Laser-Induced Deposition of Plasmonic Ag and Pt Nanoparticles, and Periodic Arrays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Laser-Induced Deposition
2.3. Samples Characterization
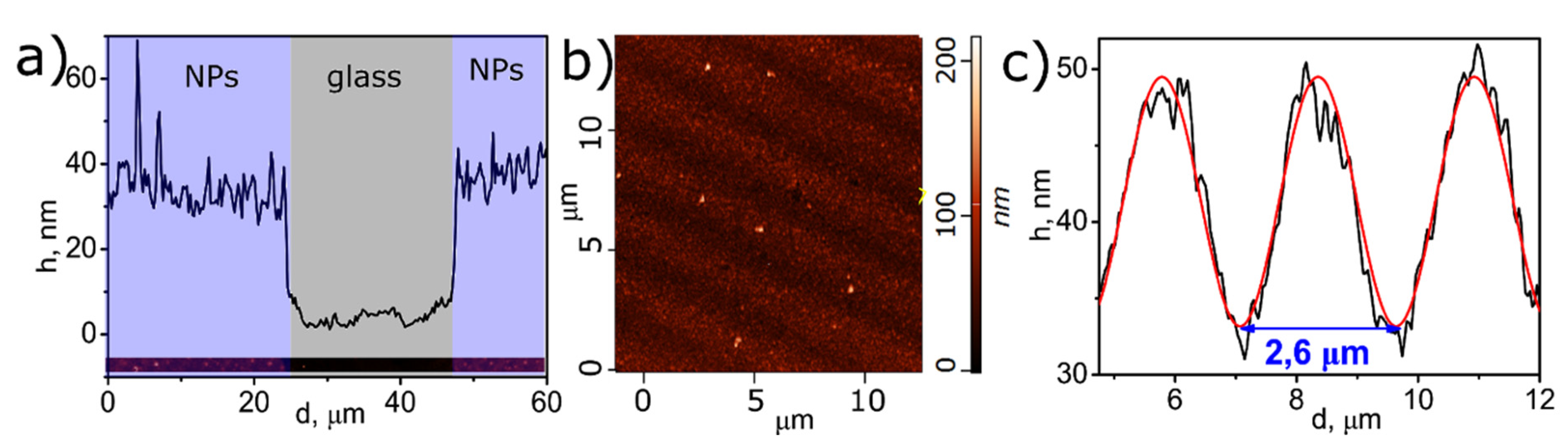
3. Results and Discussion
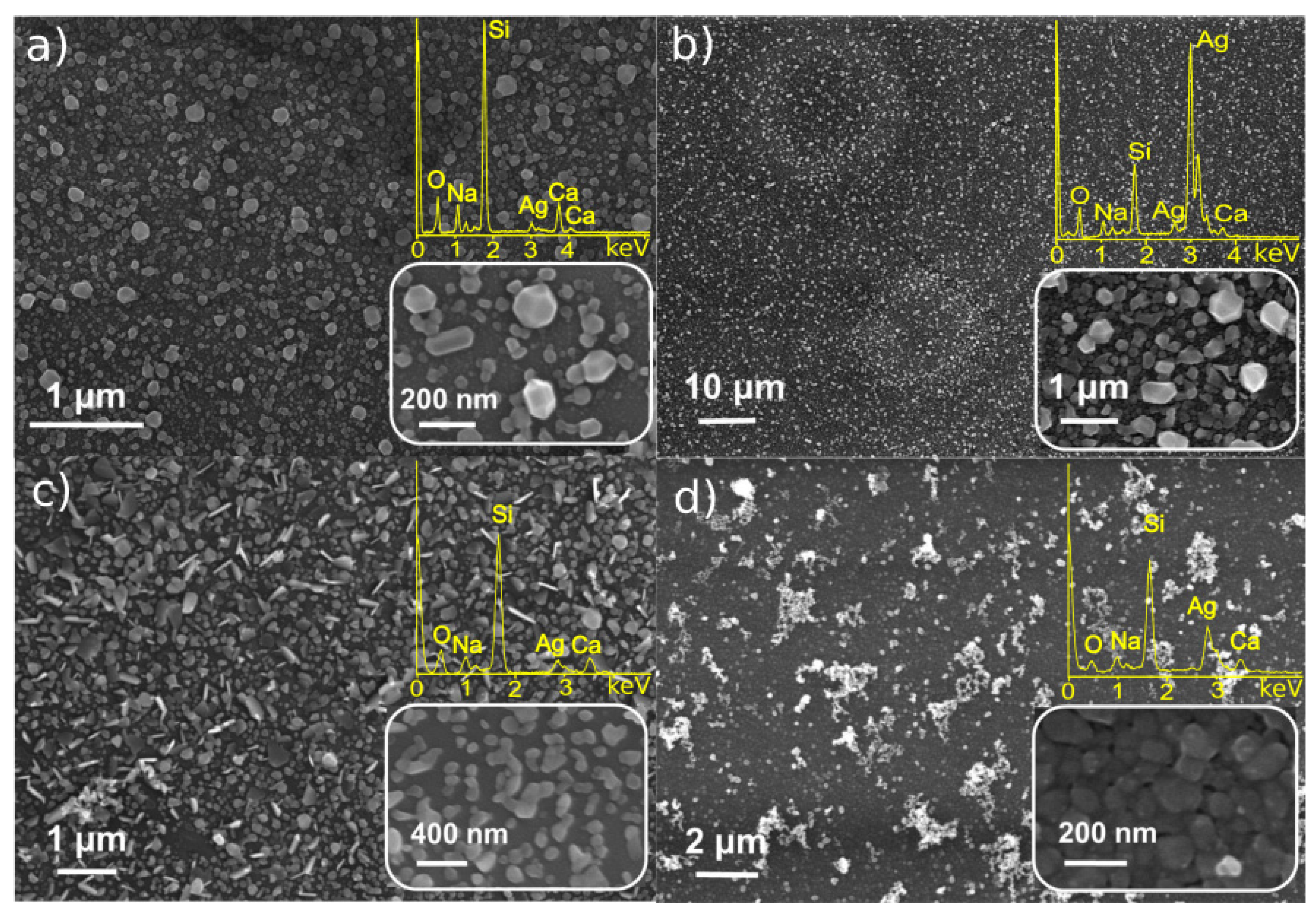
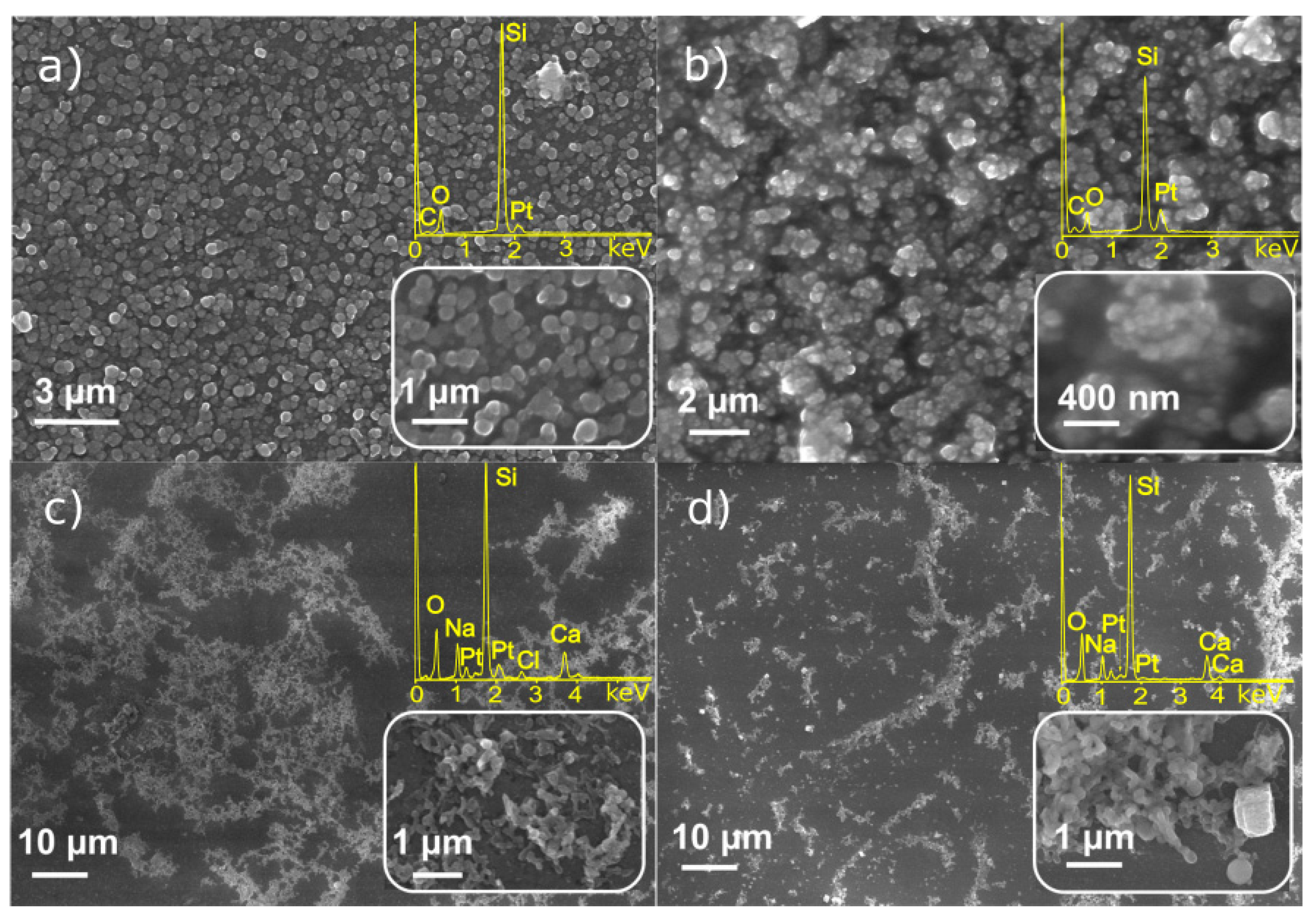
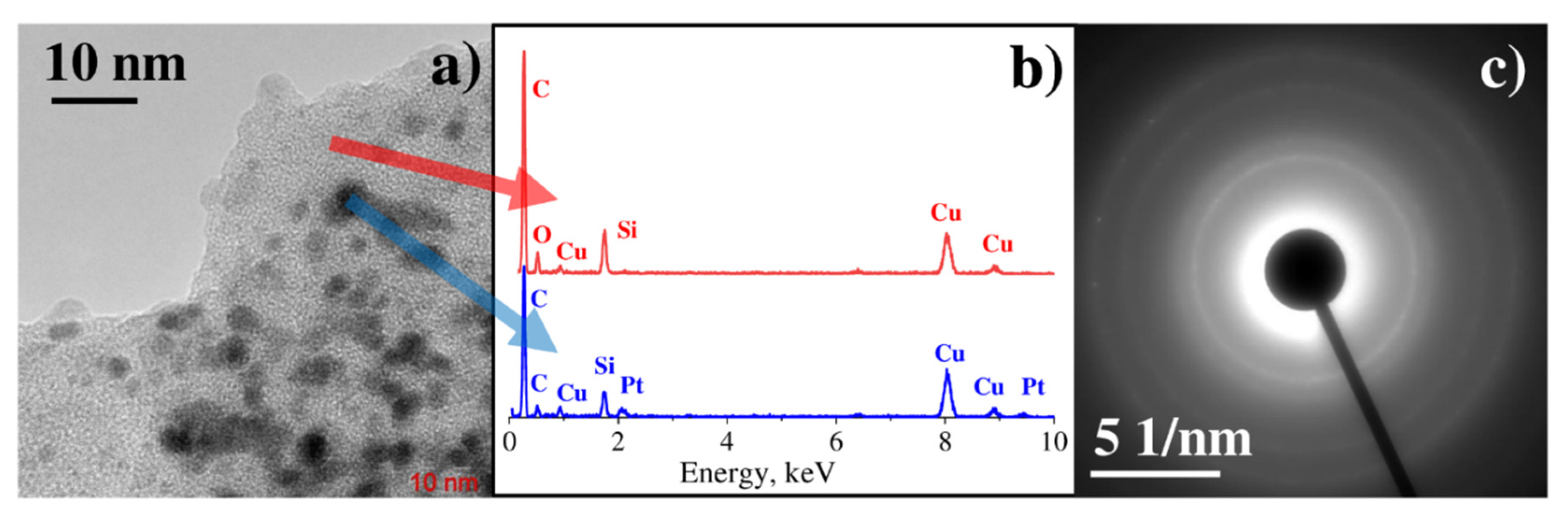
3.1. Characterization of Monometallic Ag, Pt Nanostructures Obtained by LID
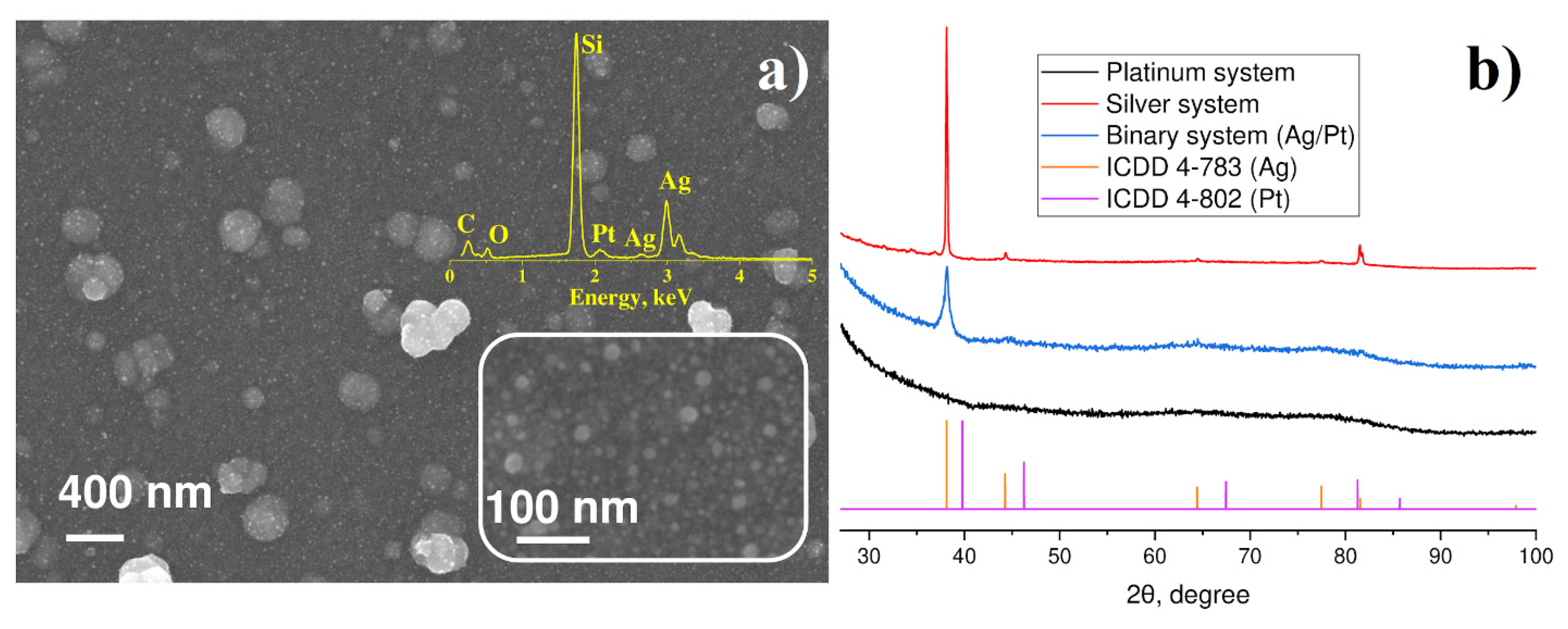
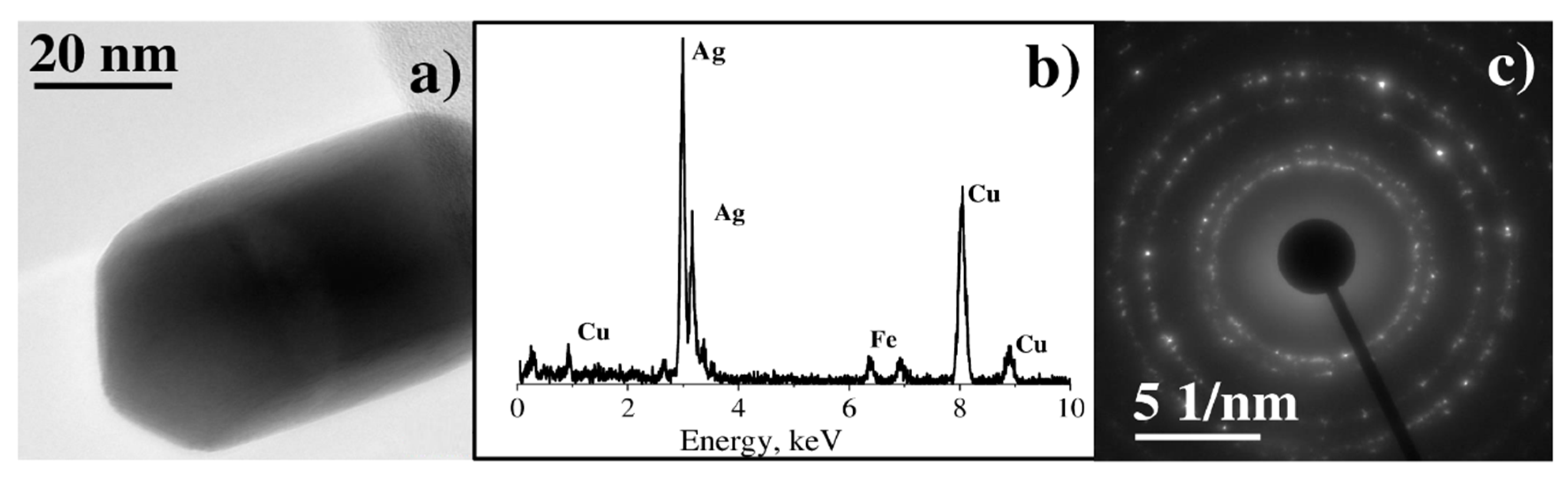
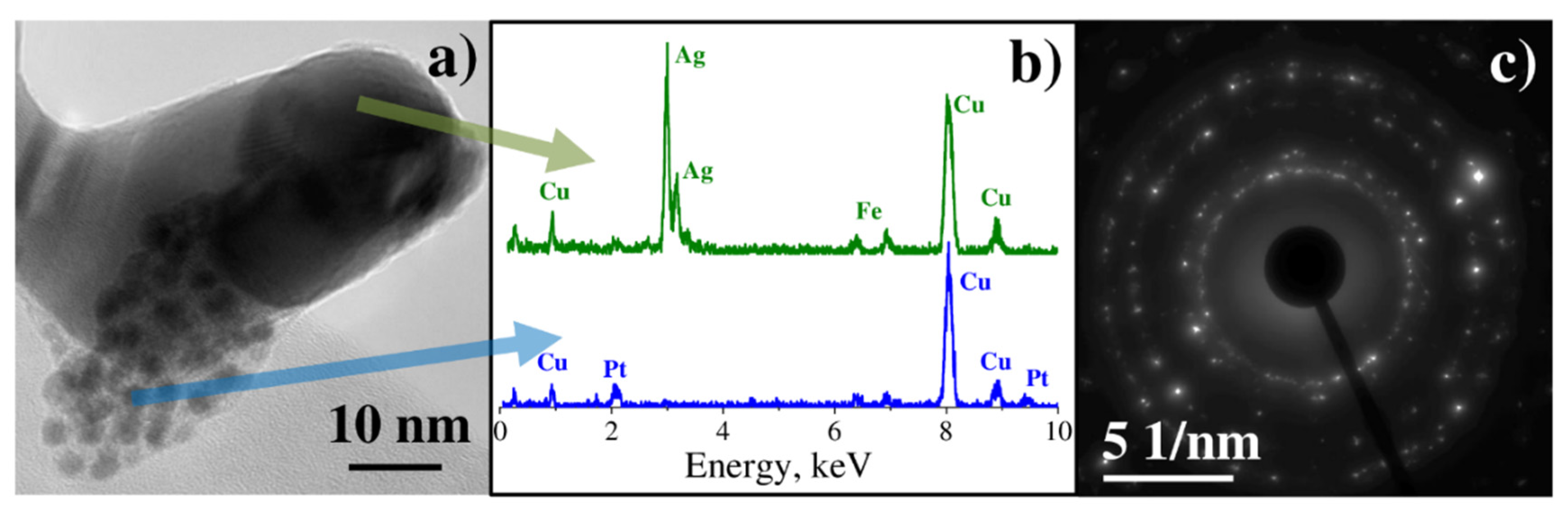
3.2. Characterization of Binary Pt-Ag System Obtained by LID
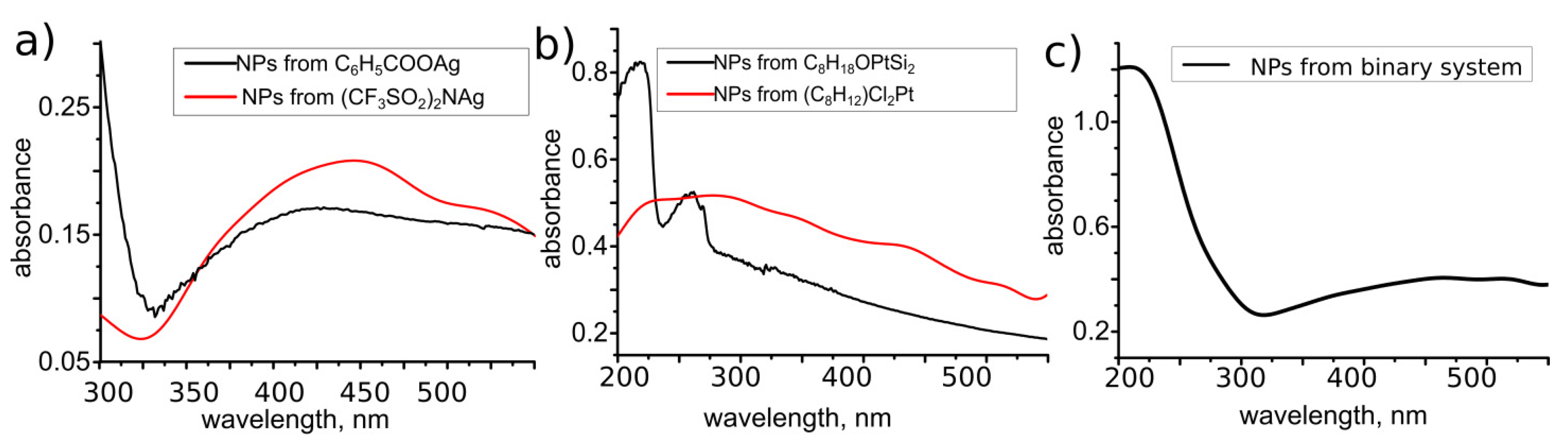
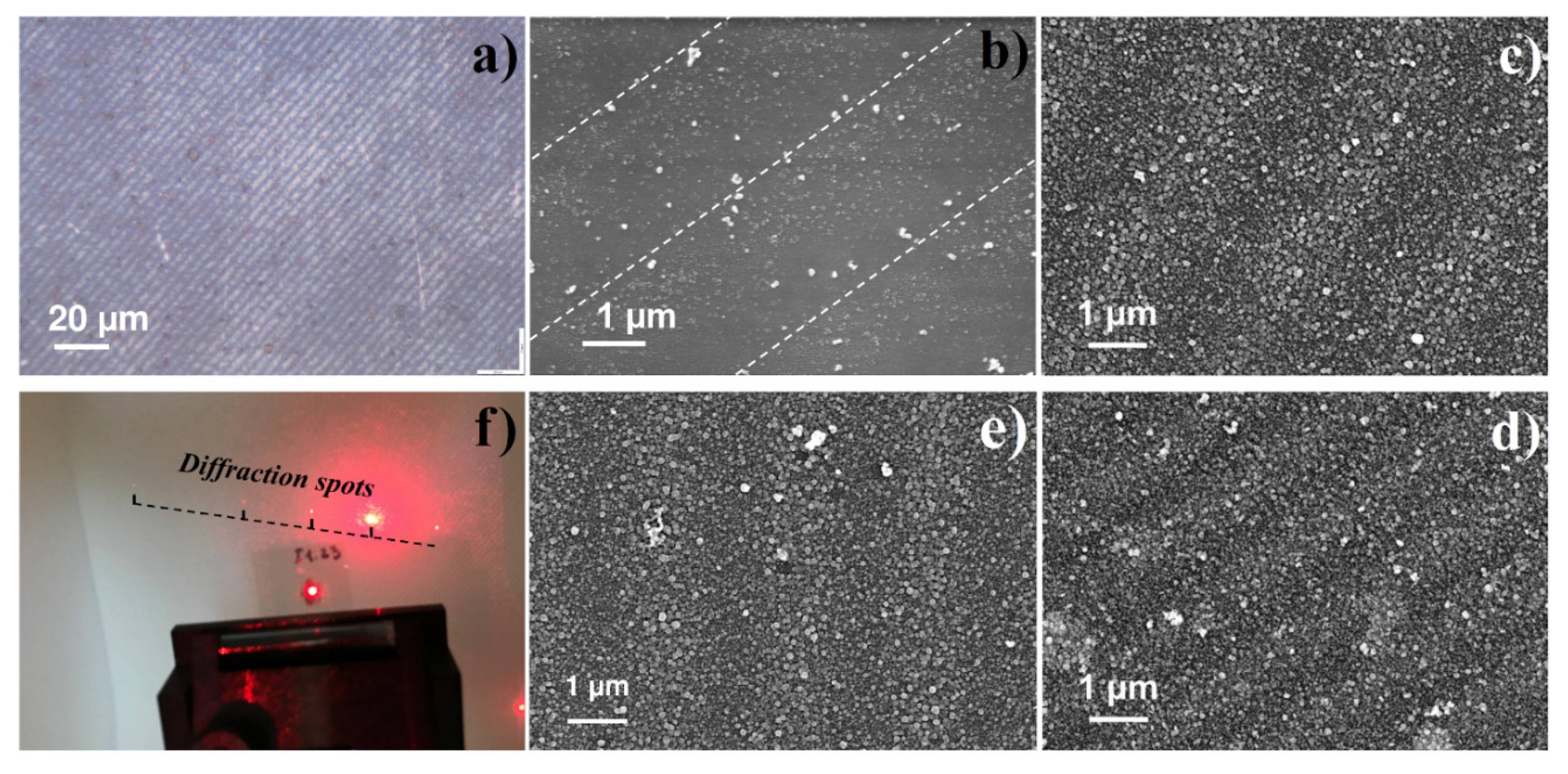
3.3. LID-Obtained Structures for Optical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, F.; Yang, J.; Lu, S.-H.; Niu, K.-Y.; Liu, Y.; Sun, J.; Du, X.-W. Laser synthesis of gold/oxide nanocomposites. J. Mater. Chem. 2010, 20, 1103–1106. [Google Scholar] [CrossRef]
- Marzun, G.; Levish, A.; Mackert, V.; Kallio, T.; Barcikowski, S.; Wagener, P. Laser synthesis, structure and chemical properties of colloidal nickel-molybdenum nanoparticles for the substitution of noble metals in heterogeneous catalysis. J. Colloid Interface Sci. 2017, 489, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhao, Y.; Xu, X.; Chen, J.; Wang, X.; Yu, J.; Wang, J.; Wu, W. Realization of Tunable Localized Surface Plasmon Resonance of Cu@Cu2O Core–Shell Nanoparticles by the Pulse Laser Deposition Method. ACS Omega 2019, 4, 14404–14410. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Magara, H.; Herbani, Y.; Ito, A.; Sato, S. Fabrication of gold-platinum nanoparticles by intense, femtosecond laser irradiation of aqueous solution. In Proceedings of the Conference on Lasers and Electro-Optics/International Quantum Electronics Conference, Munich, Germany, 14–19 June 2009; Paper JWA2. Optical Society of America: Washington, DC, USA, 2009; p. JWA2. [Google Scholar]
- Nakamura, T.; Herbani, Y.; Sato, S. Fabrication of solid-solution gold-platinum nanoparticles with controllable compositions by high-intensity laser irradiation of solution. J. Nanopart. Res. 2012, 14, 785. [Google Scholar] [CrossRef]
- Guay, J.-M.; Calà Lesina, A.; Côté, G.; Charron, M.; Poitras, D.; Ramunno, L.; Berini, P.; Weck, A. Laser-induced plasmonic colours on metals. Nat. Commun. 2017, 8, 16095. [Google Scholar] [CrossRef] [PubMed]
- Mikšys, J.; Arutinov, G.; Römer, G.R.B.E. Pico- to nanosecond pulsed laser-induced forward transfer (LIFT) of silver nanoparticle inks: A comparative study. Appl. Phys. A 2019, 125, 814. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Hu, J.; Jiang, L.; Xu, C.; Li, X.; Gao, Y.; Qu, L. Shaped femtosecond laser induced photoreduction for highly controllable Au nanoparticles based on localized field enhancement and their SERS applications. Nanophotonics 2020, 9, 691–702. [Google Scholar] [CrossRef]
- Manshina, A.; Povolotskiy, A.; Ivanova, T.; Kurochkin, A.; Tver’yanovich, Y.; Kim, D.; Kim, M.; Kwon, S.C. Laser-assisted metal deposition from CuSO4-based electrolyte solution. Laser Phys. Lett. 2007, 4, 163–167. [Google Scholar] [CrossRef]
- Seo, J.M.; Kwon, K.-K.; Song, K.Y.; Chu, C.N.; Ahn, S.-H. Deposition of Durable Micro Copper Patterns into Glass by Combining Laser-Induced Backside Wet Etching and Laser-Induced Chemical Liquid Phase Deposition Methods. Materials 2020, 13, 2977. [Google Scholar] [CrossRef]
- Peng, C.; Richard Liu, C.; Voothaluru, R.; Ou, C.-Y.; Liu, Z. An Exploratory Investigation of the Mechanical Properties of the Nanostructured Porous Materials Deposited by Laser-Induced Chemical Solution Synthesis. J. Micro Nanomanuf. 2017, 5. [Google Scholar] [CrossRef]
- Bjerneld, E.J.; Murty, K.V.G.K.; Prikulis, J.; Käll, M. Laser-Induced Growth of Ag Nanoparticles from Aqueous Solutions. ChemPhysChem 2002, 3, 116–119. [Google Scholar] [CrossRef]
- Bjerneld, E.J.; Svedberg, F.; Käll, M. Laser-Induced Growth and Deposition of Noble-Metal Nanoparticles for Surface-Enhanced Raman Scattering. Nano Lett. 2003, 3, 593–596. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, X.; Yang, C. Laser-induced pattened deposition of silver nanoparticles on glass. In Proceedings of the International Conference on Advanced Infocomm Technology 2011 (ICAIT 2011), Wuhan, China, 11–14 July 2011; p. 26. [Google Scholar] [CrossRef]
- Ming-Shan, L.; Chang-Xi, Y. Laser-Induced Silver Nanoparticles Deposited on Optical Fiber Core for Surface-Enhanced Raman Scattering. Chin. Phys. Lett. 2010, 27, 044202. [Google Scholar] [CrossRef]
- Meng, X.; Bi, Z.; Shang, G. Laser-induced Ag nanoparticles deposition on optical fiber probes for TERS. In Proceedings of the the International Photonics and Optoelectronics Meeting (POEM), Wuhan, China, 31 October–3 November 2018; Paper OT4A.74. Optical Society of America: Washington, DC, USA, 2018; p. OT4A.74. [Google Scholar]
- Povolotskiy, A.; Povolotckaia, A.; Petrov, Y.; Manshina, A.; Tunik, S. Laser-induced synthesis of metallic silver-gold nanoparticles encapsulated in carbon nanospheres for surface-enhanced Raman spectroscopy and toxins detection. Appl. Phys. Lett. 2013, 103, 113102. [Google Scholar] [CrossRef]
- Vasileva, A.; Haschke, S.; Mikhailovskii, V.; Gitlina, A.; Bachmann, J.; Man’shina, A. Direct laser-induced deposition of AgPt@C nanoparticles on 2D and 3D substrates for electrocatalytic glucose oxidation. Nano-Struct. Nano-Objects 2020, 24, 100547. [Google Scholar] [CrossRef]
- Manshina, A.; Povolotskiy, A.; Ivanova, T.; Kurochkin, A.; Tver’yanovich, Y.; Kim, D.; Kim, M.; Kwon, S.C. CuCl2-based liquid electrolyte precursor for laser-induced metal deposition. Laser Phys. Lett. 2006, 4, 242. [Google Scholar] [CrossRef]
- Manshina, A. Laser-Induced Deposition of Metal and Hybrid Metal-Carbon Nanostructures. In Progress in Photon Science: Recent Advances; Yamanouchi, K., Tunik, S., Makarov, V., Eds.; Springer Series in Chemical Physics; Springer International Publishing: Cham, Switzerland, 2019; pp. 387–403. ISBN 978-3-030-05974-3. [Google Scholar]
- Bashouti, M.Y.; Manshina, A.; Povolotckaia, A.; Povolotskiy, A.; Kireev, A.; Petrov, Y.; Mačković, M.; Spiecker, E.; Koshevoy, I.; Tunik, S.; et al. Direct laser writing of μ-chips based on hybrid C-Au-Ag nanoparticles for express analysis of hazardous and biological substances. Lab Chip 2015, 15, 1742–1747. [Google Scholar] [CrossRef] [Green Version]
- Bashouti, M.Y.; Povolotckaia, A.V.; Povolotskiy, A.V.; Tunik, S.P.; Christiansen, S.H.; Leuchs, G.; Manshina, A.A. Spatially-controlled laser-induced decoration of 2D and 3D substrates with plasmonic nanoparticles. RSC Adv. 2016, 6, 75681–75685. [Google Scholar] [CrossRef]
- Schlicht, S.; Kireev, A.; Vasileva, A.; Grachova, E.V.; Tunik, S.P.; Manshina, A.A.; Bachmann, J. A model electrode of well-defined geometry prepared by direct laser-induced decoration of nanoporous templates with Au-Ag@C nanoparticles. Nanotechnology 2017, 28. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Lvanova, T.Y.; Ivanov, D.A.; Kireev, A.A.; Mamonova, D.V.; Golyeva, E.V.; Mikhailov, M.D.; Manshina, A.A. In-situ laser-induced synthesis of associated YVO4:Eu3+@SiO2@Au-Ag/C nanohybrids with enhanced luminescence. J. Solid State Chem. 2018, 258, 835–840. [Google Scholar] [CrossRef]
- Haschke, S.; Pankin, D.; Mikhailovskii, V.; Barr, M.K.S.; Both-Engel, A.; Manshina, A.; Bachmann, J. Nanoporous water oxidation electrodes with a low loading of laser-deposited Ru/C exhibit enhanced corrosion stability. Beilstein J. Nanotechnol. 2019, 10, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Povolotckaia, A.; Pankin, D.; Petrov, Y.; Vasileva, A.; Kolesnikov, I.; Sarau, G.; Christiansen, S.; Leuchs, G.; Manshina, A. Plasmonic carbon nanohybrids from laser-induced deposition: Controlled synthesis and SERS properties. J. Mater. Sci. 2019, 54, 8177–8186. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals, 6th ed.; Elsevier: Oxford, UK, 2009; ISBN 978-1-85617-567-8. [Google Scholar]
- Smirnova, T.N.; Kokhtych, L.M.; Kutsenko, A.S.; Sakhno, O.V.; Stumpe, J. The fabrication of periodic polymer/silver nanoparticle structures: In situ reduction of silver nanoparticles from precursor spatially distributed in polymer using holographic exposure. Nanotechnology 2009, 20, 405301. [Google Scholar] [CrossRef] [PubMed]
- Spreadborough, J.; Christian, J.W. High-temperature X-ray diffractometer. J. Sci. Instrum. 1959, 36, 116–118. [Google Scholar] [CrossRef]
- Herbani, Y.; Nakamura, T.; Sato, S. Synthesis of platinum-based binary and ternary alloy nanoparticles in an intense laser field. J. Colloid Interface Sci. 2012, 375, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chiamori, H.C.; Liu, G.L.; Lin, L.; Chen, F.F. Nanofabricated plasmonic nano-bio hybrid structures in biomedical detection. Nanotechnol. Rev. 2012, 1, 213–233. [Google Scholar] [CrossRef]
- Seo, D.; Park, J.C.; Song, H. Polyhedral Gold Nanocrystals with Oh Symmetry: From Octahedra to Cubes. J. Am. Chem. Soc. 2006, 128, 14863–14870. [Google Scholar] [CrossRef]
- Pastoriza–Santos, I.; Sánchez–Iglesias, A.; García de Abajo, F.J.; Liz–Marzán, L.M. Environmental Optical Sensitivity of Gold Nanodecahedra. Adv. Funct. Mater. 2007, 17, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Rubio, A.S. Modified Au-Based Nanomaterials Studied by Surface Plasmon Resonance Spectroscopy; Springer Theses; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-19401-1. [Google Scholar]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble metal-based bimetallic nanoparticles: The effect of the structure on the optical, catalytic and photocatalytic properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Gharibshahi, E.; Saion, E. Influence of Dose on Particle Size and Optical Properties of Colloidal Platinum Nanoparticles. Int. J. Mol. Sci. 2012, 13, 14723–14741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharibshahi, E.; Saion, E.; Johnston, R.L.; Ashraf, A. Theory and experiment of optical absorption of platinum nanoparticles synthesized by gamma radiation. Appl. Radiat. Isot. 2019, 147, 204–210. [Google Scholar] [CrossRef] [PubMed]

| Solvent | Precursor | |||
|---|---|---|---|---|
| C6H5COOAg | (CF3SO2)2NAg | C8H18OPtSi2 | (C8H12)Cl2Pt | |
| C2H4Cl2 | 4.5 | 2.6 | 1.3 | 1.3 |
| CH3OH | 0.4 | 0.8 | 26.2 | 0.2 |
| C6H14 | 2.2 | 4.2 | 3.9 | - |
| C3H8O | 1.1 | 2.8 | 13.1 | 3.0 |
| H2O | 1.7 | 0.5 | - | - |
| C2H3N | 1.3 | 1.3 | 3.9 | 2.7 |
| CH2Cl2 | 1.3 | 0.8 | 2.9 | 5.3 |
| C2H5OH | 0.9 | 3.3 | 3.9 | 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamonova, D.V.; Vasileva, A.A.; Petrov, Y.V.; Danilov, D.V.; Kolesnikov, I.E.; Kalinichev, A.A.; Bachmann, J.; Manshina, A.A. Laser-Induced Deposition of Plasmonic Ag and Pt Nanoparticles, and Periodic Arrays. Materials 2021, 14, 10. https://doi.org/10.3390/ma14010010
Mamonova DV, Vasileva AA, Petrov YV, Danilov DV, Kolesnikov IE, Kalinichev AA, Bachmann J, Manshina AA. Laser-Induced Deposition of Plasmonic Ag and Pt Nanoparticles, and Periodic Arrays. Materials. 2021; 14(1):10. https://doi.org/10.3390/ma14010010
Chicago/Turabian StyleMamonova, Daria V., Anna A. Vasileva, Yuri V. Petrov, Denis V. Danilov, Ilya E. Kolesnikov, Alexey A. Kalinichev, Julien Bachmann, and Alina A. Manshina. 2021. "Laser-Induced Deposition of Plasmonic Ag and Pt Nanoparticles, and Periodic Arrays" Materials 14, no. 1: 10. https://doi.org/10.3390/ma14010010
APA StyleMamonova, D. V., Vasileva, A. A., Petrov, Y. V., Danilov, D. V., Kolesnikov, I. E., Kalinichev, A. A., Bachmann, J., & Manshina, A. A. (2021). Laser-Induced Deposition of Plasmonic Ag and Pt Nanoparticles, and Periodic Arrays. Materials, 14(1), 10. https://doi.org/10.3390/ma14010010






