Investigation of Porous Metal-Based 3D-Printed Anode GDLs for Tubular High Temperature Proton Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Experimental
2.1. Production of Metallic Porous Tubular Anode GDLs
2.2. Preparation of the Membrane Electrode Assemblies and Single Tubular Fuel Cell
2.2.1. Preparation of Catalyst Layer
2.2.2. Preparation of the Sol-Gel PBI-H3PO4 Membrane
2.2.3. Cathode GDL and Electric Connection
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Surface Morphology of the Produced PBF-LB Test Elements
3.2. Tubular HT-PEMFC Performance
3.3. Short-Term Stability Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. Approaches and Recent Development of Polymer Electrolyte Membranes for Fuel Cells Operating above 100 °C. Chem. Mater. 2003, 15, 4896–4915. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef] [Green Version]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrog. Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Lysenko, V.A. Current trends in the design of gas-diffusion layers for fuel cells. Fibre Chem. 2008, 40, 226–233. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-W.; Popov, B.N. A review of gas diffusion layer in PEM fuel cells: Materials and designs. Int. J. Hydrog. Energy 2012, 37, 5850–5865. [Google Scholar] [CrossRef]
- Bai, L.; Lloyd, G.A. Fuel Cell Having Metalized Gas Diffusion Layer. U.S. Patent 6,716,549, 6 April 2004. [Google Scholar]
- Hussain, N.; Van Steen, E.; Tanaka, S.; Levecque, P. Metal based gas diffusion layers for enhanced fuel cell performance at high current densities. J. Power Sources 2017, 337, 18–24. [Google Scholar] [CrossRef]
- Blanco, M.; Wilkinson, D.P.; Wang, H. Perforated Metal Sheets as Gas Diffusion Layers for Proton Exchange Membrane Fuel Cells. Electrochem. Solid State Lett. 2012, 15, B20. [Google Scholar] [CrossRef]
- Abele, E.; Stoffregen, H.; Lang, S.; Hampe, M.J. Elektrode zum Betrieb einer Brennstoffzelle und Verfahren zur ihrer Herstellung. EP 2 744 028 A1, 18 June 2014. [Google Scholar]
- Stoffregen, H.A.; Fischer, J.; Siedelhofer, C.; Abele, E. (Eds.) Selective Laser Melting of Porous Structures. In Proceedings of the 22nd Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, SFF 2011, Austin, TX, USA, 8–10 August 2011; pp. 680–695. [Google Scholar]
- Peng, T.; Chen, C. Influence of energy density on energy demand and porosity of 316L stainless steel fabricated by selective laser melting. Int. J. Precis. Eng. Manuf. Green Technol. 2018, 5, 55–62. [Google Scholar] [CrossRef]
- Lang, S. Entwicklung tubularer Mitteltemperatur-Brennstoffzellen—Experimentelle Untersuchungen, Modellierung und Numerische Simulation; Dr. Hut: Munich, Germany, 2016; ISBN 3843924414. [Google Scholar]
- Abele, E.; Reiber, T.; Hampe, M.; Bermúdez Agudelo, M.C.; Menz, F. Tailoring and investigation of defined porosity properties in thin-walled 316L structures using laser-based powder bed fusion. Prog. Addit. Manuf. 2019, 4, 451–463. [Google Scholar] [CrossRef]
- Bermúdez Agudelo, M.C.; Hampe, M.J. (Eds.) Development and investigation of a tubular HT-PEM-fuel cell with 3D-printed anode GDL. In Proceedings of the 8th European Fuel Cell Technology & Applications Piero Lunghi Conference - EFC19, Naples, Italy, 9–11 December 2019; pp. 329–330. [Google Scholar]
- Xu, F.-L.; Duan, J.-Z.; Lin, C.-G.; Hou, B.-R. Influence of marine aerobic biofilms on corrosion of 316L stainless steel. J. Iron Steel Res. Int. 2015, 22, 715–720. [Google Scholar] [CrossRef]
- Dewidar, M.M.; Khalil, K.A.; Lim, J.K. Processing and mechanical properties of porous 316L stainless steel for biomedical applications. Trans. Nonferrous Metals Soc. China 2007, 17, 468–473. [Google Scholar] [CrossRef]
- Dudek, A.; Włodarczyk, R. Effect of sintering atmosphere on properties of porous stainless steel for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 434–439. [Google Scholar] [CrossRef]
- Davies, D.P.; Adcock, P.L.; Turpin, M.; Rowen, S.J. Stainless steel as a bipolar plate material for solid polymer fuel cells. J. Power Sources 2000, 86, 237–242. [Google Scholar] [CrossRef]
- Wang, H. Stainless steel as bipolar plate material for polymer electrolyte membrane fuel cells. J. Power Sources 2003, 115, 243–251. [Google Scholar] [CrossRef]
- Kumar, A.; Reddy, R.G. Materials and design development for bipolar/end plates in fuel cells. J. Power Sources 2004, 129, 62–67. [Google Scholar] [CrossRef]
- Jang, J.H.; Joo, B.D.; Mun, S.M.; Van Tyne, C.J.; Moon, Y.H. Application of a direct laser melting process for fabricating a micropattern in bipolar plates used in direct methanol fuel cells. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2011, 225, 1784–1791. [Google Scholar] [CrossRef]
- Dawson, R.J.; Patel, A.J.; Rennie, A.E.W.; White, S. An investigation into the use of additive manufacture for the production of metallic bipolar plates for polymer electrolyte fuel cell stacks. J. Appl. Electrochem. 2015, 45, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Miranda, G.; Faria, S.; Bartolomeu, F.; Pinto, E.; Madeira, S.; Mateus, A.; Carreira, P.; Alves, N.; Silva, F.S.; Carvalho, O. Predictive models for physical and mechanical properties of 316L stainless steel produced by selective laser melting. Mater. Sci. Eng. A 2016, 657, 43–56. [Google Scholar] [CrossRef]
- Lee, K.I.; Lee, S.W.; Park, M.S.; Chu, C.N. The development of air-breathing proton exchange membrane fuel cell (PEMFC) with a cylindrical configuration. Int. J. Hydrog. Energy 2010, 35, 11844–11854. [Google Scholar] [CrossRef]
- Bullecks, B.; Rengaswamy, R.; Bhattacharyya, D.; Campbell, G. Development of a cylindrical PEM fuel cell. Int. J. Hydrog. Energy 2011, 36, 713–719. [Google Scholar] [CrossRef]
- Abele, E.; Stoffregen, H.A.; Kniepkamp, M.; Lang, S.; Hampe, M. Selective Laser Melting for Manufacturing of Thin-walled Porous Elements. J. Mater. Process. Technol. 2014, 114–121. [Google Scholar] [CrossRef]
- Spierings, A.B.; Schneider, M.; Eggenberger, R. Comparison of density measurement techniques for additive manufactured metallic parts. Rapid Prototyp. J. 2011, 17, 380–386. [Google Scholar] [CrossRef]
- The International Nickel Co. Inc. (INCO). Corrosion Resistance of the Austenitic Chromium-Nickel Stainless Steels in Chemical Environments. Publication No 2828. Available online: https://www.nickelinstitute.org/media/1662/corrosionresistanceoftheausteniticchromium_nickelstainlesssteelsinchemicalenvironments_2828_.pdf (accessed on 27 April 2020).
- Lizlovs, E.A. Corrosion Behavior of Types 304 and 316 Stainless Steel in Hot 85% Phosphoric Acid. Corrosion 1969, 25, 389–393. [Google Scholar] [CrossRef]
- Sánchez Tovar, R.; Montañés Sanjuan, M.T.; Garcia-Anton, J.; Guenbour, A. Galvanic Corrosion of the Base AISI 316l/Micro-Plasma Arc Welded AISI 316l in Polluted Phosphoric Acid Media at Different Temperatures. Int. J. Electrochem. Sci. 2011, 6, 3656–3670. [Google Scholar]
- Sánchez-Tovar, R.; Montañés, M.T.; García-Antón, J.; Guenbour, A.; Ben-Bachir, A. Corrosion behaviour of micro-plasma arc welded stainless steels in H3PO4 under flowing conditions at different temperatures. Corros. Sci. 2011, 53, 1237–1246. [Google Scholar] [CrossRef]
- Li, Y.L.; Xiang, S.; Zeng, H.T.; Wang, J.P.; Wang, Q.D. The Corrosion Behavior of 304L and 316L Stainless Steel in Food Grade Phosphoric Acid Solutions. AMM 2011, 109, 28–31. [Google Scholar] [CrossRef]
- Loto, C.A.; Popoola, A.P.I.; Fayomi, O.S.; Loto, R.T. Corrosion Polarization Behaviour of Type 316 Stainless Steel in Strong Acids and Acid Chlorides. Int. J. Electrochem. Sci. 2012, 7, 3787–3797. [Google Scholar]
- Zhang, Y.; Liu, F.; Chen, J.; Yuan, Y. Effects of surface quality on corrosion resistance of 316L stainless steel parts manufactured via SLM. J. Laser Appl. 2017, 29, 22306. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S. Corrosion Behaviour of 316L Stainless Steel in Boric Acid Solutions. Int. J. Electrochem. Sci. 2018, 3246–3256. [Google Scholar] [CrossRef]
- Arwati, A.I.G.; Majlan, E.H.; Daud, W.R.; Shyuan, L.K.; Arifin, K.B.; Husaini, T.; Alfa, S.; Ashidiq, F. Temperature Effects on Stainless Steel 316L Corrosion in the Environment of Sulphuric Acid (H2SO4). IOP Conf. Series Mater. Sci. Eng. 2018, 343, 12016. [Google Scholar] [CrossRef]
- Barbir, F. PEM fuel Cells. Theory and Practice, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2013; ISBN 0123877105. [Google Scholar]
- Schmidt, T.J.; Baurmeister, J. Properties of high-temperature PEFC Celtec®-P 1000 MEAs in start/stop operation mode. J. Power Sources 2008, 176, 428–434. [Google Scholar] [CrossRef]
- Siegel, C.; Bandlamudi, G.; Heinzel, A. Systematic characterization of a PBI/H3PO4 sol–gel membrane—Modeling and simulation. J. Power Sources 2011, 196, 2735–2749. [Google Scholar] [CrossRef]
- Lang, S.; Kazdal, T.J.; Kühl, F.; Hampe, M.J. Experimental investigation and numerical simulation of the electrolyte loss in a HT-PEM fuel cell. Int. J. Hydrog. Energy 2015, 40, 1163–1172. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrog. Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Danish Power Systems. MEA fact sheet: Dapazol (R)—The optimal solution for Fuel Cell stack operating at temperatures of 120–200 °C. Available online: https://daposy.com/images/files/Dapozol_MEA_fact_sheet.pdf (accessed on 9 April 2020).
- BASF New Business GmbH. Celtec®-P Membrane Electrode Assemblies for High-Temperature PEM fuel cells. Available online: https://www.basf.com/global/de/documents/about-us/Companies/new-business-gmbh/publications/e-power-management/BASF_Celtec_P_201902.pdf (accessed on 28 April 2020).
- Yazici, M.S. Passive air management for cylindrical cartridge fuel cells. J. Power Sources 2007, 166, 137–142. [Google Scholar] [CrossRef]

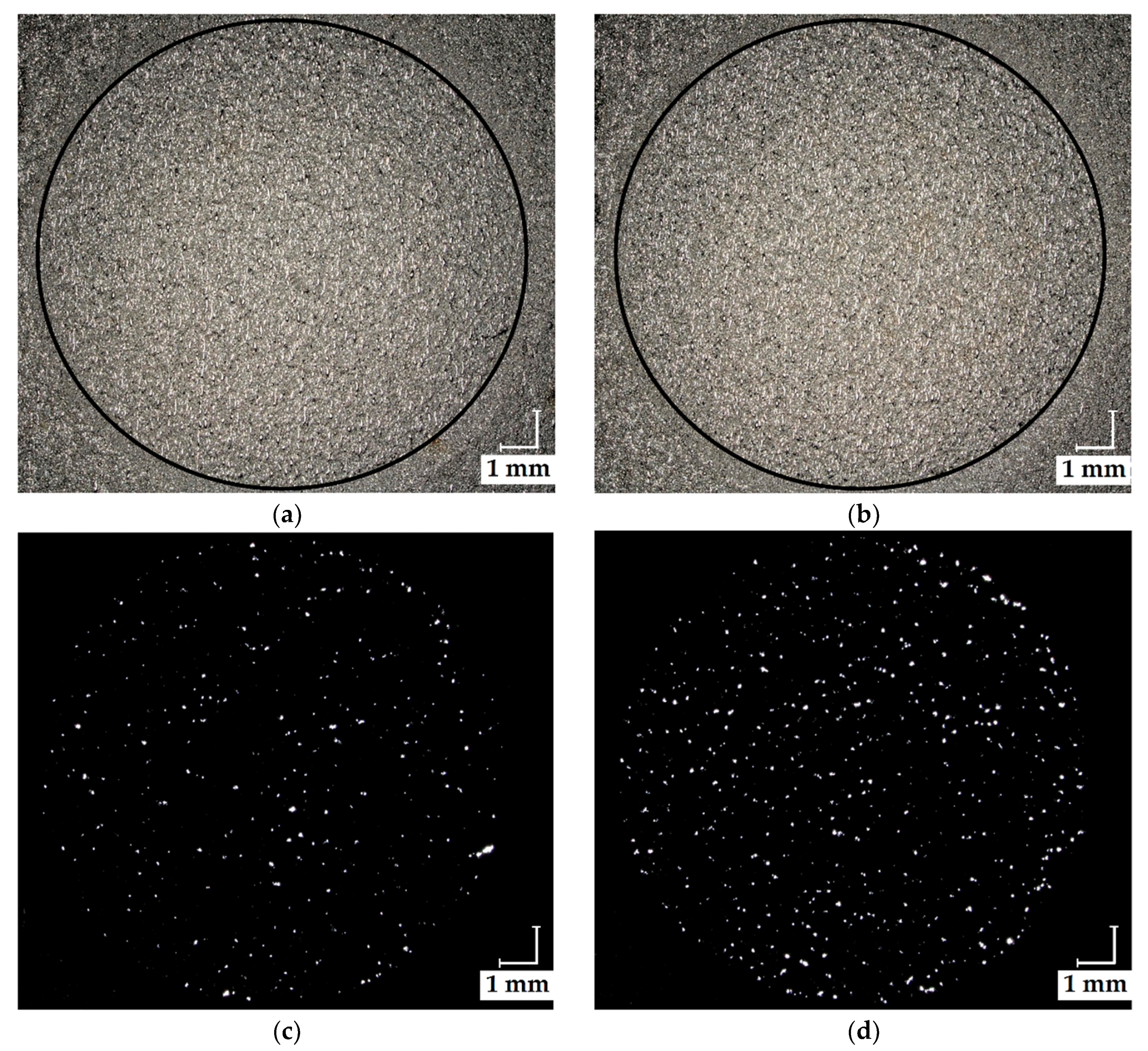

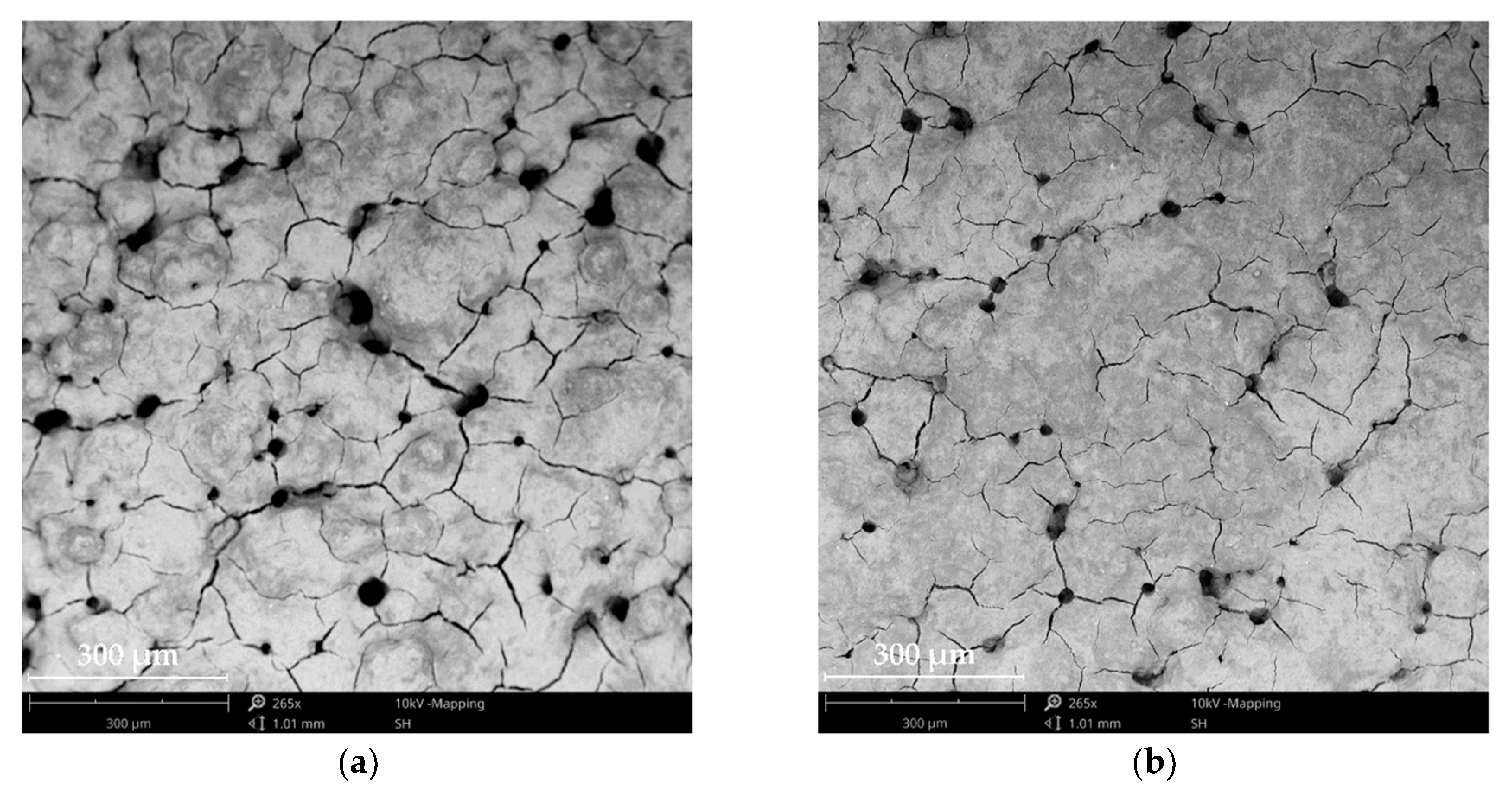
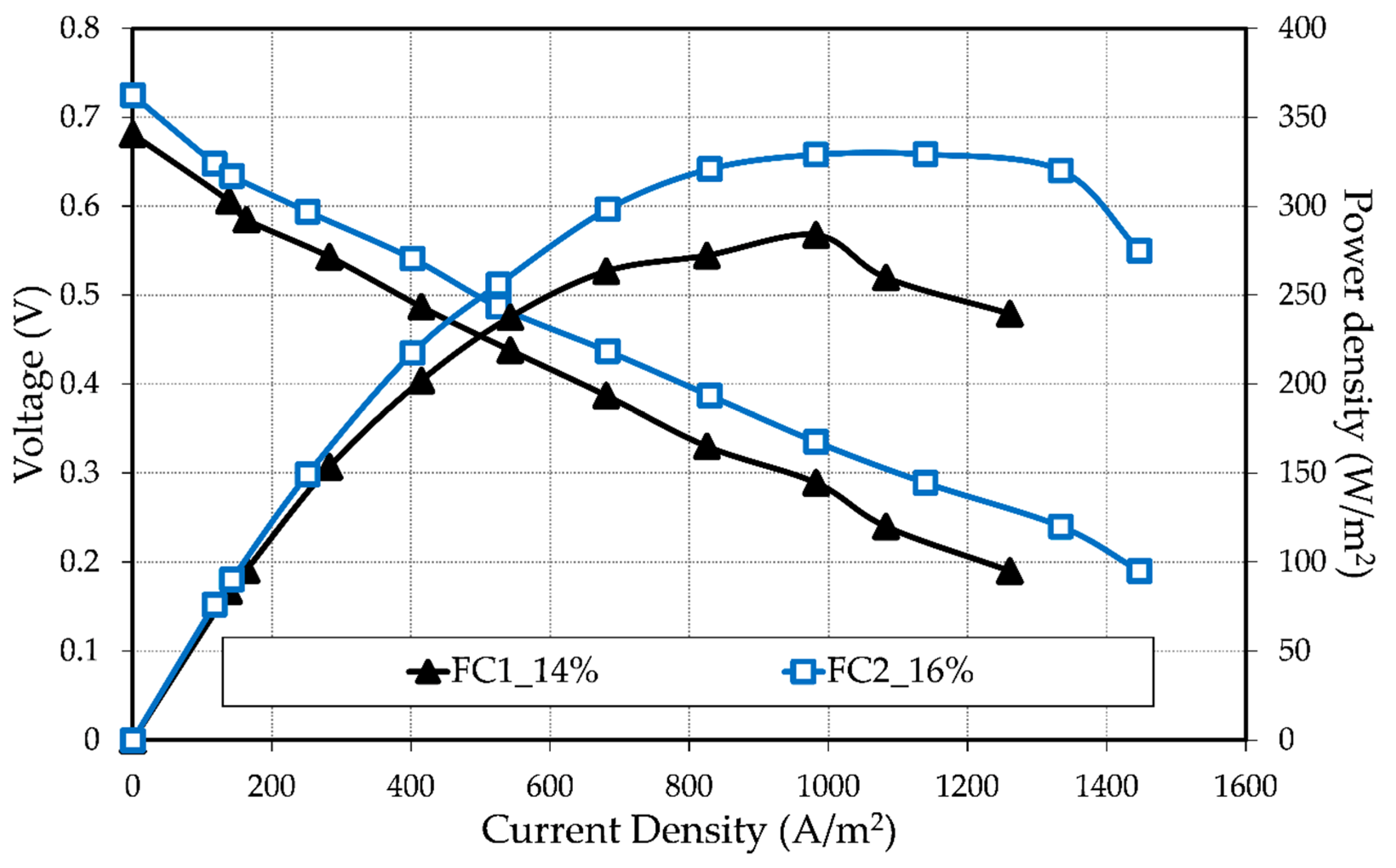

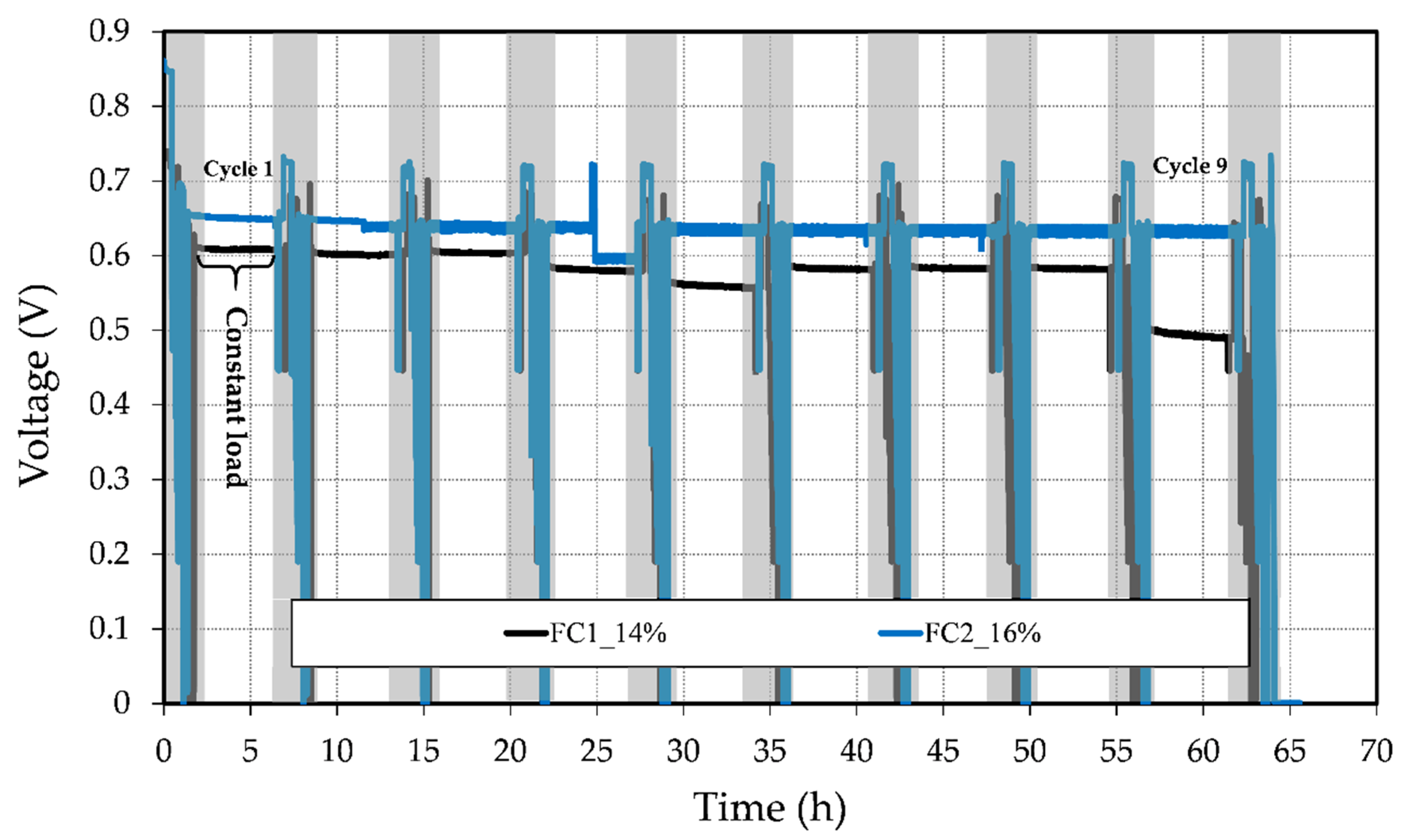
| Fuel Cell | Membrane Resistance, RΩ (mΩ) | Electrochemical Resistance, Rec (mΩ) |
|---|---|---|
| FC1_14% | 557 | 216 |
| FC2_16% | 548 | 182 |
| Fuel Cell | OCV Drop (V) | Voltage Degradation Rate (mV/h) |
|---|---|---|
| FC1_14% | 0.10 | 3.067 |
| FC2_16% | 0.13 | 0.511 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermúdez Agudelo, M.C.; Hampe, M.; Reiber, T.; Abele, E. Investigation of Porous Metal-Based 3D-Printed Anode GDLs for Tubular High Temperature Proton Exchange Membrane Fuel Cells. Materials 2020, 13, 2096. https://doi.org/10.3390/ma13092096
Bermúdez Agudelo MC, Hampe M, Reiber T, Abele E. Investigation of Porous Metal-Based 3D-Printed Anode GDLs for Tubular High Temperature Proton Exchange Membrane Fuel Cells. Materials. 2020; 13(9):2096. https://doi.org/10.3390/ma13092096
Chicago/Turabian StyleBermúdez Agudelo, María Catalina, Manfred Hampe, Thorsten Reiber, and Eberhard Abele. 2020. "Investigation of Porous Metal-Based 3D-Printed Anode GDLs for Tubular High Temperature Proton Exchange Membrane Fuel Cells" Materials 13, no. 9: 2096. https://doi.org/10.3390/ma13092096





