Hybrid Pectin-Based Sorbents for Cesium Ion Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Sorbent Preparation and Charactrization
2.3.1. Prussian Blue Preparation
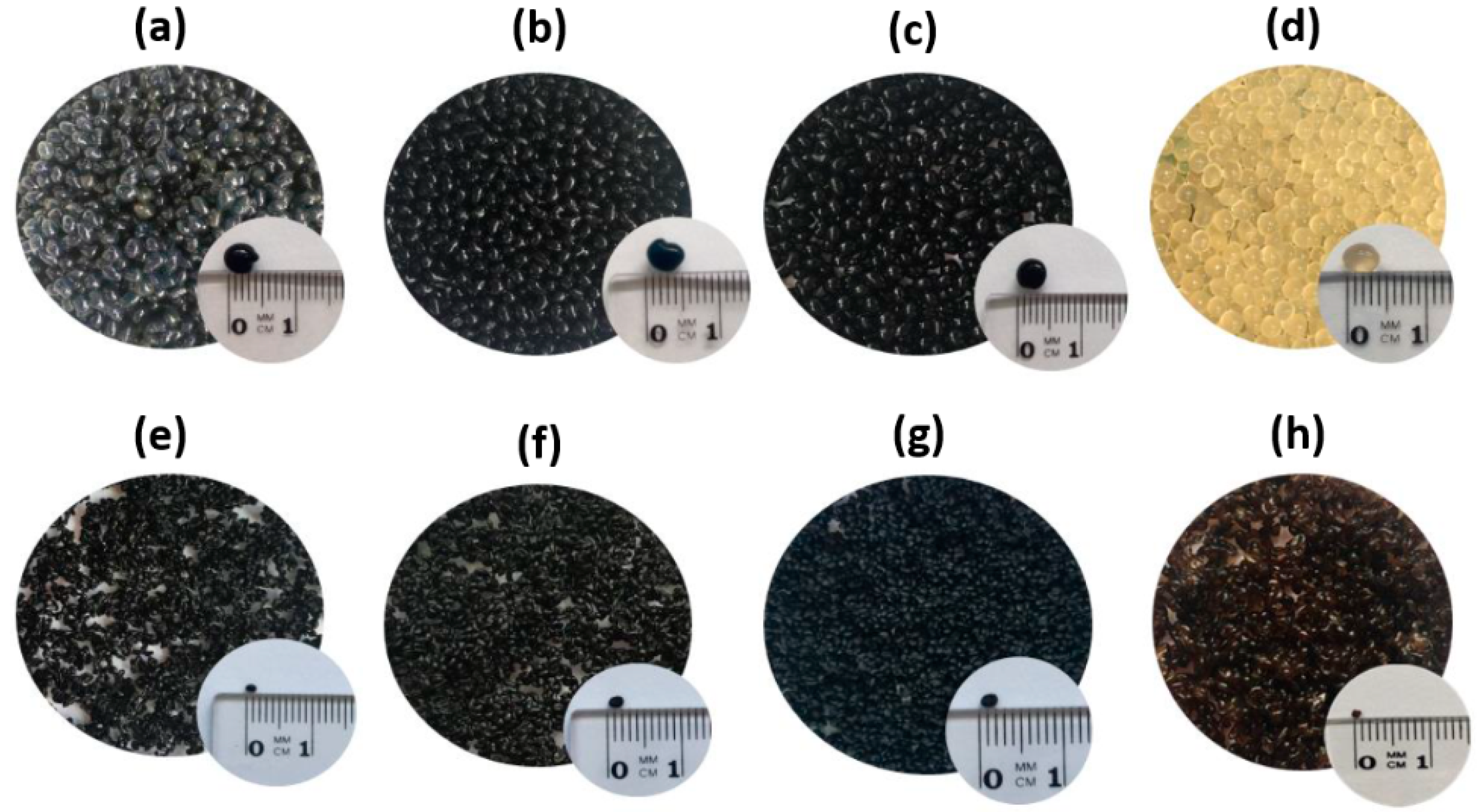
2.3.2. Hybrid Sorbents’ Preparation
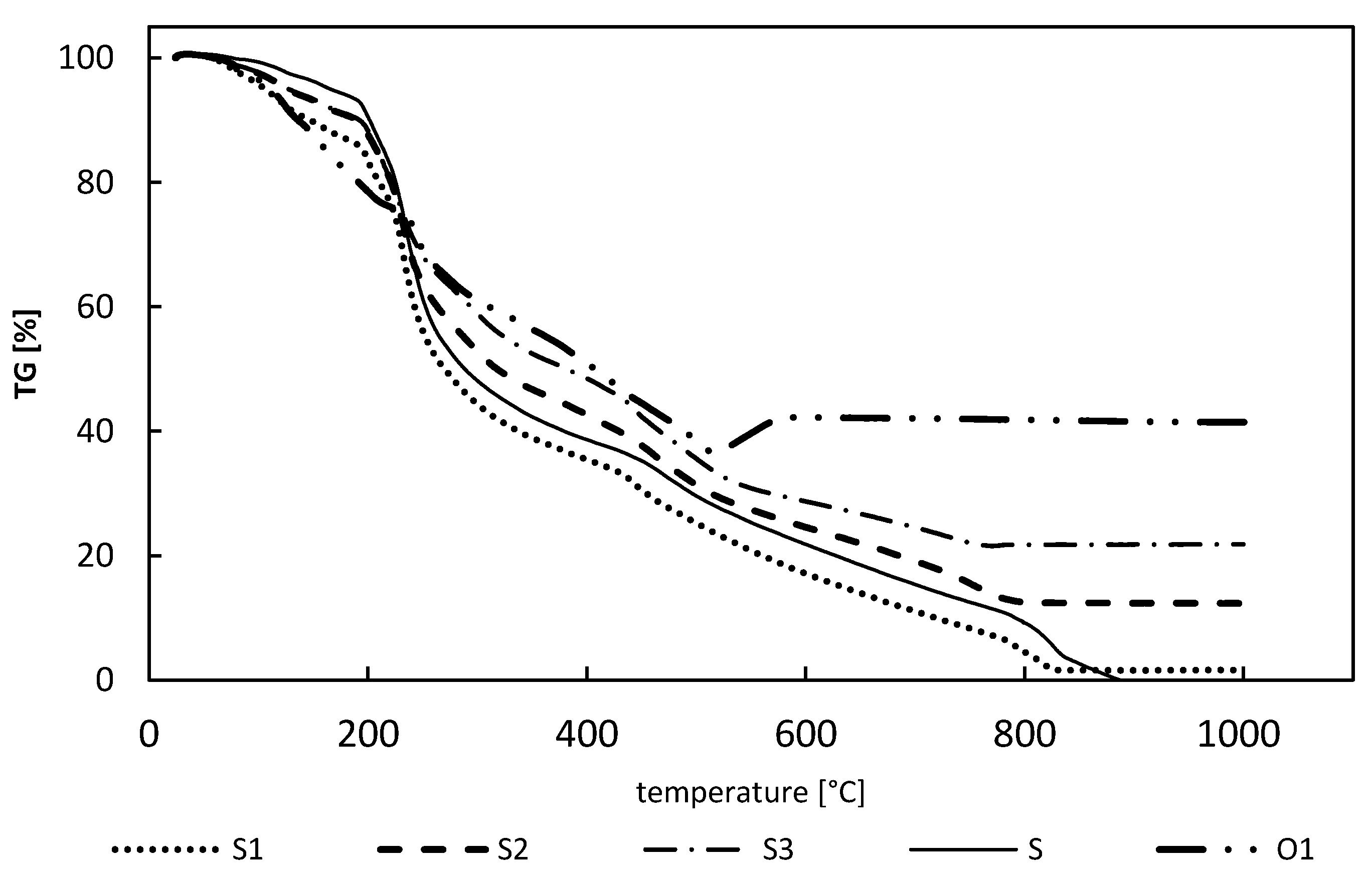
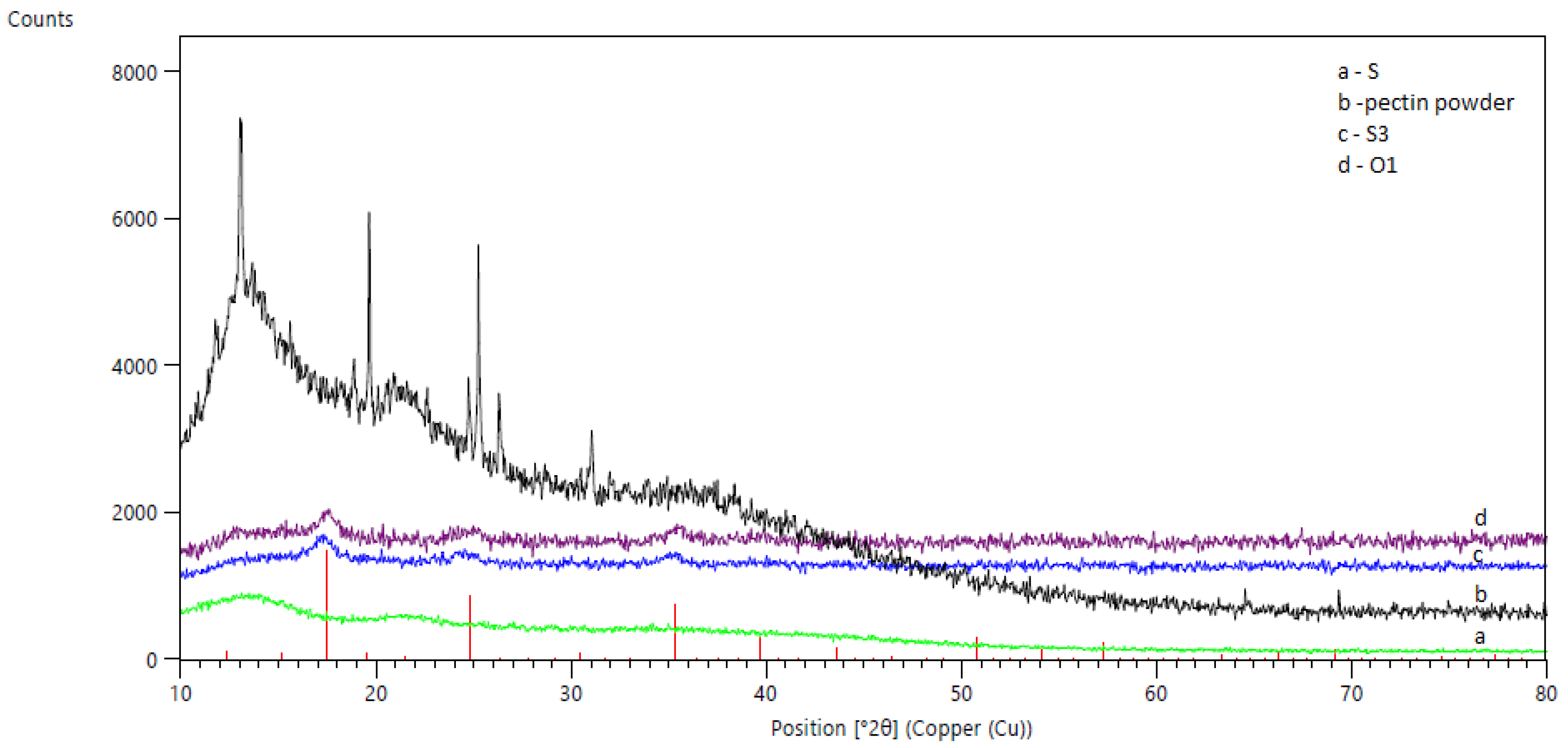
2.3.3. Materials Characterization
- ms—mass of the swollen sorbent [g],
- md—mass of the dry sorbent [g].
- mL—the mass of iron leached from a known amount of sorbent into solution,
- mFe—the mass of iron in the hybrid sorbent (calculated from TG analysis).
2.4. Preliminary Sorption Studies
2.4.1. General Sorption Examination
- c0—the initial concentration of cesium ions in the solution [mg/L],
- c—the final concentration of cesium ions in the solution [mg/L],
- V—the volume of the solution [L],
- m—the mass of the dry biosorbent [g].
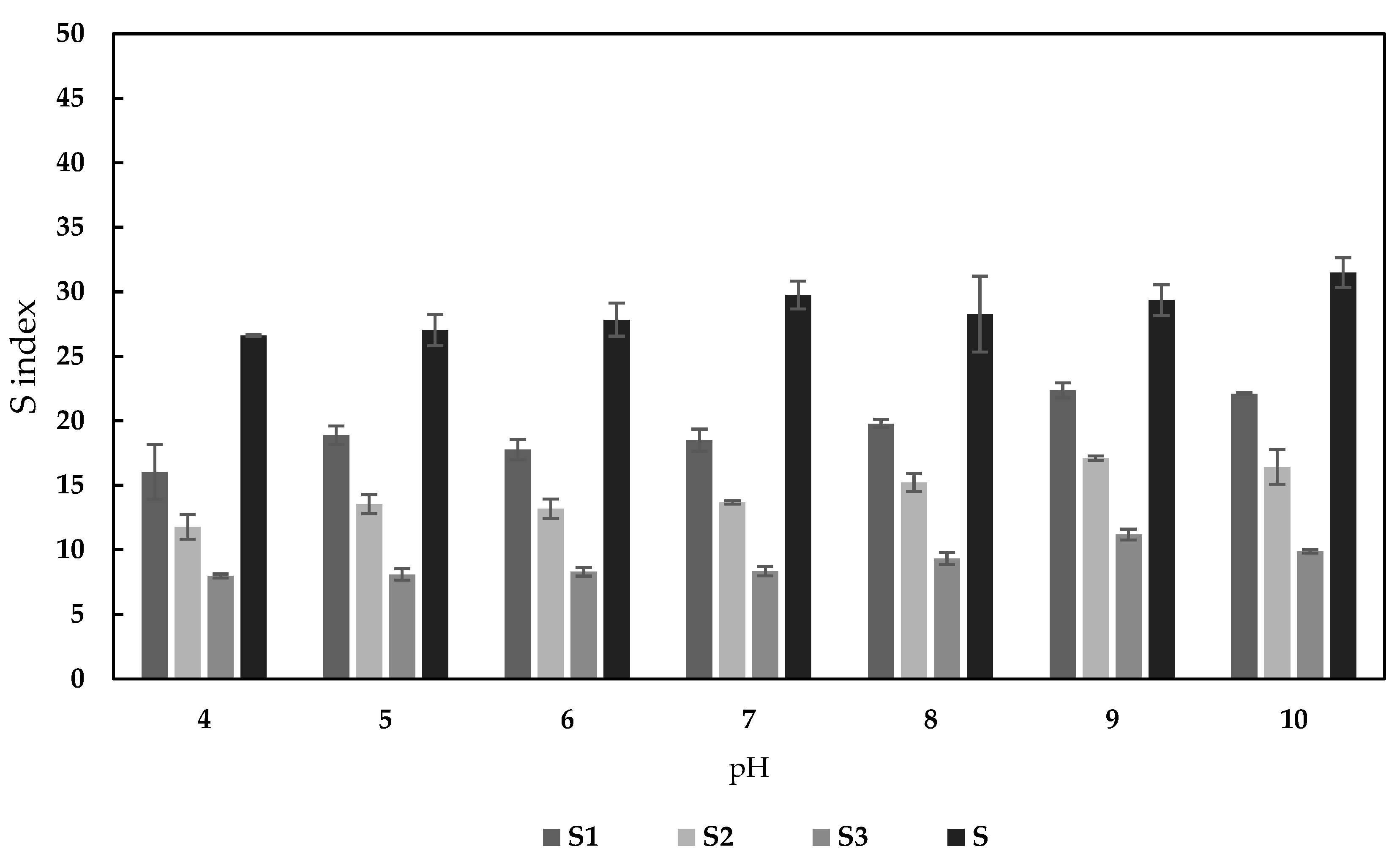
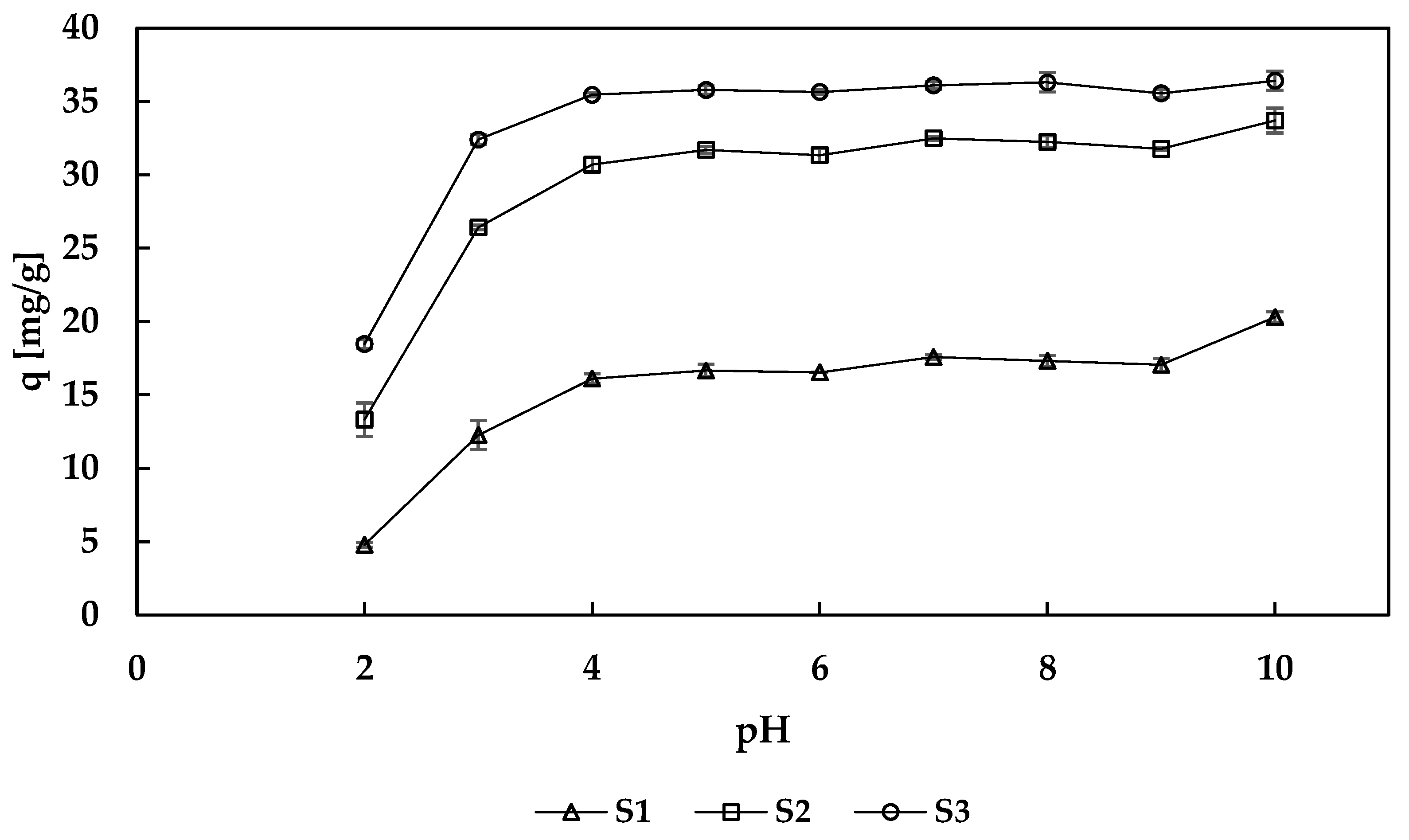
2.4.2. The Effect of the pH on Sorption Properties
2.4.3. The Effect of the Sorbent Dose on Sorption Capacity
- c0—the initial concentration of cesium ions in the solution [mg/L],
- c—the final concentration of cesium ions in the solution [mg/L].
3. Results
3.1. Sorbent Preparation and Charactrization
3.1.1. Prussian Blue Preparation and Characterization
3.1.2. Hybrid Sorbents’ Preparation and Characterization
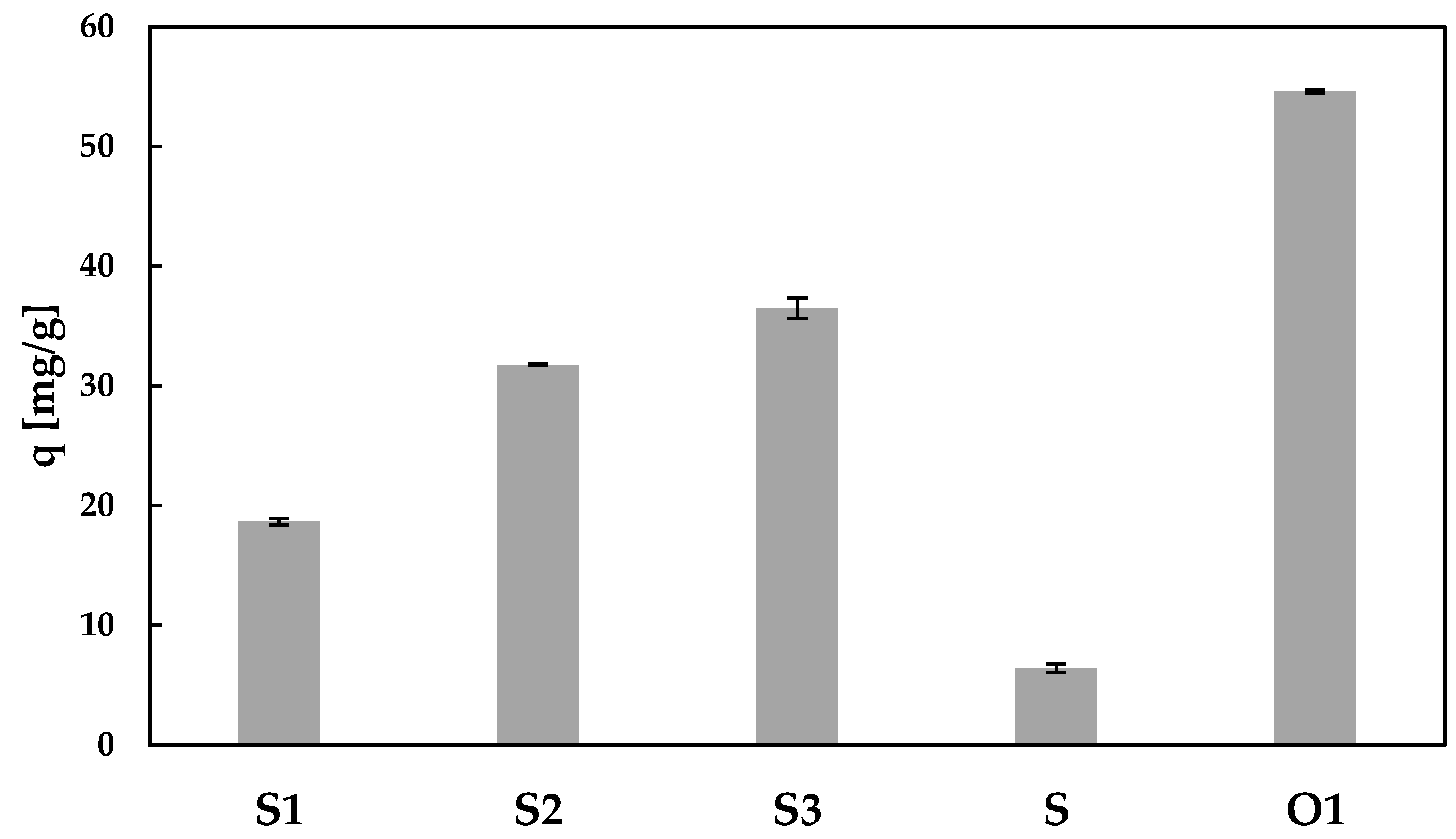
3.2. Preliminary Sorption Studies
3.2.1. Effect of the pH on Sorption Properties
3.2.2. Effect of the Sorbent Dose on Sorption Capacity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dancause, K.N.; Yevtushok, L.; Lapchenko, S.; Shumlyansky, I.; Shevchenko, G.; Wertelecki, W.; Garruto, R.M. Chronic radiation exposure in the Rivne-Polissia region of Ukraine: Implications for birth defects. Am. J. Hum. Biol. 2010, 22, 667–674. [Google Scholar] [CrossRef]
- Sekitani, Y.; Hayashida, N.; Karevskaya, I.V.; Vasilitsova, O.A.; Kozlovsky, A.; Omiya, M.; Yamashita, S.; Takamura, N. Evaluation of 137Cs body burden in inhabitants of Bryansk Oblast, Russian federation, where a high incidence of thyroid cancerwas observed after the accident at the Chernobyl nuclear power plant. Radiat. Prot. Dosim. 2010, 141, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheha, R.R. Synthesis and characterization of magnetic hexacyanoferrate (II) polymeric nanocomposite for separation of cesium from radioactive waste solutions. J. Colloid Interface Sci. 2012, 388, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska-Trznadel, G. Radioactive solutions treatment by hybrid complexation-UF/NF process. J. Memb. Sci. 2003, 225, 25–39. [Google Scholar] [CrossRef]
- Zakrzewska-Trznadel, G. Membrane processes for environmental protection: Applications in nuclear. Nukleonika 2006, 51, 101–111. [Google Scholar]
- Vincent, T.; Vincent, C.; Barré, Y.; Guari, Y.; Le Saout, G.; Guibal, E. Immobilization of metal hexacyanoferrates in chitin beads for cesium sorption: Synthesis and characterization. J. Mater. Chem. A 2014, 2, 10007. [Google Scholar] [CrossRef]
- Wiley, J.R. Decontamination of Alkaline Radioactive Waste by Ion Exchange. Ind. Eng. Chem. Process Des. Dev. 1978, 17, 67–71. [Google Scholar] [CrossRef]
- Ararem, A.; Bouras, O.; Bouzidi, A. Batch and continuous fixed-bed column adsorption of Cs+ and Sr2+ onto montmorillonite-iron oxide composite: Comparative and competitive study. J. Radioanal. Nucl. Chem. 2013, 298, 537–545. [Google Scholar] [CrossRef]
- Balarama Krishna, M.V.; Rao, S.V.; Arunachalam, J.; Murali, M.S.; Kumar, S.; Manchanda, V.K. Removal of 137Cs and 90Sr from actual low level radioactive waste solutions using moss as a phyto-sorbent. Sep. Purif. Technol. 2004, 38, 149–161. [Google Scholar] [CrossRef]
- Caccin, M.; Giacobbo, F.; Da Ros, M.; Besozzi, L.; Mariani, M. Adsorption of uranium, cesium and strontium onto coconut shell activated carbon. J. Radioanal. Nucl. Chem. 2013, 297, 9–18. [Google Scholar] [CrossRef]
- Chakraborty, D.; Maji, S.; Bandyopadhyay, A.; Basu, S. Biosorption of cesium-137 and strontium-90 by mucilaginous seeds of Ocimum basilicum. Bioresour. Technol. 2007, 98, 2949–2952. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Removal of Pb2+, Ag+, Cs+ and Sr2+ from aqueous solution by brewery’s waste biomass. J. Hazard. Mater. 2008, 151, 65–70. [Google Scholar] [CrossRef]
- Ding, D.; Zhao, Y.; Yang, S.; Shi, W.; Zhang, Z.; Lei, Z.; Yang, Y. Adsorption of cesium from aqueous solution using agricultural residue—Walnut shell: Equilibrium, kinetic and thermodynamic modeling studies. Water Res. 2013, 47, 2563–2571. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.O.; Lee, S.M.; Jeon, C. Adsorption characteristics of sericite for cesium ions from an aqueous solution. Chem. Eng. Res. Des. 2014, 92, 368–374. [Google Scholar] [CrossRef]
- Sangvanich, T.; Sukwarotwat, V.; Wiacek, R.J.; Grudzien, R.M.; Fryxell, G.E.; Addleman, R.S.; Timchalk, C.; Yantasee, W. Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functionalized mesoporous silica. J. Hazard. Mater. 2010, 182, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Thammawong, C.; Opaprakasit, P.; Tangboriboonrat, P.; Sreearunothai, P. Prussian blue-coated magnetic nanoparticles for removal of cesium from contaminated environment. J. Nanoparticle Res. 2013, 15, 1689. [Google Scholar] [CrossRef]
- Yavari, R.; Huang, Y.D.; Ahmadi, S.J. Adsorption of cesium (I) from aqueous solution using oxidized multiwall carbon nanotubes. J. Radioanal. Nucl. Chem. 2011, 287, 393–401. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pathak, S.K.; Kumar, M.; Tripathi, S.C.; Bajaj, P.N. Potassium cobalthexacyanoferrate-gel beads for cesium removal: Kinetics and sorption studies. RSC Adv. 2013, 3, 22102. [Google Scholar] [CrossRef]
- Vipin, A.K.; Ling, S.; Fugetsu, B. Sodium cobalt hexacyanoferrate encapsulated in alginate vesicle with CNT for both cesium and strontium removal. Carbohydr. Polym. 2014, 111, 477–484. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Zhai, J.; Sun, L.; Zhao, Y.; Yu, H. Magnetic prussian blue/graphene oxide nanocomposites caged in calcium alginate microbeads for elimination of cesium ions from water and soil. Chem. Eng. J. 2014, 246, 10–19. [Google Scholar] [CrossRef]
- Nilchi, A.; Saberi, R.; Moradi, M.; Azizpour, H.; Zarghami, R. Adsorption of cesium on copper hexacyanoferrate-PAN composite ion exchanger from aqueous solution. Chem. Eng. J. 2011, 172, 572–580. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Walter, R.H.; Taylor, S. The Chemistry and Technology of Pectin; Elsevier Science: Amsterdam, The Netherlands, 2012; ISBN 9780080926445. [Google Scholar]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Harel, P.; Mignot, L.; Sauvage, J.-P.; Junter, G.-A. Cadmium removal from dilute aqueous solution by gel beads of sugar beet pectin. Ind. Crops Prod. 1998, 7, 239–247. [Google Scholar] [CrossRef]
- Mata, Y.N.; Blázquez, M.L.; Ballester, A.; González, F.; Muñoz, J.A. Sugar-beet pulp pectin gels as biosorbent for heavy metals: Preparation and determination of biosorption and desorption characteristics. Chem. Eng. J. 2009, 150, 289–301. [Google Scholar] [CrossRef]
- Bok-Badura, J.; Jakóbik-Kolon, A.; Karoń, K.; Mitko, K. Sorption studies of heavy metal ions on pectin-nano-titanium dioxide composite adsorbent. Sep. Sci. Technol. 2018, 53, 1034–1044. [Google Scholar] [CrossRef]
- Jakóbik-Kolon, A.; Szybaj, A.; Mitko, K.; Bok-Badura, J. Zinc ion removal on hybrid pectin-based beads containing modified poly(methyl methacrylate) waste. Molecules 2017, 22, 2274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadam, A.A.; Jang, J.; Lee, D.S. Facile synthesis of pectin-stabilized magnetic graphene oxide Prussian blue nanocomposites for selective cesium removal from aqueous solution. Bioresour. Technol. 2016, 216, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.M.; Jeon, E.K.; Baek, K. Selective and irreversible adsorption mechanism of cesium on illite. Appl. Geochem. 2017, 85, 188–193. [Google Scholar] [CrossRef]
- Khandaker, S.; Toyohara, Y.; Kamida, S.; Kuba, T. Adsorptive removal of cesium from aqueous solution using oxidized bamboo charcoal. Water Resour. Ind. 2018, 19, 35–46. [Google Scholar] [CrossRef]
- Vincent, T.; Taulemesse, J.M.; Dauvergne, A.; Chanut, T.; Testa, F.; Guibal, E. Thallium(I) sorption using Prussian blue immobilized in alginate capsules. Carbohydr. Polym. 2014, 99, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Poudel, K.; Yong, C.S.; Kim, J.O. Prussian blue nanoparticles: Synthesis, surface modification, and application in cancer treatment. Int. J. Pharm. 2018, 549, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Torad, N.L.; Hu, M.; Imura, M.; Naito, M.; Yamauchi, Y. Large Cs adsorption capability of nanostructured Prussian Blue particles with high accessible surface areas. J. Mater. Chem. 2012, 22, 18261. [Google Scholar] [CrossRef]
- Hu, B.; Fugetsu, B.; Yu, H.; Abe, Y. Prussian blue caged in spongiform adsorbents using diatomite and carbon nanotubes for elimination of cesium. J. Hazard. Mater. 2012, 217–218, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.R.; Chang, Y.R.; Liu, X.; Kawamoto, T.; Tanaka, H.; Kitajima, A.; Parajuli, D.; Takasaki, M.; Yoshino, K.; Chen, M.L.; et al. Prussian blue (PB) granules for cesium (Cs) removal from drinking water. Sep. Purif. Technol. 2015, 143, 146–151. [Google Scholar] [CrossRef]
- Vipin, A.K.; Hu, B.; Fugetsu, B. Prussian blue caged in alginate/calcium beads as adsorbents for removal of cesium ions from contaminated water. J. Hazard. Mater. 2013, 258–259, 93–101. [Google Scholar] [CrossRef]
- Jang, J.; Lee, D.S. Magnetic Prussian Blue Nanocomposites for Effective Cesium Removal from Aqueous Solution. Ind. Eng. Chem. Res. 2016, 55, 3852–3860. [Google Scholar] [CrossRef]
- Ricci, F.; Palleschi, G. Sensor and biosensor preparation, optimisation and applications of Prussian Blue modified electrodes. Biosens. Bioelectron. 2005, 21, 389–407. [Google Scholar] [CrossRef]
- Ishizaki, M.; Akiba, S.; Ohtani, A.; Hoshi, Y.; Ono, K.; Matsuba, M.; Togashi, T.; Kananizuka, K.; Sakamoto, M.; Takahashi, A.; et al. Proton-exchange mechanism of specific Cs+ adsorption via lattice defect sites of Prussian blue filled with coordination and crystallization water molecules. Dalt. Trans. 2013, 42, 16049. [Google Scholar] [CrossRef]


| Prussian Blue Powder | Ratio * | Conditions of Drying and Mixing | Sorption Capacity [mg/g] |
|---|---|---|---|
| O | 1:1.15 | - | - |
| O1 | 1:1.5 | 40 °C, 1 h | 54.6 ± 0.1 |
| O2 | 1:2.3 | 40 °C, 1 h | 55.0 ± 0.4 |
| O3 | 1:1.5 | 40 °C, 24 h | 51.4 ± 1.0 |
| O4 | 1:1.5 | 80 °C, 1 h | 41.8 ± 1.6 |
| O5 | 1:1.5 | 105 °C, 1 h | 36.3 ± 0.4 |
| % of Iron Leached from S1 Hybrid Sorbent (%) | % of Iron Leached from S2 Hybrid Sorbent (%) | % of Iron Leached from S3 Hybrid Sorbent (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| days | 1 | 4 | 7 | 1 | 4 | 7 | 1 | 4 | 7 |
| pH | |||||||||
| 2 | 0.69 | 1.05 | 1.33 | 0.97 | 1.45 | 1.80 | 1.10 | 1.89 | 2.53 |
| 3 | 0.02 | 0.01 | <0.01 | 0.01 | 0.01 | 0.01 | 0.01 | <0.01 | <0.01 |
| 4 | 0.04 | 0.03 | 0.03 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 5 | 0.06 | 0.04 | 0.03 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 6 | 0.04 | 0.03 | 0.03 | 0.02 | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 7 | 0.05 | 0.04 | 0.03 | 0.04 | 0.02 | 0.01 | <0.01 | <0.01 | <0.01 |
| 8 | 0.04 | 0.03 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | <0.01 | <0.01 |
| 9 | 0.07 | 0.06 | 0.09 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 10 | 0.06 | 0.06 | 0.06 | 0.05 | 0.04 | 0.21 | 0.01 | <0.01 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bok-Badura, J.; Jakóbik-Kolon, A.; Kazek-Kęsik, A.; Karoń, K. Hybrid Pectin-Based Sorbents for Cesium Ion Removal. Materials 2020, 13, 2160. https://doi.org/10.3390/ma13092160
Bok-Badura J, Jakóbik-Kolon A, Kazek-Kęsik A, Karoń K. Hybrid Pectin-Based Sorbents for Cesium Ion Removal. Materials. 2020; 13(9):2160. https://doi.org/10.3390/ma13092160
Chicago/Turabian StyleBok-Badura, Joanna, Agata Jakóbik-Kolon, Alicja Kazek-Kęsik, and Krzysztof Karoń. 2020. "Hybrid Pectin-Based Sorbents for Cesium Ion Removal" Materials 13, no. 9: 2160. https://doi.org/10.3390/ma13092160





