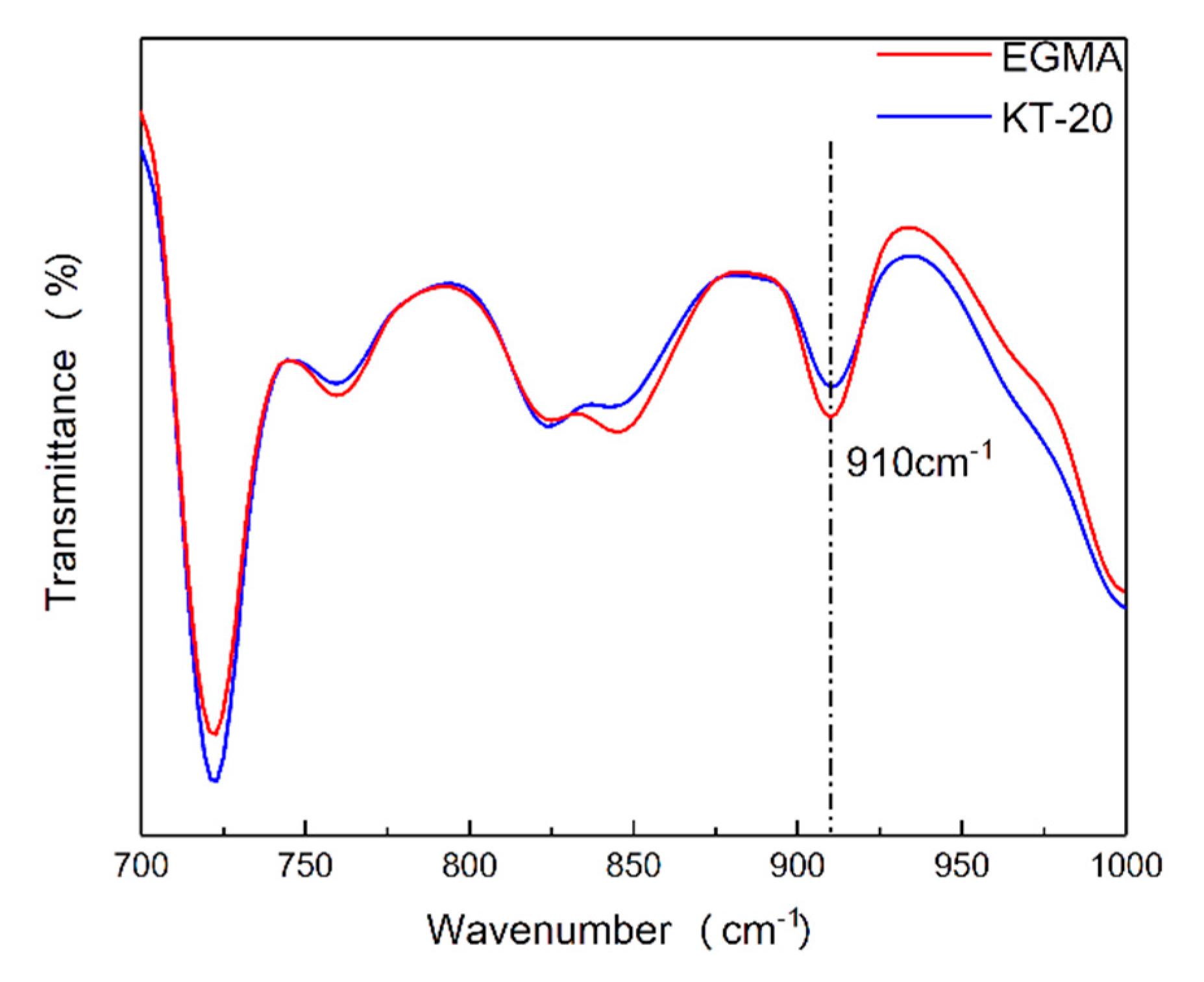
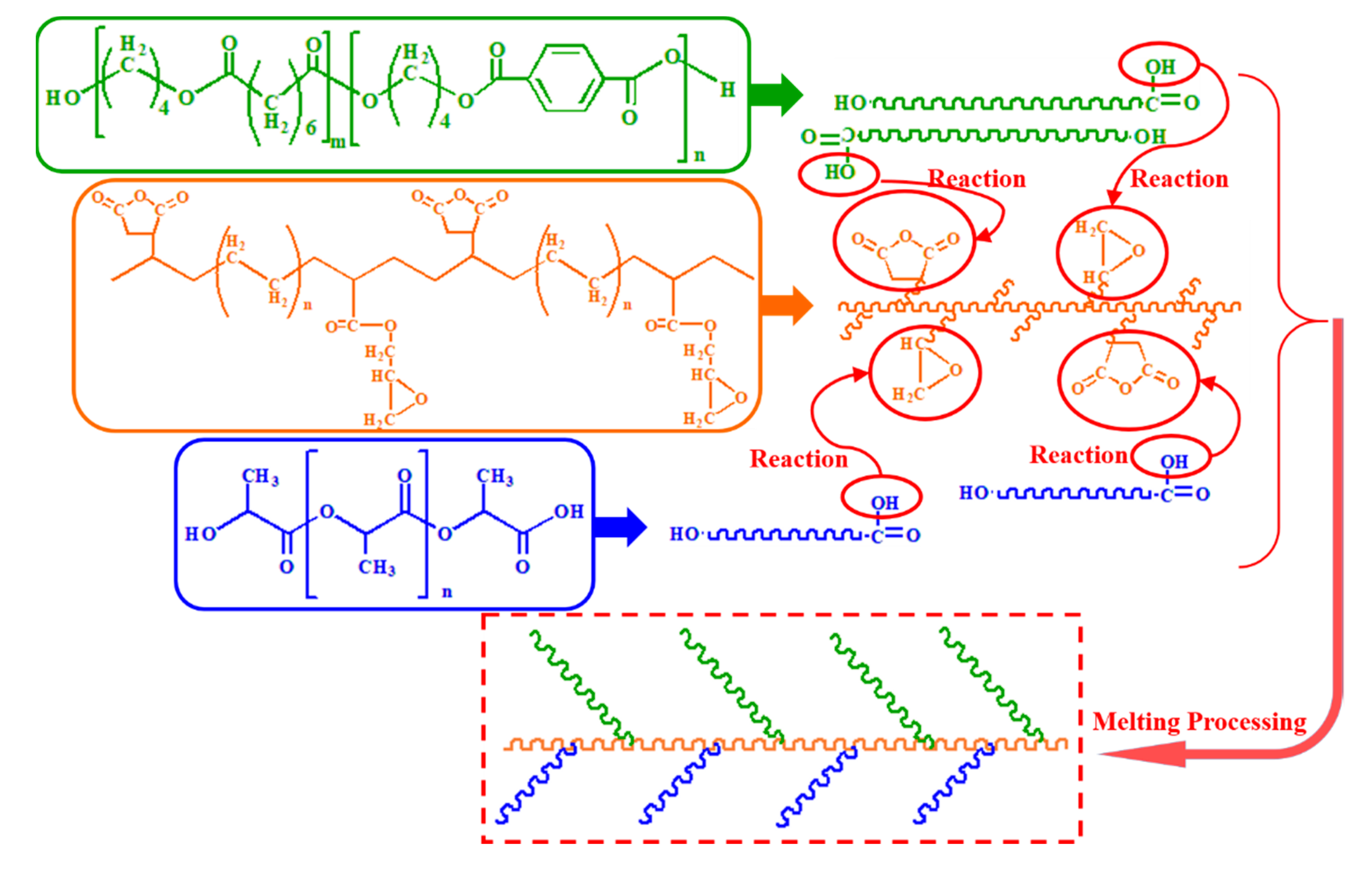
2.4. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5. Rheological Characterization Measurements
2.6. Mechanical Testing
3. Results and Discussion
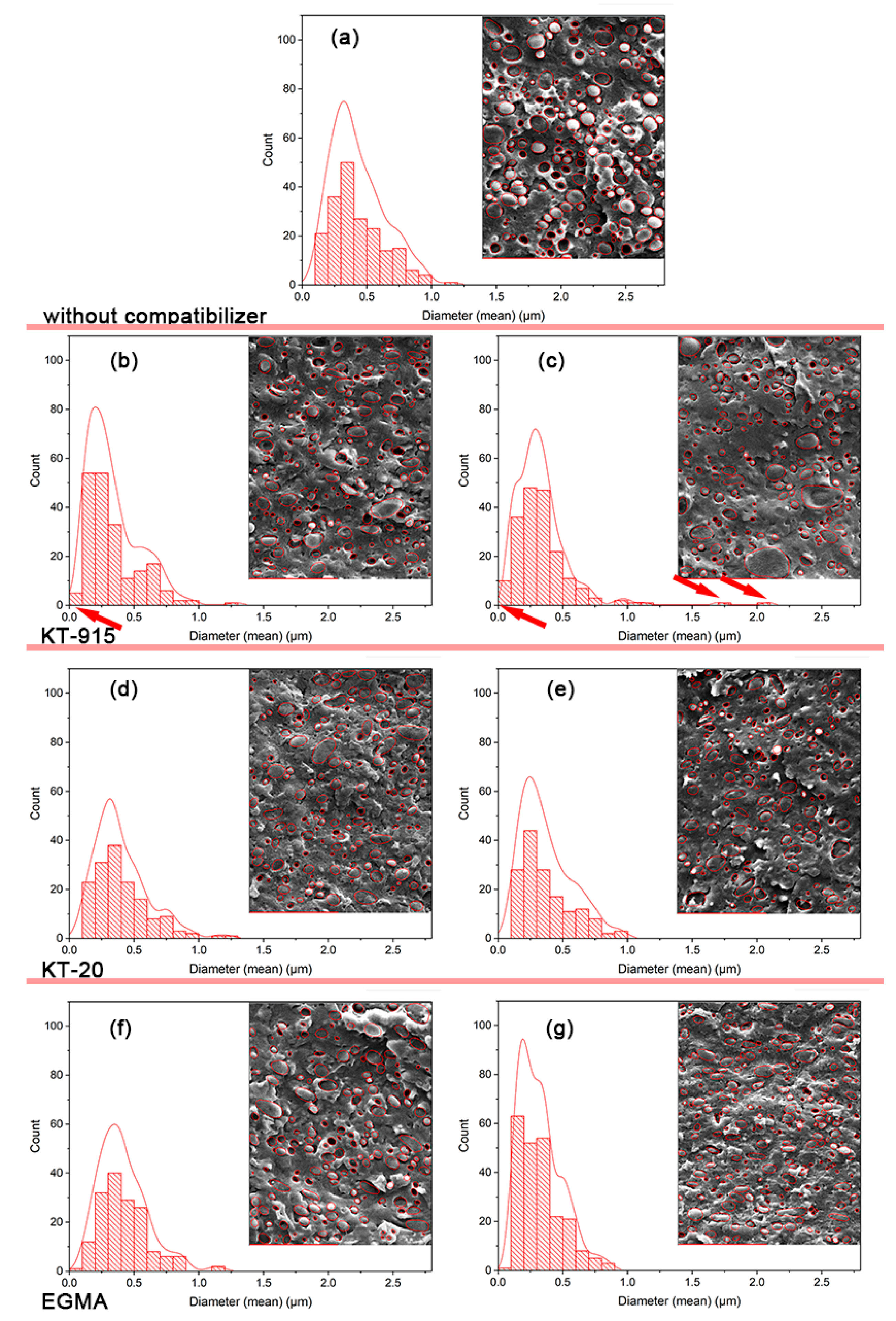
3.1. Morphological Analysis
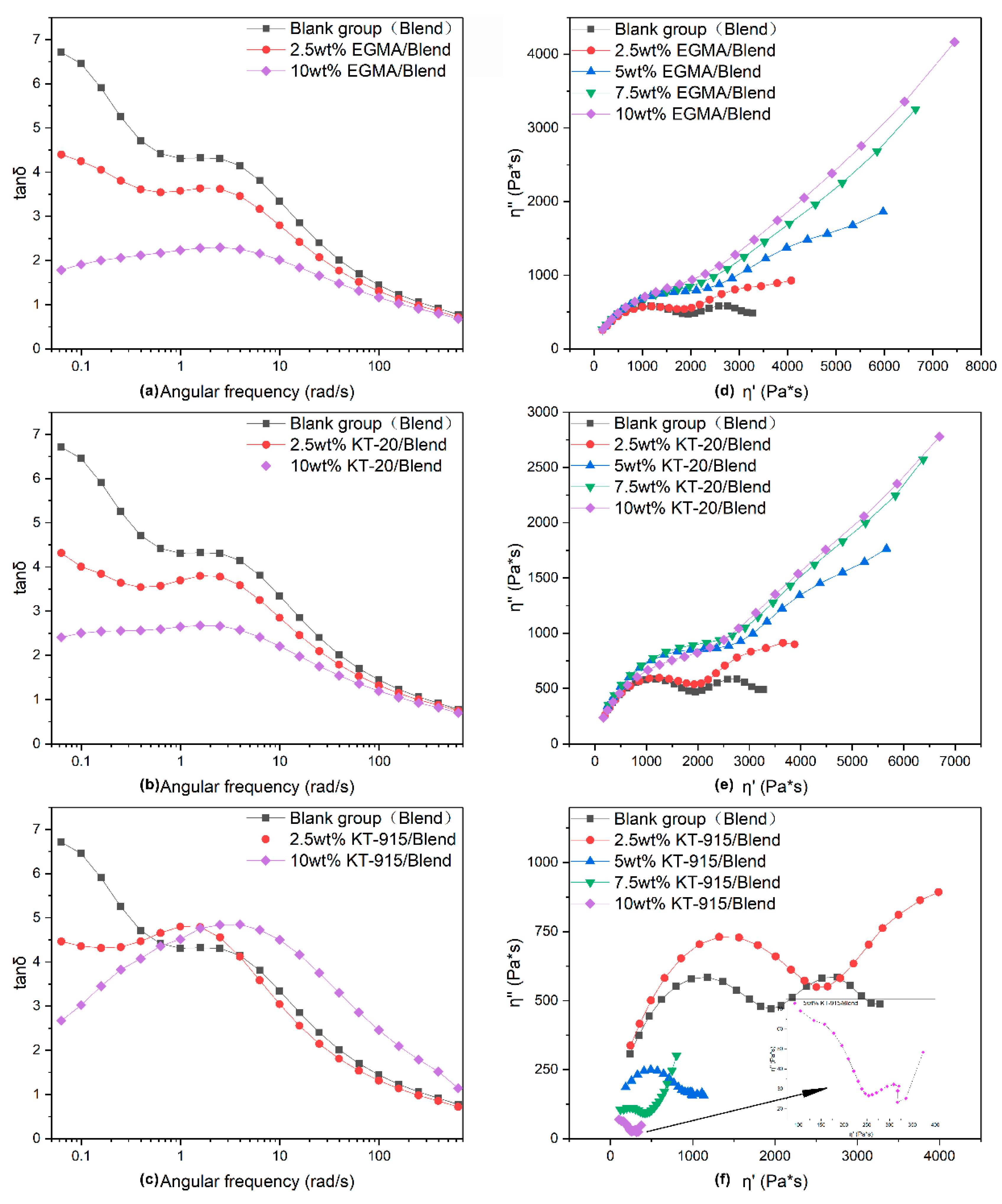
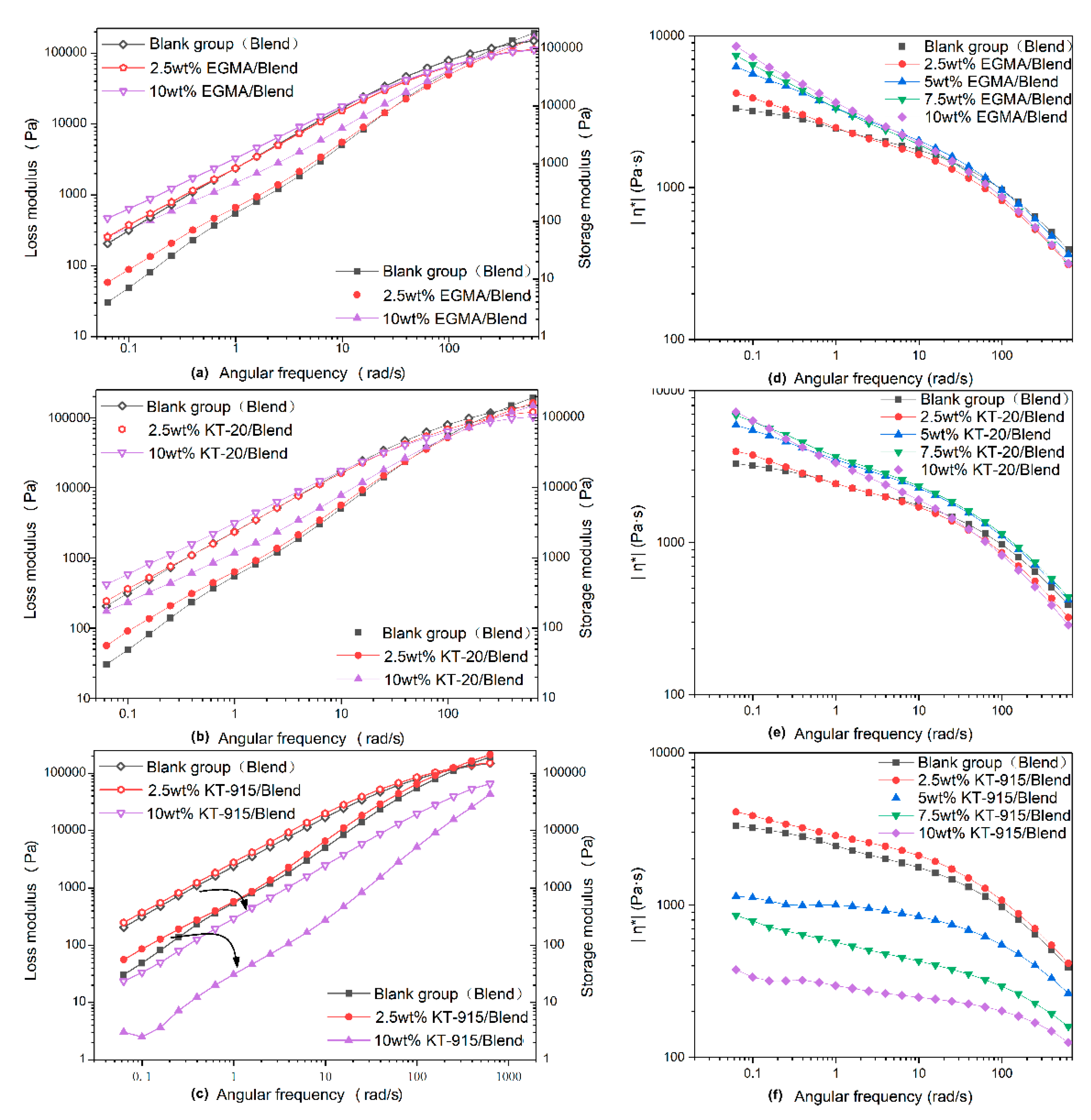
3.2. Dynamic Rheology Properties Analysis
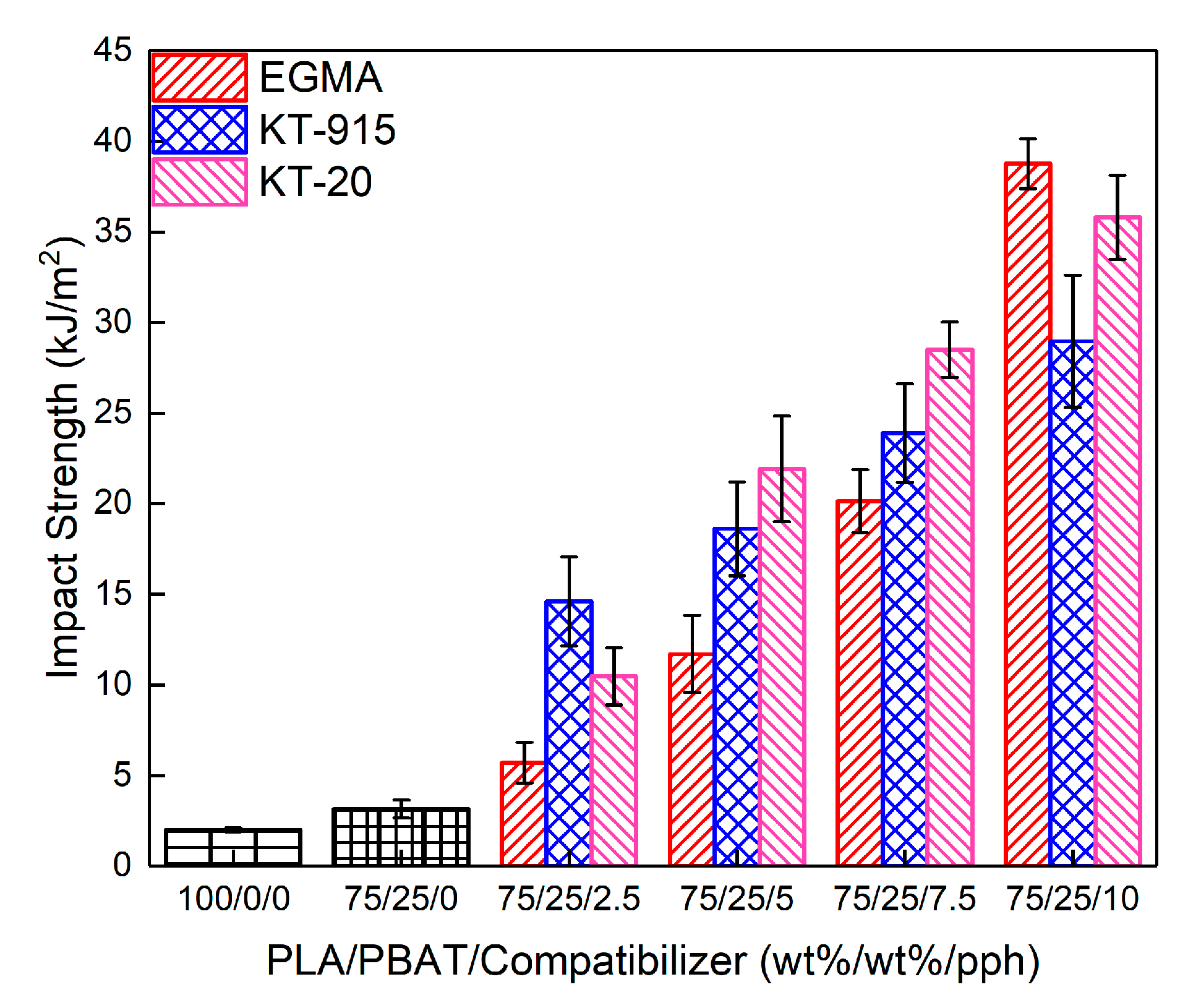
3.4. Mechanical Properties Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albertsson, A.; Varma, I.K. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Sawyer, D.J. Bioprocessing no longer a field of dreams. Macromol. Symp. 2003, 201, 271–282. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Ouchi, T.; Ohya, Y. Design of lactide copolymers as biomaterials. J. Polym. Sci. Pol. Chem. 2004, 42, 453–462. [Google Scholar] [CrossRef]
- Zhu, K.; Zhu, W.; Gu, Y.; Shen, Z.; Chen, W.; Zhu, G. Synthesis and characterization of poly(butylene adipate-co-terephthalate) catalyzed by rare earth Stearates. Chin. J. Chem. 2007, 25, 1581–1583. [Google Scholar] [CrossRef]
- Moustafa, H.; El, K.N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT bionanocomposites with antimicrobial natural rosin for green packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Lu, B.; Wang, P.; Wang, G.; Ji, J. PLA-PBAT-PLA tri-block copolymers: Effective compatibilizers for promotion of the mechanical and rheological properties of PLA/PBAT blends. Polym. Degrad. Stabil. 2018, 147, 41–48. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, B.; Bian, X.; Feng, L.; Zhang, H.; Duan, R.; Sun, J.; Pang, X.; Chen, W.; Chen, X. Synergistic effect of PLA–PBAT–PLA tri-block copolymers with two molecular weights as compatibilizers on the mechanical and rheological properties of PLA/PBAT blends. RSC Adv. 2015, 5, 73842–73849. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Rheological, morphological, and interfacial properties of compatibilized PLA/PBAT blends. Rheol. Acta 2014, 53, 501–517. [Google Scholar] [CrossRef]
- Kim, T.J.; Kim, T.H.; Kim, S.G.; Seo, K.H. Structural, thermal, and mechanical properties of PLA/PBAT/MEA blend. Polym. Korea 2016, 40, 371–379. [Google Scholar] [CrossRef]
- Ko, S.W.; Hong, M.K.; Park, B.J.; Gupta, R.K.; Choi, H.J.; Bhattacharya, S.N. Morphological and rheological characterization of multi-walled carbon nanotube/PLA/PBAT blend nanocomposites. Polym. Bull. 2009, 63, 125–134. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of biodegradable Polylactide/Poly(butylene adipate-co-terephthalate) blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, T.; Kontani, T.; Ohya, Y. Modification of polylactide upon physical properties by solution-cast blends from polylactide and polylactide-grafted dextran. Polymer 2003, 44, 3927–3933. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Pei, X.; Yu, L.; Feng, Y. Genome-shuffling improved acid tolerance and l-lactic acid volumetric productivity in Lactobacillus rhamnosus. J. Biotechnol. 2007, 129, 510–515. [Google Scholar] [CrossRef]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Murariu, M.; Da Silva Ferreira, A.; Pluta, M.; Bonnaud, L.; Alexandre, M.; Dubois, P. Polylactide (PLA)–CaSO4 composites toughened with low molecular weight and polymeric ester-like plasticizers and related performances. Eur. Polym. J. 2008, 44, 3842–3852. [Google Scholar] [CrossRef]
- Xue, B.; He, H.; Huang, Z.; Zhu, Z.; Xue, F.; Liu, S.; Liu, B. Fabrication of super-tough ternary blends by melt compounding of poly(lactic acid) with poly(butylene succinate) and ethylene-methyl acrylate-glycidyl methacrylate. Compos. Part B Eng. 2019, 172, 743–749. [Google Scholar] [CrossRef]
- Xue, B.; He, H.; Zhu, Z.; Li, J.; Huang, Z.; Wang, G.; Chen, M.; Zhan, Z. A facile fabrication of high toughness poly(lactic acid) via reactive extrusion with poly(butylene succinate) and ethylene-methyl acrylate-glycidyl methacrylate. Polym. Basel 2018, 10, 1401. [Google Scholar] [CrossRef]
- Teamsinsungvon, A.; Jarapanyacheep, R.; Ruksakulpiwat, Y.; Jarukumjorn, K. Melt processing of maleic anhydride grafted poly(lactic acid) and its compatibilizing effect on poly(lacticacid)/poly(butylene adipate-co-terephthalate) blend and their composite. Polym. Sci. Ser. A+ 2017, 59, 384–396. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Xu, J.; Yan, Z.; Zhong, G.; Li, Z. Largely enhanced mechanical performance of poly(butylene succinate) multiple system via shear stress-induced orientation of the hierarchical structure. J. Mater. Chem. A 2018, 6, 13373–13385. [Google Scholar] [CrossRef]
- Zhou, S.; Niu, B.; Xie, X.; Ji, X.; Zhong, G.; Hsiao, B.S.; Li, Z. Interfacial shish-kebabs lengthened by coupling effect of in situ flexible nanofibrils and intense shear flow: Achieving hierarchy to conquer the conflicts between strength and toughness of polylactide. ACS Appl. Mater. Interfaces 2017, 9, 10148–10159. [Google Scholar] [CrossRef] [PubMed]
- Abbate, M.; Liello, V.D.; Martuscelli, E.; Musto, P.; Ragosta, G.; Scarinzi, G. Molecular and mechanical characterization of reactive ethylene-propylene elastomers and their use in PA6-based blends. Polymer 1992, 33, 2940–2948. [Google Scholar] [CrossRef]
- Mohanty, S.; Nayak, S.K. Biodegradable nanocomposites of poly(butylene adipate-co-terephthalate) (pbat) and organically modified layered silicates. J. Polym. Environ. 2012, 20, 195–207. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, S.C. Side chain extension of polypropylene by aliphatic diamine and isocyanate. Macromol. Symp. 2004, 214, 289–298. [Google Scholar] [CrossRef]
- Kumar, M.; Mohanty, S.; Nayak, S.K.; Rahail Parvaiz, M. Effect of glycidyl methacrylate (GMA) on the thermal, mechanical and morphological property of biodegradable PLA/PBAT blend and its nanocomposites. Bioresour. Technol. 2010, 101, 8406–8415. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, S.; Sun, C.; Wan, L.; Tan, H.; Zhang, Y. Effect of MAH-g-PLA on the properties of wood fiber/polylactic acid composites. Polym. Basel 2017, 9, 591. [Google Scholar] [CrossRef]
- Phetwarotai, W.; Phusunti, N.; Aht-Ong, D. Preparation and characteristics of poly(butylene adipate-co-terephthalate)/polylactide blend films via synergistic efficiency of plasticization and compatibilization. Chin. J. Polym. Sci. 2019, 37, 68–78. [Google Scholar] [CrossRef]
- Hao, M.Y.; Wu, H.W.; Zhu, Z.H. In situ reactive interfacial compatibilization of polylactide/sisal fiber biocomposites via melt-blending with an epoxy-functionalized terpolymer elastomer. RSC Adv. 2017, 7, 32399–32412. [Google Scholar] [CrossRef]
- He, H.; Xue, F.; Jia, P.; He, G.; Huang, Z.; Liu, S.; Xue, B. Linear low-density polyethylene/poly(ethylene terephthalate) blends compatibilization prepared by an eccentric rotor extruder: A morphology, mechanical, thermal, and rheological study. J. Appl. Polym. Sci. 2018, 135, 46489. [Google Scholar] [CrossRef]
- Zou, W.; Chen, R.; Zhang, G.; Zhang, H.; Qu, J. Mechanical, thermal and rheological properties and morphology of poly (lactic acid)/poly (propylene carbonate) blends prepared by vane extruder. Polym. Adv. Technol. 2016, 27, 1430–1437. [Google Scholar] [CrossRef]
- Graebling, D.; Muller, R.; Palierne, J.F. Linear viscoelastic behavior of some incompatible polymer blends in the melt. Interpretation of data with a model of emulsion of viscoelastic liquids. Macromolecules 1993, 26, 320–329. [Google Scholar] [CrossRef]
- Dil, E.J.; Carreau, P.J.; Favis, B.D. Morphology, miscibility and continuity development in poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. Polymer 2015, 68, 202–212. [Google Scholar]
- Ajji, A.; Choplin, L.; Prud’Homme, R.E. Rheology of polystyrene/poly(vinyl methyl ether)blends near the phase transition. J. Polym. Sci. Part B Polym. Phys. 1991, 29, 1573–1578. [Google Scholar] [CrossRef]
- Tian, J.; Yu, W.; Zhou, C. The preparation and rheology characterization of long chain branching polypropylene. Polymer 2006, 47, 7962–7969. [Google Scholar] [CrossRef]
- Winter, H.H.; Chambon, F. Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Fang, H.; Jiang, F.; Wu, Q.; Ding, Y.; Wang, Z. Supertough polylactide materials prepared through in situ reactive blending with PEG-based diacrylate monomer. ACS Appl. Mater. Interfaces 2014, 6, 13552–13563. [Google Scholar] [CrossRef]
- Hristov, V.; Vasileva, S. Dynamic mechanical and thermal properties of modified poly(propylene) wood fiber composites. Macromol. Mater. Eng. 2003, 288, 798–806. [Google Scholar] [CrossRef]
- Mohanty, S.; Nayak, S.K. Interfacial, dynamic mechanical, and thermal fiber reinforced behavior of MAPE treated sisal fiber reinforced HDPE composites. J. Appl. Polym. Sci. 2006, 102, 3306–3315. [Google Scholar] [CrossRef]
- Hristov, V.N.; Krumova, M.; Vasileva, S.; Michler, G.H. Modified polypropylene wood flour composites. II. Fracture, deformation, and mechanical properties. J. Appl. Polym. Sci. 2004, 92, 1286–1292. [Google Scholar] [CrossRef]
- Xu, H.; Fang, H.; Bai, J.; Zhang, Y.; Wang, Z. Preparation and characterization of high-melt-strength polylactide with long-chain branched structure through gamma-radiation-induced chemical reactions. Ind. Eng. Chem. Res. 2014, 53, 1150–1159. [Google Scholar] [CrossRef]
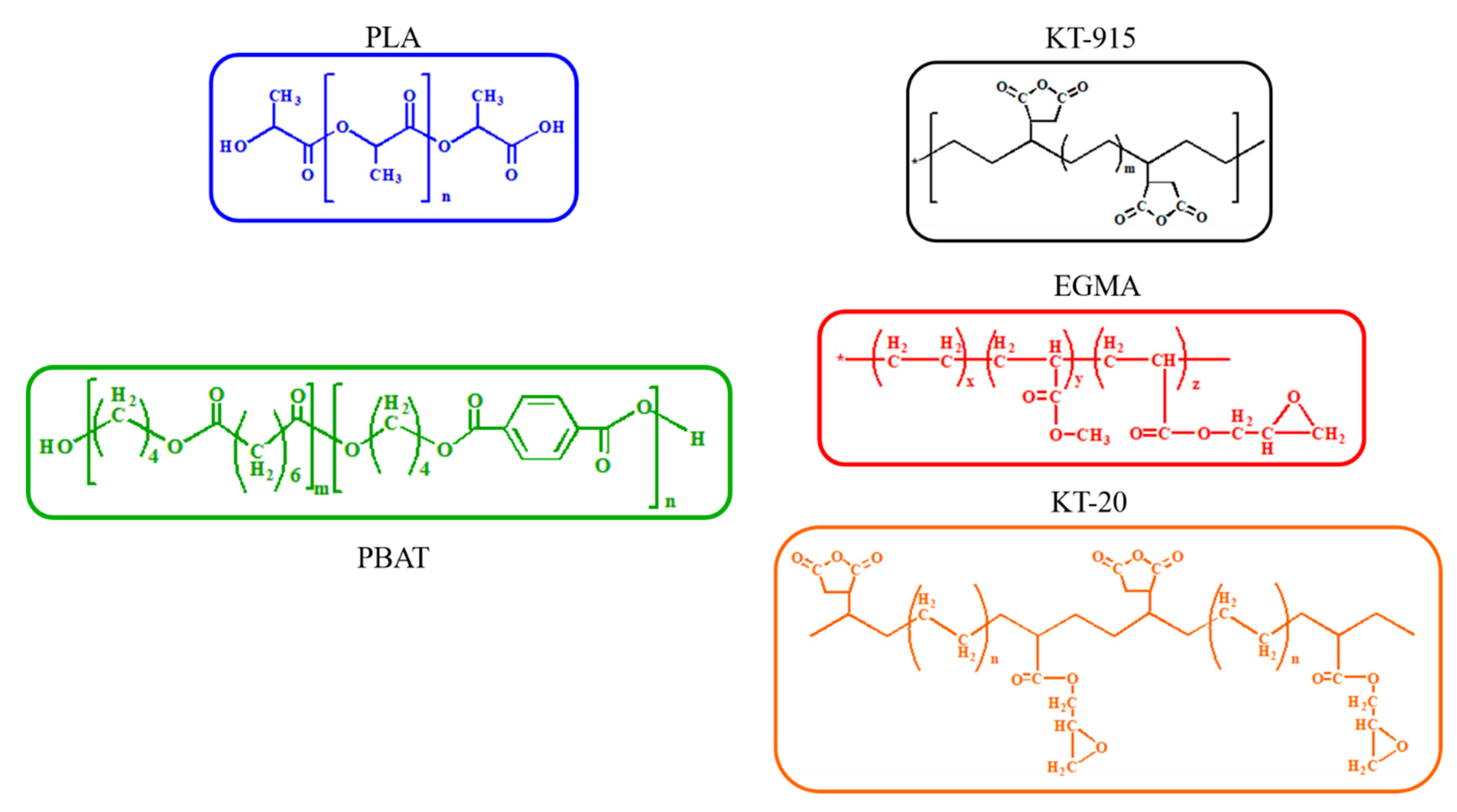

| Material | Trademark | Density(g/cm−3) | MFI(190 °C, 2.16 kg) | Factory |
|---|---|---|---|---|
| PLA (semi-crystalline material containing 2% D-lactide units) | SC M310–A2-HP | 1.24 | 6 g/10 min | COFCO Biomaterial Co., Ltd. Jilin China |
| PBAT | PBAT | 1.20 | 43 g/10 min | Kingfa SCI. & TECH. Co., Ltd. Guangzhou, China |
| Compatibilizer1: EGMA | AX 8900 | 0.95 | 6.0 g/10 min | Arkema Investment Co., Ltd. (Shanghai, China) |
| Compatibilizer2: KT-20 | KT-20 | 0.94 | 1.3 g/10 min | Shenyang Ketong Plastic Co., Ltd. Shenyang, China |
| Compatibilizer3: KT-915 | KT-915 | 0.85 | 0.87 g/10 min | Shenyang Ketong Plastic Co., Ltd. Shenyang, China |
| Abbreviation | Various Matrix Weight |
|---|---|
| Blank group (Blend) | PLA/PBAT (75 wt%/25 wt%) |
| 2.5 wt% EGMA/Blend | PLA/PBAT/EGMA (75 wt%/25 wt%/2.5 wt%) |
| 5 wt% EGMA/Blend | PLA/PBAT/EGMA (75 wt%/25 wt%/5 wt%) |
| 7.5 wt% EGMA/Blend | PLA/PBAT/EGMA (75 wt%/25 wt%/7.5 wt%) |
| 10 wt% EGMA/Blend | PLA/PBAT/EGMA (75 wt%/25 wt%/10 wt%) |
| 2.5 wt% KT-915/Blend | PLA/PBAT/KT-915 (75 wt%/25 wt%/2.5 wt%) |
| 5 wt% KT-915/Blend | PLA/PBAT/KT-915 (75 wt%/25 wt%/5 wt%) |
| 7.5 wt% KT-915/Blend | PLA/PBAT/KT-915 (75 wt%/25 wt%/7.5 wt%) |
| 10 wt% KT-915/Blend | PLA/PBAT/KT-915 (75 wt%/25 wt%/10 wt%) |
| 2.5 wt% KT-20/Blend | PLA/PBAT/KT-20 (75 wt%/25 wt%/2.5 wt%) |
| 5 wt% KT-20/Blend | PLA/PBAT/KT-20 (75 wt%/25 wt%/5 wt%) |
| 7.5 wt% KT-20/Blend | PLA/PBAT/KT-20 (75 wt%/25 wt%/7.5 wt%) |
| 10 wt% KT-20/Blend | PLA/PBAT/KT-20 (75 wt%/25 wt%/10 wt%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Wang, G.; Chen, M.; Xiong, C.; Li, Y.; Tong, Y. Effect of Different Compatibilizers on the Properties of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate) Blends Prepared under Intense Shear Flow Field. Materials 2020, 13, 2094. https://doi.org/10.3390/ma13092094
He H, Wang G, Chen M, Xiong C, Li Y, Tong Y. Effect of Different Compatibilizers on the Properties of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate) Blends Prepared under Intense Shear Flow Field. Materials. 2020; 13(9):2094. https://doi.org/10.3390/ma13092094
Chicago/Turabian StyleHe, Hezhi, Guozhen Wang, Ming Chen, Chengtian Xiong, Yi Li, and Yi Tong. 2020. "Effect of Different Compatibilizers on the Properties of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate) Blends Prepared under Intense Shear Flow Field" Materials 13, no. 9: 2094. https://doi.org/10.3390/ma13092094
APA StyleHe, H., Wang, G., Chen, M., Xiong, C., Li, Y., & Tong, Y. (2020). Effect of Different Compatibilizers on the Properties of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate) Blends Prepared under Intense Shear Flow Field. Materials, 13(9), 2094. https://doi.org/10.3390/ma13092094




