Metal Oxide Compact Electron Transport Layer Modification for Efficient and Stable Perovskite Solar Cells
Abstract
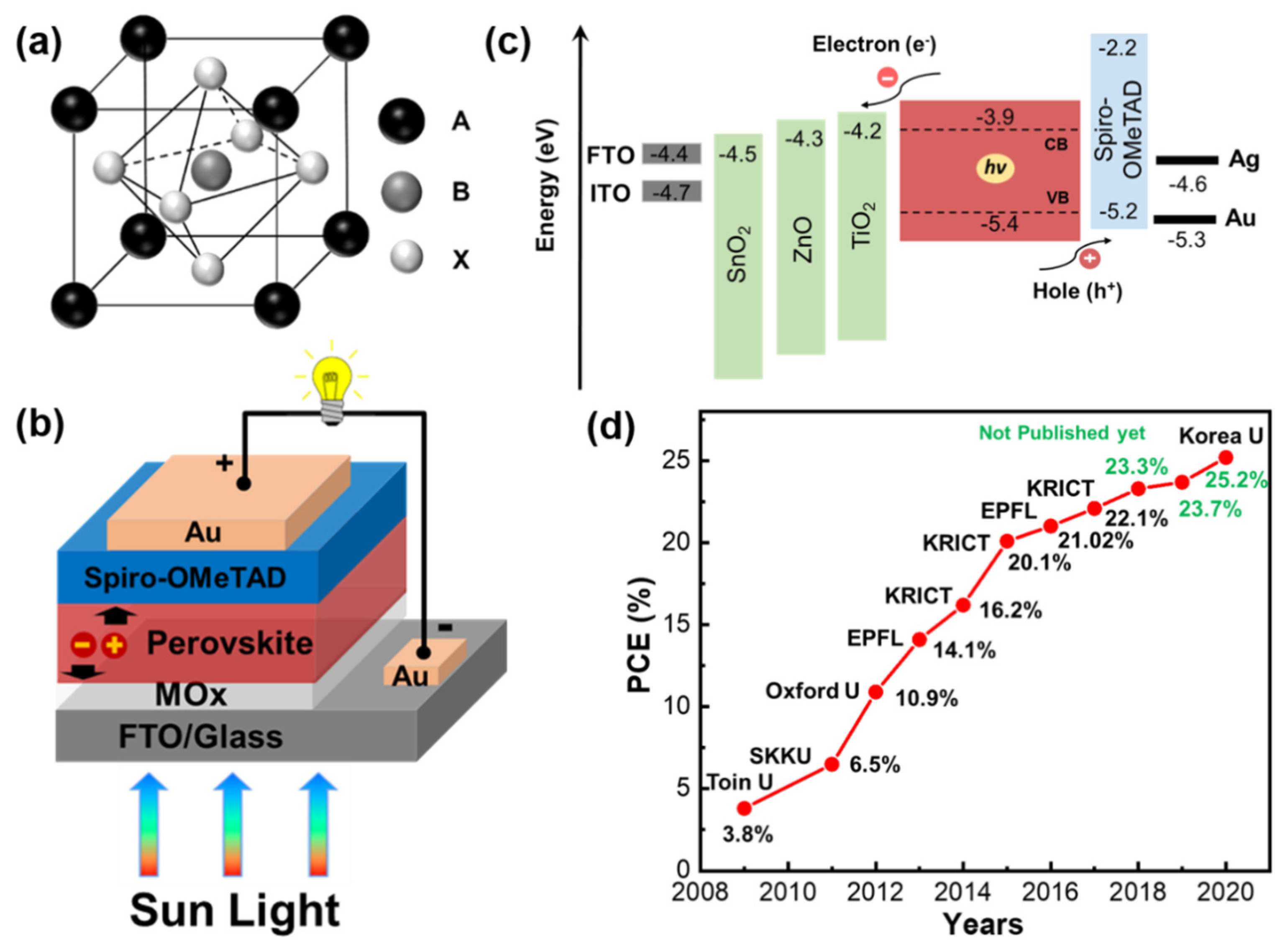
:1. Introduction
2. Metal Oxide Compact Electron Transport Layers
3. Titanium Dioxide (TiO2)
3.1. TiO2 Compact Layers (CLs)
3.1.1. Sol–Gel Technique
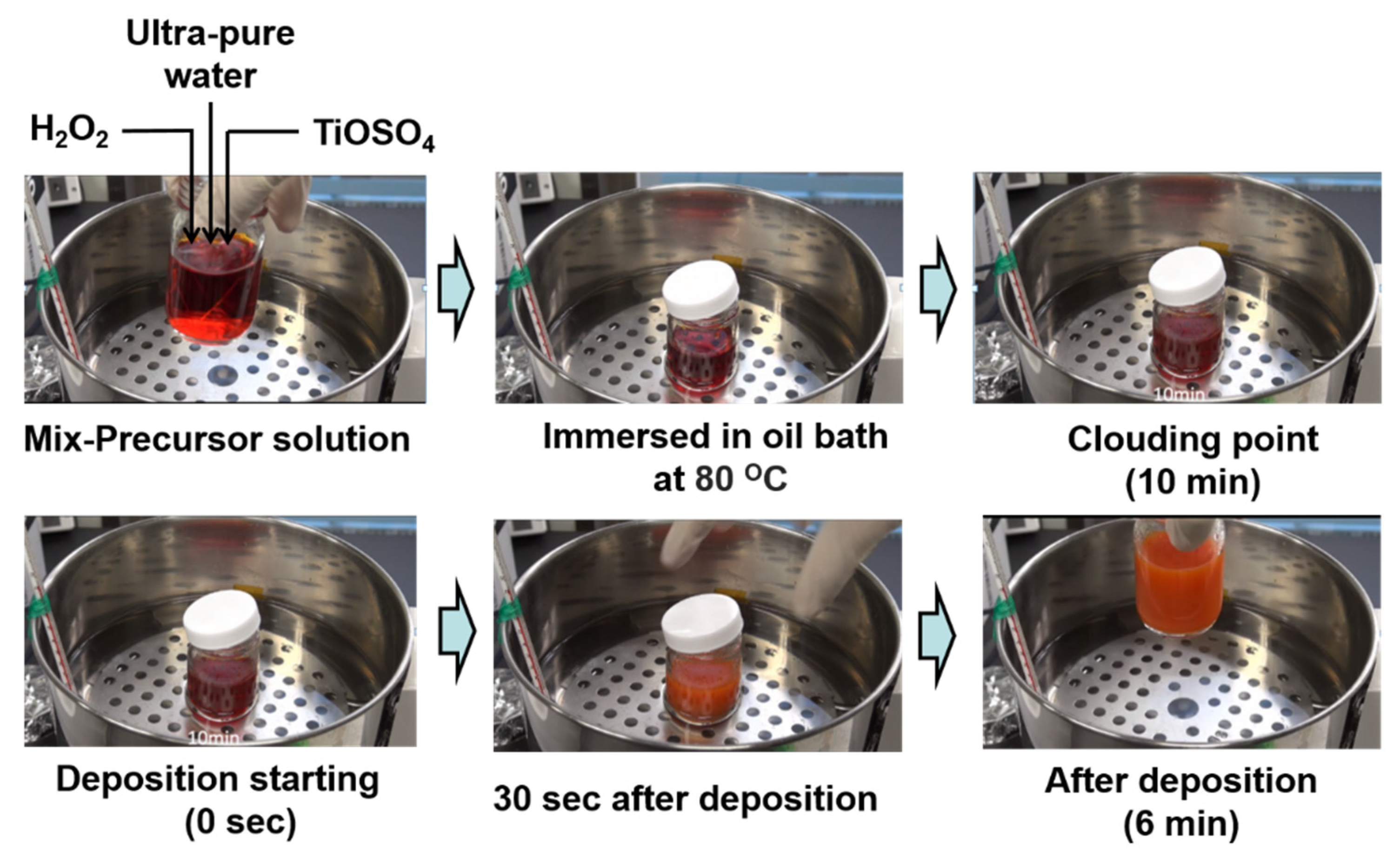
3.1.2. Chemical Bath Deposition (CBD)
3.1.3. Inkjet Printing Approaches
3.2. Surface Modification by TiO2 Nanoparticles
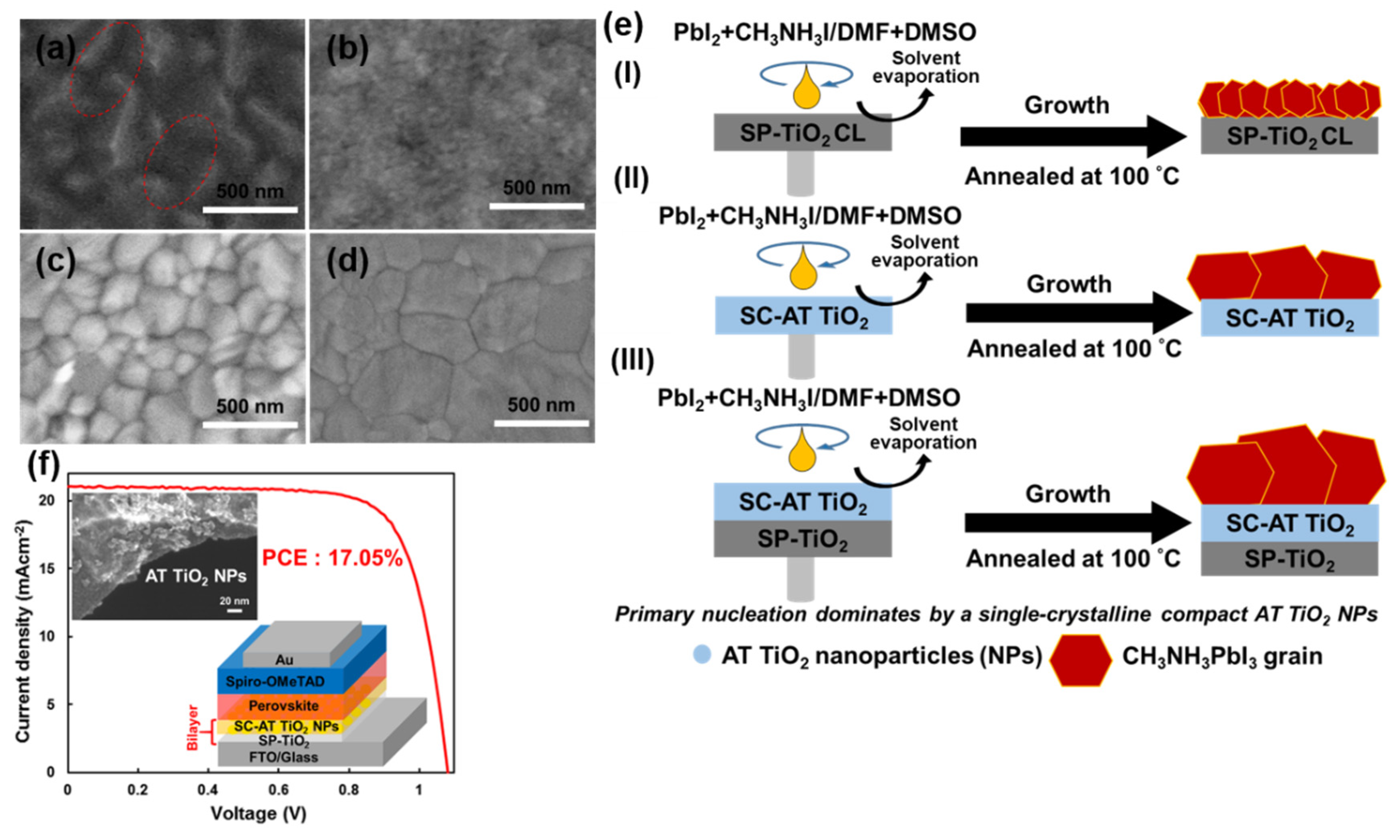
3.2.1. Anatase TiO2 Nanoparticles (NPs)
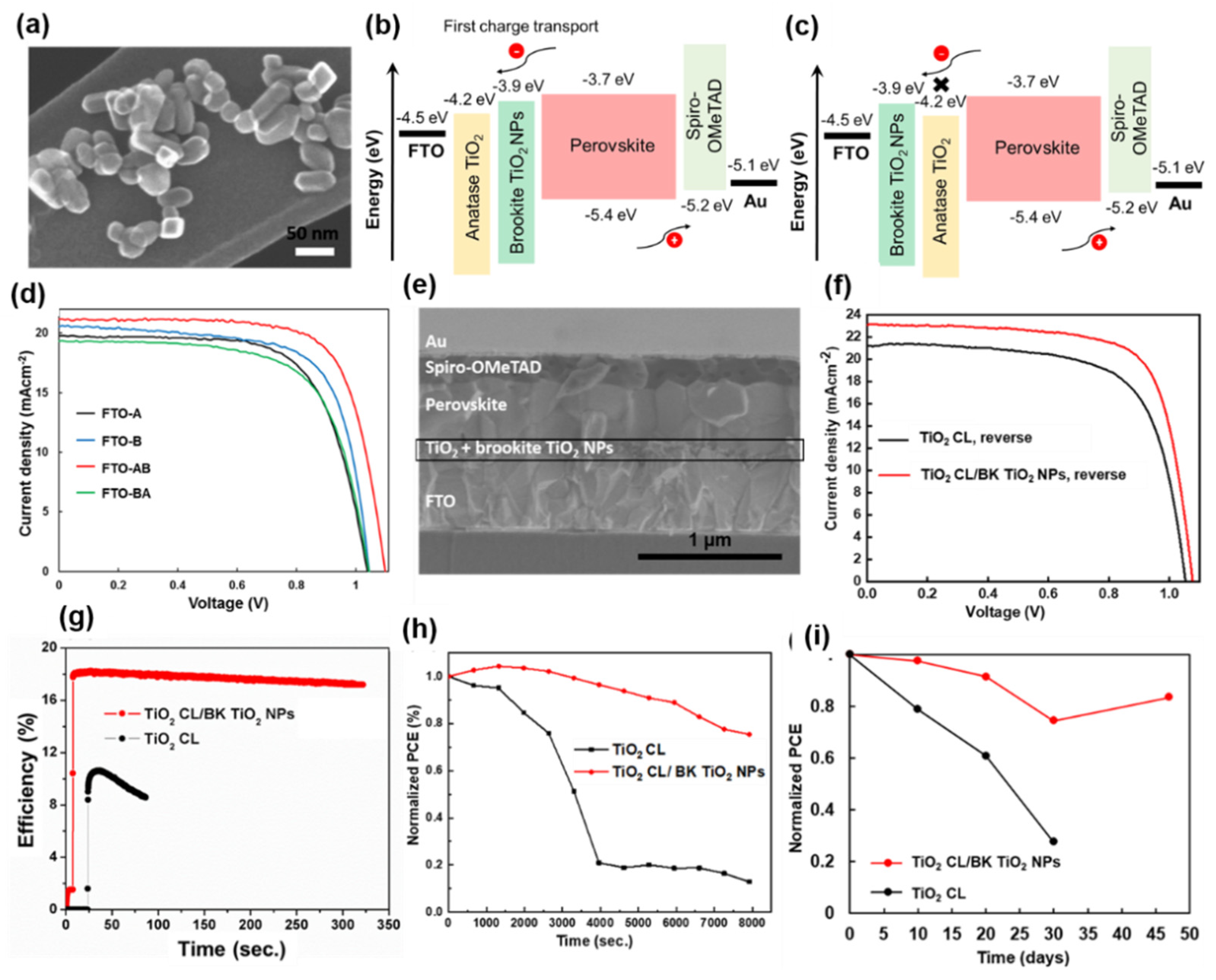
3.2.2. Brookite TiO2 NPs
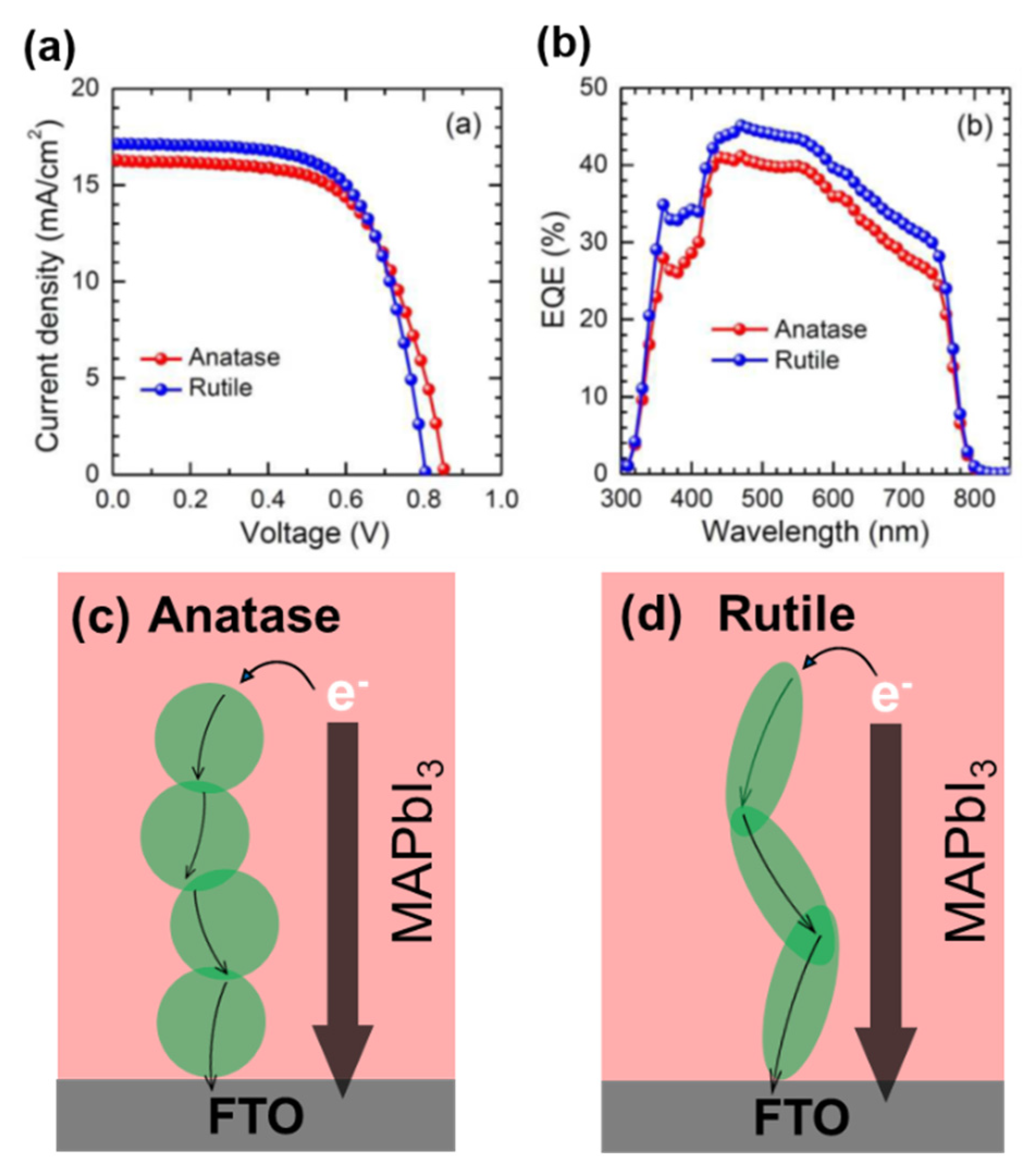
3.2.3. Rutile TiO2 NPs
3.3. Mesoporous TiO2
3.4. Tin Dioxide
3.5. Zinc Oxide
4. Other Metal Oxides
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beggs, C. Energy: Management, Supply and Conservation; Elsevier Ltd.: Oxford, UK, 2012. [Google Scholar]
- Priya, S.; Inman, D.J. Energy Harvesting Technologies; Springer Science: New York, NY, USA, 2008. [Google Scholar]
- Green, M.A. Thin-film solar cells: Review of materials, technologies and commercial status. J. Mater. Sci. Mater. Electron. 2007, 18, 15–19. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Green, M.A. The path to 25% silicon solar cell efficiency: History of silicon cell evolution. Prog. Photovolt. Res. Appl. 2009, 17, 183–189. [Google Scholar] [CrossRef]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Cui, D.; Ren, X.; Sun, J.; Liu, X.; Zhang, J.; Wei, Q.; Fan, H.; Yu, F.; et al. Two-inch-sized perovskite CH3NH3PbX3 (X = Cl, Br, I) crystals: Growth and characterization. Adv. Mater. 2015, 27, 5176–5183. [Google Scholar] [CrossRef]
- Luo, D.; Yang, W.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z.; et al. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef] [Green Version]
- Snaith, H.J. Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/assets/pdfs/pv-efficiencies-04-14-2020.pdf (accessed on 14 April 2020).
- Yang, J.; Yuan, Z.; Liu, X.; Braun, S.; Li, Y.; Tang, J.; Gao, F.; Duan, C.-G.; Fahlman, M.; Bao, Q. Oxygen- and water-induced energetics degradation in organometal halide perovskites. ACS Appl. Mater. Interfaces 2018, 10, 16225–16230. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; You, J.; Yang, Y. Addressing the stability issue of perovskite solar cells for commercial applications. Nat. Commun. 2018, 9, 5265. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhou, Y.; Jiang, Q.; Ye, Q.; Chu, Z.; Li, X.; Yang, X.; Yin, Z.; You, J. Solvent-controlled growth of inorganic perovskite films in dry environment for efficient and stable solar cells. Nat. Commun. 2018, 9, 2225. [Google Scholar] [CrossRef]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jena, A.K.; Kulkarni, A.; Sanehira, Y.; Ikegami, M.; Miyasaka, T. Stabilization of α-CsPbI3 in ambient room temperature conditions by incorporating Eu into CsPbI3. Chem. Mater. 2018, 30, 6668–6674. [Google Scholar] [CrossRef]
- Pant, N.; Kulkarni, A.; Yanagida, M.; Shirai, Y.; Miyasaka, T.; Miyano, K. Investigating the growth of CH3NH3PbI3 Thin Films on RF-sputtered NiOx for inverted planar perovskite solar cells: Effect of CH3NH3+ halide additives versus CH3NH3+ halide vapor annealing. Adv. Mater. Interfaces 2020, 7. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kulkarni, A.; Kim, G.M.; Öz, S.; Jena, A.K. Perovskite solar cells: Can we go organic-free, lead-free, and dopant-free? Adv. Energy Mater. 2020, 10, 1902500. [Google Scholar] [CrossRef]
- Zong, Y.; Zhou, Y.; Zhang, Y.; Li, Z.; Zhang, L.; Ju, M.-G.; Chen, M.; Pang, S.; Zeng, X.C.; Padture, N.P. Continuous grain-boundary functionalization for high-efficiency perovskite solar cells with exceptional stability. Chem 2018, 4, 1404–1415. [Google Scholar] [CrossRef] [Green Version]
- Saidaminov, M.I.; Kim, J.; Jain, A.; Quintero-Bermudez, R.; Tan, H.; Long, G.; Tan, F.; Johnston, A.; Zhao, Y.; Voznyy, O.; et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat. Energy 2018, 3, 648–654. [Google Scholar] [CrossRef]
- Chaudhary, B.; Kulkarni, A.; Jena, A.K.; Ikegami, M.; Udagawa, Y.; Kunugita, H.; Ema, K.; Miyasaka, T. Poly(4-vinylpyridine)-based interfacial passivation to enhance voltage and moisture stability of lead halide perovskite solar cells. ChemSusChem 2017, 10, 2473–2479. [Google Scholar] [CrossRef]
- Yang, J.-M.; Luo, Y.; Bao, Q.; Li, Y.-Q.; Tang, J.-X. Hall of fame article: Recent advances in energetics and stability of metal halide perovskites for optoelectronic applications. Adv. Mater. Interfaces 2019, 6, 1801351. [Google Scholar] [CrossRef] [Green Version]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Yi, C.; Luo, J.; Décoppet, J.-D.; Zhang, F.; Zakeeruddin, S.M.; Li, X.; Hagfeldt, A.; Grätzel, M. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy 2016, 1, 16142. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Marks, T.J.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Wuβler, M.; Fabregat-Santiago, F.; Lakus-Wollny, K.; Mankel, E.; Mayer, T.; Jaegermann, W.; Mora-Seró, I. Role of the selective contacts in the performance of lead halide perovskite solar cells. J. Phys. Chem. Lett. 2014, 5, 680–685. [Google Scholar] [CrossRef]
- Moehl, T.; Im, J.H.; Lee, Y.H.; Domanski, K.; Giordano, F.; Zakeeruddin, S.M.; Dar, M.I.; Heiniger, L.-P.; Nazeeruddin, M.K.; Park, N.-G.; et al. Strong photocurrent amplification in perovskite solar cells with a porous TiO2 blocking layer under reverse bias. J. Phys. Chem. Lett. 2014, 5, 3931–3936. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Fang, G.; Wang, J.; Qin, P.; Tao, H.; Lei, H.; Liu, Q.; Dai, X.; Zhao, X. Perovskite solar cell with an efficient TiO2 compact film. ACS Appl. Mater. Interfaces 2014, 6, 15959–15965. [Google Scholar] [CrossRef]
- Han, J.; Kwon, H.; Kim, E.; Kim, D.-W.; Son, H.J. Interfacial engineering of a ZnO electron transporting layer using self-assembled monolayers for high performance and stable perovskite solar cells. J. Mater. Chem. A 2020, 8, 2105–2113. [Google Scholar] [CrossRef]
- Anaraki, E.H.; Kermanpur, A.; Steier, L.; Domanski, K.; Matsui, T.; Tress, W.R.; Saliba, M.; Abate, A.; Grätzel, M.; Hagfeldt, A.; et al. Highly efficient and stable planar perovskite solar cells by solution-processed tin oxide. Energy Environ. Sci. 2016, 9, 3128–3134. [Google Scholar] [CrossRef]
- Azmi, R.; Hadmojo, W.T.; Sinaga, S.; Lee, C.-L.; Yoon, S.C.; Jung, I.H.; Jang, S.-Y. High-efficiency low-temperature ZnO based perovskite solar cells based on highly polar, nonwetting self-assembled molecular layers. Adv. Energy Mater. 2018, 8, 1701683. [Google Scholar] [CrossRef]
- Mejía Escobar, M.A.; Pathak, S.; Liu, J.; Snaith, H.J.; Jaramillo, F. ZrO2/TiO2 Electron collection layer for efficient meso-superstructured hybrid perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Gheno, A.; Pham, T.T.T.; Di Bin, C.; Bouclé, J.; Ratier, B.; Vedraine, S. Printable WO3 electron transporting layer for perovskite solar cells: Influence on device performance and stability. Sol. Energy Mater. Sol. Cells 2017, 161, 347–354. [Google Scholar] [CrossRef]
- Ling, X.; Yuan, J.; Liu, D.; Wang, Y.; Zhang, Y.; Chen, S.; Wu, H.; Jin, F.; Wu, F.; Shi, G.; et al. Room-temperature processed Nb2O5 as the electron-transporting layer for efficient planar perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 23181–23188. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M.; Gr, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kim, H.-S.; Mora-Seró, I.; González-Pedro, V.; Fabregat-Santiago, F.; Juarez-Perez, E.J.; Park, N.-G.; Bisquert, J. Mechanism of carrier accumulation in perovskite thin-absorber solar cells. Nat. Commun. 2013, 4, 2242. [Google Scholar] [CrossRef] [Green Version]
- Snaith, H.J.; Abate, A.; Ball, J.M.; Eperon, G.E.; Leijtens, T.; Noel, N.K.; Stranks, S.D.; Wang, J.T.-W.; Wojciechowski, K.; Zhang, W. Anomalous hysteresis in perovskite solar cells. J. Phys. Chem. Lett. 2014, 5, 1511–1515. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Pathak, S.; Abate, A.; Lee, M.M.; Snaith, H.J. Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat. Commun. 2013, 4, 2885. [Google Scholar] [CrossRef]
- Ito, S.; Tanaka, S.; Manabe, K.; Nishino, H. Effects of surface blocking layer of Sb2S3 on nanocrystalline TiO2 for CH3NH3PbI3 perovskite solar cells. J. Phys. Chem. C 2014, 118, 16995–17000. [Google Scholar] [CrossRef]
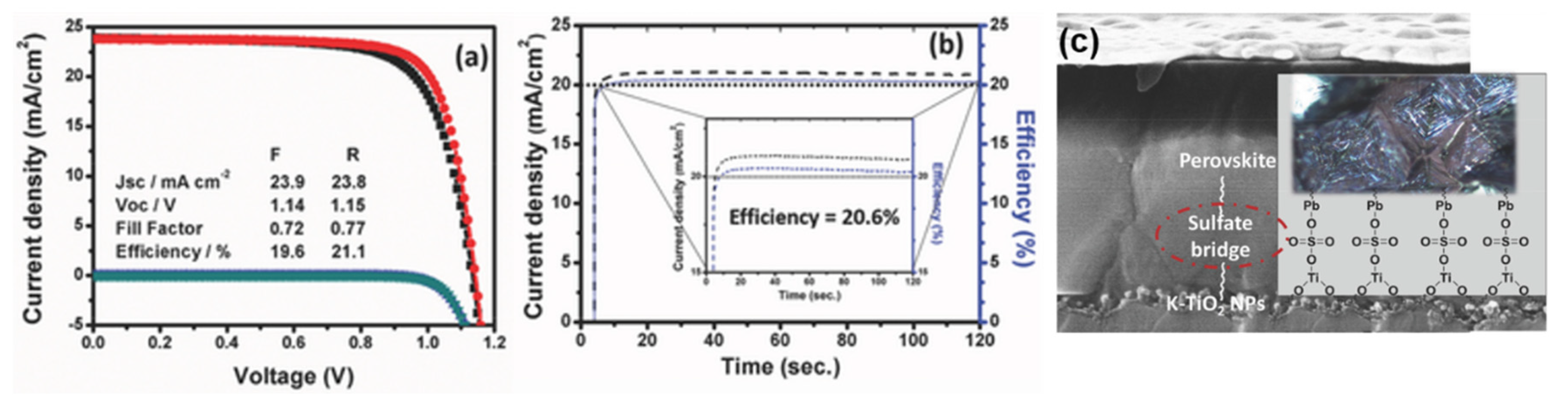
- Singh, T.; Öz, S.; Sasinska, A.; Frohnhoven, R.; Mathur, S.; Miyasaka, T. Sulfate-assisted interfacial engineering for high yield and efficiency of triple cation perovskite solar cells with alkali-doped TiO2 electron-transporting layers. Adv. Funct. Mater. 2018, 28, 1706287. [Google Scholar] [CrossRef]
- Mohammadian-Sarcheshmeh, H.; Mazloum-Ardakani, M. Recent advancements in compact layer development for perovskite solar cells. Heliyon 2018, 4, e00912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Yamamoto, K.; Furumoto, Y.; Kuwabara, T.; Takahashi, K.; Taima, T. Enhanced photovoltaic performance of perovskite solar cells via modification of surface characteristics using a fullerene interlayer. Chem. Lett. 2015, 44, 1735–1737. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Karakawa, M.; Yamamoto, K.; Kusumi, T.; Yonezawa, K.; Kuwabara, T.; Takahashi, K.; Taima, T. Interface engineering of compact-TiOx in planar perovskite solar cells using low-temperature processable high-mobility fullerene derivative. Sol. Energy Mater. Sol. Cells 2018, 178, 1–7. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic–organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, X.; Chen, H.; Zhang, K.; Qin, C.; Liu, J.; Peng, W.; Islam, A.; Bi, E.; Ye, F.; et al. Highly compact TiO2 layer for efficient hole-blocking in perovskite solar cells. Appl. Phys. Express 2014, 7, 052301. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, Y.; Dai, Q.; Song, H. Radio frequency magnetron sputtering deposition of TiO2 thin films and their perovskite solar cell applications. Sci. Rep. 2015, 5, 17684. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, T.; Arai, S. Titanium dioxide thin films prepared by chemical vapor deposition. Sol. Energy Mater. Sol. Cells 1992, 26, 323–329. [Google Scholar] [CrossRef]
- Su, T.-S.; Hsieh, T.-Y.; Hong, C.-Y.; Wei, T.-C. Electrodeposited ultrathin TiO2 blocking layers for efficient perovskite solar cells. Sci. Rep. 2015, 5, 16098. [Google Scholar] [CrossRef]
- Cojocaru, L.; Uchida, S.; Sanehira, Y.; Nakazaki, J.; Kubo, T.; Segawa, H. Surface treatment of the compact TiO2 layer for efficient planar heterojunction perovskite solar cells. Chem. Lett. 2015, 44, 674–676. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Deng, X.; Wilkes, G.C.; Chen, A.Z.; Prasad, N.S.; Gupta, M.C.; Choi, J.J. Room-temperature processing of TiOx electron transporting layer for perovskite solar cells. J. Phys. Chem. Lett. 2017, 8, 3206–3210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Q.; Hong, Z.; Zhou, H.; Xu, X.; De Marco, N.; Sun, P.; Zhao, Z.; Cheng, Y.-B.; Yang, Y. Low-temperature TiOx compact layer for planar heterojunction perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 11076–11083. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Yonezawa, K.; Yamamoto, K.; Ripolles, T.S.; Karakawa, M.; Kuwabara, T.; Takahashi, K.; Hayase, S.; Taima, T. Improved reproducibility and intercalation control of efficient planar inorganic perovskite solar cells by simple alternate vacuum deposition of PbI2 and CsI. ACS Omega 2017, 2, 4464–4469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Xie, L.; Kim, W.B.; Lee, D.G.; Jung, H.S.; Liu, S. Chemical bath deposition of co-doped TiO2 electron transport layer for hysteresis-suppressed high-efficiency planar perovskite solar cells. Sol. RRL 2019, 3, 1900176. [Google Scholar] [CrossRef]
- Kuwabara, T.; Sugiyama, H.; Kuzuba, M.; Yamaguchi, T.; Takahashi, K. Inverted bulk-heterojunction organic solar cell using chemical bath deposited titanium oxide as electron collection layer. Org. Electron. 2010, 11, 1136–1140. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, T.; Shahiduzzaman, M.; Yamamoto, K.; Lebel, O.; Nunzi, J.-M. Interfacial modification of the electron collecting layer of low-temperature solution-processed organometallic halide photovoltaic cells using an amorphous perylenediimide. Sol. Energy Mater. Sol. Cells 2017, 160, 294–300. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Yamamoto, K.; Furumoto, Y.; Kuwabara, T.; Takahashi, K.; Taima, T. Ionic liquid-assisted growth of methylammonium lead iodide spherical nanoparticles by a simple spin-coating method and photovoltaic properties of perovskite solar cells. RSC Adv. 2015, 5, 77495–77500. [Google Scholar] [CrossRef]
- Taima, T.; Shahiduzzaman, M.; Yamamoto, K.; Furumoto, Y.; Kuwabara, T.; Takahashi, K. Planar heterojunction type perovskite solar cells based on TiOx compact layer fabricated by chemical bath deposition. In Oxide-based Materials and Devices VII; International Society for Optics and Photonics: Bellingham, WA, USA, 2016; Volume 9749, p. 97491G. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Sakuma, T.; Kaneko, T.; Tomita, K.; Isomura, M.; Taima, T.; Umezu, S.; Iwamori, S. Oblique electrostatic inkjet-deposited TiO2 electron transport layers for efficient planar perovskite solar cells. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Ashikawa, H.; Kuniyoshi, M.; Visal, S.; Sakakibara, S.; Kaneko, T.; Katsumata, T.; Taima, T.; Iwamori, S.; Isomura, M.; et al. Compact TiO2/Anatase TiO2 single-crystalline nanoparticle electron-transport bilayer for efficient planar perovskite solar cells. ACS Sustain. Chem. Eng. 2018, 6, 12070–12078. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Visal, S.; Kuniyoshi, M.; Kaneko, T.; Umezu, S.; Katsumata, T.; Iwamori, S.; Kakihana, M.; Taima, T.; Isomura, M.; et al. Low-temperature-processed brookite-based TiO2 heterophase junction enhances performance of planar perovskite solar cells. Nano Lett. 2018, 19, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Kulkarni, A.; Visal, S.; Wang, L.; Nakano, M.; Karakawa, M.; Takahashi, K.; Umezu, S.; Masuda, A.; Iwamori, S.; et al. A single-phase brookite TiO2 nanoparticle bridge enhances the stability of perovskite solar cells. Sustain. Energy Fuels 2020, 4, 2009–2017. [Google Scholar] [CrossRef]
- Li, X.; Dai, S.-M.; Zhu, P.; Deng, L.-L.; Xie, S.-Y.; Cui, Q.; Chen, H.; Wang, N.; Lin, H. Efficient perovskite solar cells depending on TiO2 Nanorod Arrays. ACS Appl. Mater. Interfaces 2016, 8, 21358–21365. [Google Scholar] [CrossRef]
- Lee, Y.; Paek, S.; Cho, K.T.; Oveisi, E.; Gao, P.; Lee, S.; Park, J.-S.; Zhang, Y.; Humphry-Baker, R.; Asiri, A.M.; et al. Enhanced charge collection with passivation of the tin oxide layer in planar perovskite solar cells. J. Mater. Chem. A 2017, 5, 12729–12734. [Google Scholar] [CrossRef]
- Kim, G.M.; Ishii, A.; Öz, S.; Miyasaka, T. MACl-assisted Ge doping of Pb-hybrid perovskite: A universal route to stabilize high performance perovskite solar cells. Adv. Energy Mater. 2020, 10, 1903299. [Google Scholar] [CrossRef]
- Song, S.; Kang, G.; Pyeon, L.; Lim, C.; Lee, G.-Y.; Park, T.; Choi, J. Systematically optimized bilayered electron transport layer for highly efficient planar perovskite solar cells (η = 21.1%). ACS Energy Lett. 2017, 2, 2667–2673. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Wang, K.; Wu, C.; Zhu, X.; Feng, J.; Ren, X.; Fang, G.; Priya, S.; Liu, S. High efficiency planar-type perovskite solar cells with negligible hysteresis using EDTA-complexed SnO2. Nat. Commun. 2018, 9, 3239. [Google Scholar] [CrossRef] [Green Version]
- Zuo, L.; Chen, Q.; De Marco, N.; Hsieh, Y.-T.; Chen, H.; Sun, P.; Chang, S.-Y.; Zhao, H.; Dong, S.; Yang, Y. Tailoring the Interfacial Chemical Interaction for High-Efficiency Perovskite Solar Cells. Nano Lett. 2016, 17, 269–275. [Google Scholar] [CrossRef]
- Ke, W.; Zhao, D.; Xiao, C.; Wang, C.; Cimaroli, A.J.; Grice, C.; Yang, M.; Li, Z.; Jiang, C.-S.; Al-Jassim, M.; et al. Cooperative tin oxide fullerene electron selective layers for high-performance planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 14276–14283. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, J.; Liu, J.; Wei, L.; Liu, Z.; Guan, L.; Cao, G. Stabilization of organometal halide perovskite films by SnO2 coating with inactive surface hydroxyl groups on ZnO nanorods. J. Power Sources 2017, 339, 51–60. [Google Scholar] [CrossRef]
- Tomita, K.; Kobayashi, M.; Petrykin, V.; Yin, S.; Sato, T.; Yoshimura, M.; Kakihana, M. Hydrothermal synthesis of TiO2 nano-particles using novel water-soluble titanium complexes. J. Mater. Sci. 2007, 43, 2217–2221. [Google Scholar] [CrossRef]
- Gan, X.; Wang, O.; Liu, K.; Du, X.; Guo, L.; Liu, H. 2D homologous organic-inorganic hybrids as light-absorbers for planer and nanorod-based perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 162, 93–102. [Google Scholar] [CrossRef]
- He, X.; Wu, J.; Tu, Y.; Xie, Y.; Dong, J.; Jia, J.; Wei, Y.; Lan, Z. TiO2 single crystalline nanorod compact layer for high-performance CH3NH3PbI3 perovskite solar cells with an efficiency exceeding 17%. J. Power Sources 2016, 332, 366–371. [Google Scholar] [CrossRef]
- Wei, D.; Ji, J.; Song, D.; Li, M.; Cui, P.; Li, Y.; Mbengue, J.M.; Zhou, W.; Ning, Z.; Park, N.-G. A TiO2 embedded structure for perovskite solar cells with anomalous grain growth and effective electron extraction. J. Mater. Chem. A 2017, 5, 1406–1414. [Google Scholar] [CrossRef]
- Choi, M.; Lim, J.; Baek, M.; Choi, W.; Kim, W.; Yong, K. Investigating the unrevealed photocatalytic activity and stability of nanostructured brookite TiO2 film as an environmental photocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 16252–16260. [Google Scholar] [CrossRef]
- Zhao, M.; Li, L.; Lin, H.; Yang, L.; Li, G. A facile strategy to fabricate large-scale uniform brookite TiO2 nanospindles with high thermal stability and superior electrical properties. Chem. Commun. 2013, 49, 7046. [Google Scholar] [CrossRef]
- Mo, S.-D.; Ching, W.Y. Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase, and brookite. Phys. Rev. B 1995, 51, 13023–13032. [Google Scholar] [CrossRef]
- Kogo, A.; Sanehira, Y.; Numata, Y.; Ikegami, M.; Miyasaka, T. Amorphous metal oxide blocking layers for highly efficient low-temperature brookite TiO2-based perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 2224–2229. [Google Scholar] [CrossRef]
- Qiu, J.; Qiu, Y.; Yan, K.; Zhong, M.; Mu, C.; Yan, H.; Yang, S. All-solid-state hybrid solar cells based on a new organometal halide perovskite sensitizer and one-dimensional TiO2 nanowire arrays. Nanoscale 2013, 5, 3245–3248. [Google Scholar] [CrossRef]
- Yang, M.; Guo, R.; Kadel, K.; Liu, Y.; O’Shea, K.; Bone, R.; Wang, X.; He, J.; Li, W. Improved charge transport of Nb-doped TiO2 nanorods in methylammonium lead iodide bromide perovskite solar cells. J. Mater. Chem. A 2014, 2, 19616–19622. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, Z.; Tao, X.; Sun, H.; Chen, W.; Zhou, X. Sn-doped TiO2 nanorod arrays and application in perovskite solar cells. RSC Adv. 2014, 4, 64001–64005. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, J.-W.; Yantara, N.; Boix, P.P.; Kulkarni, S.A.; Mhaisalkar, S.; Grätzel, M.; Park, N.-G. High Efficiency Solid-State Sensitized Solar Cell-Based on Submicrometer Rutile TiO2 nanorod and CH3NH3PbI3 Perovskite Sensitizer. Nano Lett. 2013, 13, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Lee, T.-Y.; Yoo, P.J.; Grätzel, M.; Mhaisalkar, S.; Park, N.-G. Rutile TiO2-based perovskite solar cells. J. Mater. Chem. A 2014, 2, 9251. [Google Scholar] [CrossRef]
- Wu, S.; Cheng, C.; Jin, J.; Wang, J.; Peng, T. Low-temperature processed nanostructured rutile TiO2 array films for perovskite solar cells with high efficiency and stability. Sol. RRL 2017, 2, 1700164. [Google Scholar] [CrossRef]
- Liang, C.; Wu, Z.; Li, P.; Fan, J.; Zhang, Y.; Shao, G. Chemical bath deposited rutile TiO2 compact layer toward efficient planar heterojunction perovskite solar cells. Appl. Surf. Sci. 2017, 391, 337–344. [Google Scholar] [CrossRef]
- Giordano, F.; Abate, A.; Correa-Baena, J.-P.; Saliba, M.; Matsui, T.; Im, S.H.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Hagfeldt, A.; Graetzel, M. Enhanced electronic properties in mesoporous TiO2 via lithium doping for high-efficiency perovskite solar cells. Nat. Commun. 2016, 7, 10379. [Google Scholar] [CrossRef]
- Huckaba, A.J.; Lee, Y.; Xia, R.; Paek, S.; Bassetto, V.C.; Oveisi, E.; Lesch, A.; Kinge, S.; Dyson, P.J.; Girault, H.; et al. Inkjet-printed mesoporous TiO2 and perovskite layers for high efficiency perovskite solar cells. Energy Technol. 2019, 7, 186. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.D.; Ojha, D.P.; You, J.S.; Lee, J.; Kim, J.; Lee, W.I. 50 nm sized spherical TiO2 nanocrystals for highly efficient mesoscopic perovskite solar cells. Nanoscale 2015, 7, 8898–8906. [Google Scholar] [CrossRef]
- Wang, J.T.-W.; Ball, J.M.; Barea, E.; Abate, A.; Alexander-Webber, J.; Huang, J.; Saliba, M.; Mora-Seró, I.; Bisquert, J.; Snaith, H.J.; et al. Low-temperature processed electron collection layers of graphene/TiO2 nanocomposites in thin film perovskite solar cells. Nano Lett. 2013, 14, 724–730. [Google Scholar] [CrossRef]
- Huang, A.; Lei, L.; Zhu, J.; Yu, Y.; Liu, Y.; Yang, S.; Bao, S.; Jin, P. Controllable deposition of TiO2 nanopillars at room temperature for high performance perovskite solar cells with suppressed hysteresis. Sol. Energy Mater. Sol. Cells 2017, 168, 172–182. [Google Scholar] [CrossRef]
- Shin, S.S.; Lee, S.J.; Seok, S.I. Metal oxide charge transport layers for efficient and stable perovskite solar cells. Adv. Funct. Mater. 2019, 29, 1900455. [Google Scholar] [CrossRef]
- Singh, T.; Miyasaka, T.; Singh, J. ChemInform abstract: Role of metal oxide electron-transport layer modification on the stability of high performing perovskite solar cells. ChemSusChem 2016, 47, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Guo, Y.; Wen, J.; Liu, H.; Yang, G.; Qin, P.; Fang, G. Review on the application of SnO2 in perovskite solar cells. Adv. Funct. Mater. 2018, 28, 1802757. [Google Scholar] [CrossRef]
- Song, J.; Zheng, E.; Bian, J.; Wang, X.-F.; Tian, W.; Sanehira, Y.; Miyasaka, T. Low-temperature SnO2-based electron selective contact for efficient and stable perovskite solar cells. J. Mater. Chem. A 2015, 3, 10837–10844. [Google Scholar] [CrossRef]
- Jiang, Q.; Chu, Z.; Wang, P.; Yang, X.; Liu, H.; Wang, Y.; Yin, Z.; Wu, J.; Zhang, Y.; You, J. Planar-structure perovskite solar cells with efficiency beyond 21%. Adv. Mater. 2017, 29, 1703852. [Google Scholar] [CrossRef]
- Bai, Y.; Fang, Y.; Deng, Y.; Wang, Q.; Zhao, J.; Zheng, X.; Zhang, Y.; Huang, J. Low-temperature solution-processed Sb:SnO2 nanocrystals for efficient planar perovskite solar cells. ChemSusChem 2016, 9, 2686–2691. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Kim, J.-Y.; Son, H.J.; Lee, C.-H.; Jang, S.S.; Ko, M.J. Low-temperature solution-processed Li-doped SnO2 as an effective electron transporting layer for high-performance flexible and wearable perovskite solar cells. Nano Energy 2016, 26, 208–215. [Google Scholar] [CrossRef]
- Yang, G.; Wang, C.; Lei, H.; Zheng, X.; Qin, P.; Xiong, L.; Zhao, X.; Yan, Y.; Fang, G. Interface engineering in planar perovskite solar cells: Energy level alignment, perovskite morphology control and high performance achievement. J. Mater. Chem. A 2017, 5, 1658–1666. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Zhang, X.; Wang, H.; Cui, X.; Yuan, S.; Lu, H.; Tu, L.; Zhan, Y.; Zheng, L. Boosting the performance of perovskite solar cells through a novel active passivation method. J. Mater. Chem. A 2018, 6, 15853–15858. [Google Scholar] [CrossRef]
- Ren, H.; Zou, X.; Cheng, J.; Ling, T.; Bai, X.; Chen, D. Facile solution spin-coating SnO2 thin film covering cracks of TiO2 hole blocking layer for perovskite solar cells. Coatings 2018, 8, 314. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhang, X.; Bai, S.; Liu, C.; Li, Z.; Guo, W.; Gao, F. Surface chlorination of ZnO for perovskite solar cells with enhanced efficiency and stability. Sol. RRL 2019, 3, 1900154. [Google Scholar] [CrossRef]
- Ali, F.; Pham, N.D.; Fan, L.; Tiong, V.; Dai, X.; Bell, J.M.; Wang, H.; Tesfamichael, T.; Ostrikov, K. Low hysteresis perovskite solar cells using an electron-beam evaporated WO3−x thin film as the electron transport layer. ACS Appl. Energy Mater. 2019, 2, 5456–5464. [Google Scholar] [CrossRef]
- You, Y.; Tian, W.; Min, L.; Cao, F.; Deng, K.; Li, L. TiO2/WO3 bilayer as electron transport layer for efficient planar perovskite solar cell with efficiency exceeding 20%. Adv. Mater. Interfaces 2020, 7, 1901406. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, Y.; Wang, Q.; Abate, A.; Li, Y.; Wei, M. Highly efficient Zn2SnO4 perovskite solar cells through band alignment engineering. Chem. Commun. 2019, 55, 14673–14676. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Shen, D.; Li, Y.; Abate, A.; Wei, M. Highly efficient perovskite solar cells based on a Zn2SnO4 compact layer. ACS Appl. Mater. Interfaces 2019, 11, 36553–36559. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Xu, W.; Yuan, H.; Wu, J. Mesostructured perovskite solar cells based on Zn2SnO4 single crystal mesoporous layer with efficiency of 18.32%. J. Alloys Compd. 2020, 823, 153730. [Google Scholar] [CrossRef]
- Shin, S.S.; Yang, W.S.; Noh, J.H.; Suk, J.H.; Jeon, N.J.; Park, J.H.; Kim, J.S.; Seong, W.M.; Seok, S.I. High-performance flexible perovskite solar cells exploiting Zn2SnO4 prepared in solution below 100 °C. Nat. Commun. 2015, 6, 7410. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Yin, X.; Que, M.; Yang, Y.; Liu, X.; Que, W. Bilayer photoanode approach for efficient In2O3 based planar heterojunction perovskite solar cells. J. Alloy. Compd. 2018, 735, 938–944. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, S.J.; Kim, H.S.; Park, J.-S.; Han, I.K.; Jung, J.W.; Park, M. Solution-processed indium oxide electron transporting layers for high-performance and photo-stable perovskite and organic solar cells. Nanoscale 2017, 9, 16305–16312. [Google Scholar] [CrossRef]
- Okamoto, Y.; Fukui, R.; Fukazawa, M.; Suzuki, Y. SrTiO3/TiO2 composite electron transport layer for perovskite solar cells. Mater. Lett. 2017, 187, 111–113. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, T.; Wang, Y.; Hahn, Y.-B. SrTiO3/Al2O3-graphene electron transport layer for highly stable and efficient composites-based perovskite solar cells with 20.6% efficiency. Adv. Energy Mater. 2020, 10, 1903369. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Z.; Shi, W.; Liu, Y.; Gao, H.; Mao, Y. Enhanced performance of perovskite solar cells by using ultrathin BaTiO3 interface modification. ACS Appl. Mater. Interfaces 2018, 10, 36067–36074. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Suzuki, Y. Mesoporous BaTiO3/TiO2 double layer for electron transport in perovskite solar cells. J. Phys. Chem. C 2016, 120, 13995–14000. [Google Scholar] [CrossRef]
- Shin, S.S.; Yeom, E.J.; Yang, W.S.; Hur, S.; Kim, M.G.; Im, J.; Seo, J.; Noh, J.H.; Seok, S.I. Colloidally prepared La-doped BaSnO3 electrodes for efficient, photostable perovskite solar cells. Science 2017, 356, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ye, J.; Zhang, X.; Zheng, H.; Liu, G.; Pan, X.; Dai, S. Performance enhancement of perovskite solar cells using a La-doped BaSnO3 electron transport layer. J. Mater. Chem. A 2017, 5, 3675–3682. [Google Scholar] [CrossRef]
- Mahmood, K.; Swain, B.S.; Kirmani, A.R.; Amassian, A. Highly efficient perovskite solar cells based on a nanostructured WO3–TiO2 core–shell electron transporting material. J. Mater. Chem. A 2015, 3, 9051–9057. [Google Scholar] [CrossRef]

| Surface Modification | Devices Structure | PCE (%) | Ref. |
|---|---|---|---|
| TiO2/anatase TiO2 NPs | FTO/TiO2/anatase TiO2 NPs/MAPbI3/Spiro/Au | 17.1 | [65] |
| TiO2/brookite TiO2 NPs | FTO/TiO2/brookite TiO2NPs/Cs0.05(FA0.83MA0.17)0.95Pb(I0.83Br0.17)3/Spiro/Au | 16.8 | [66] |
| TiO2/brookite TiO2 NPs | FTO/TiO2/brookite TiO2NPs/MAPbI3/Spiro/Au | 18.2 | [67] |
| TiO2/mp-TiO2 | FTO/TiO2/mp-TiO2/Cs0.05(FA0.83MA0.17)0.95Pb(I0.83Br0.17)3/Spiro/Au | 21.1 | [44] |
| TiO2/rutile TiO2 NRs | FTO/TiO2/rutile TiO2 NPs/MAPbI3/Spiro/Ag | 18.2 | [68] |
| TiO2/C60 | ITO/TiO2/C60/MAPbI3/Spiro/Ag | 9.5 | [47] |
| TiO2/PDI-glass | ITO/TiO2/PDI-glass/MAPbI3/Spiro/Au | 5.7 | [61] |
| TiO2/PNP | ITO/TiO2/PNP/MAPbI3/Spiro/Ag | 8.2 | [48] |
| TiO2/SnO2 | FTO/TiO2/SnO2/MAPbI3/PATT/Au | 19.8 | [69] |
| TiO2/SnO2 | FTO/TiO2/SnO2/FA0.83MA0.17(Ge0.03Pb0.97(I0.9Br0.1)3/Spiro/Au | 22.1 | [70] |
| TiO2/SnO2 | FTO/TiO2/SnO2/MAPbI3/Spiro/Ag | 21.1 | [71] |
| TiO2/SnO2 | ITO/TiO2/SnO2/FA0.95Cs0.05PbI3/PCBM/Ag | 21.5 | [72] |
| SnO2/SAM | FTO/SnO2/SAM/MAPbI3/Spiro/Au | 18.8 | [73] |
| SnO2/PCBM | FTO/SnO2/PCBM/MAPbI3/Spiro/Au | 19.1 | [74] |
| ZnO/SnO2 | FTO/SnO2/PCBM/MAPbI3/Spiro/Ag | 12.17 | [75] |
| ZnO/SAM | ITO/ZnO/SAM/MAPbI3/Spiro | 13.7 | [32] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahiduzzaman, M.; Fukaya, S.; Muslih, E.Y.; Wang, L.; Nakano, M.; Akhtaruzzaman, M.; Karakawa, M.; Takahashi, K.; Nunzi, J.-M.; Taima, T. Metal Oxide Compact Electron Transport Layer Modification for Efficient and Stable Perovskite Solar Cells. Materials 2020, 13, 2207. https://doi.org/10.3390/ma13092207
Shahiduzzaman M, Fukaya S, Muslih EY, Wang L, Nakano M, Akhtaruzzaman M, Karakawa M, Takahashi K, Nunzi J-M, Taima T. Metal Oxide Compact Electron Transport Layer Modification for Efficient and Stable Perovskite Solar Cells. Materials. 2020; 13(9):2207. https://doi.org/10.3390/ma13092207
Chicago/Turabian StyleShahiduzzaman, Md., Shoko Fukaya, Ersan Y. Muslih, Liangle Wang, Masahiro Nakano, Md. Akhtaruzzaman, Makoto Karakawa, Kohshin Takahashi, Jean-Michel Nunzi, and Tetsuya Taima. 2020. "Metal Oxide Compact Electron Transport Layer Modification for Efficient and Stable Perovskite Solar Cells" Materials 13, no. 9: 2207. https://doi.org/10.3390/ma13092207
APA StyleShahiduzzaman, M., Fukaya, S., Muslih, E. Y., Wang, L., Nakano, M., Akhtaruzzaman, M., Karakawa, M., Takahashi, K., Nunzi, J.-M., & Taima, T. (2020). Metal Oxide Compact Electron Transport Layer Modification for Efficient and Stable Perovskite Solar Cells. Materials, 13(9), 2207. https://doi.org/10.3390/ma13092207








