Femur Auxetic Meta-Implants with Tuned Micromotion Distribution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analytical Analysis
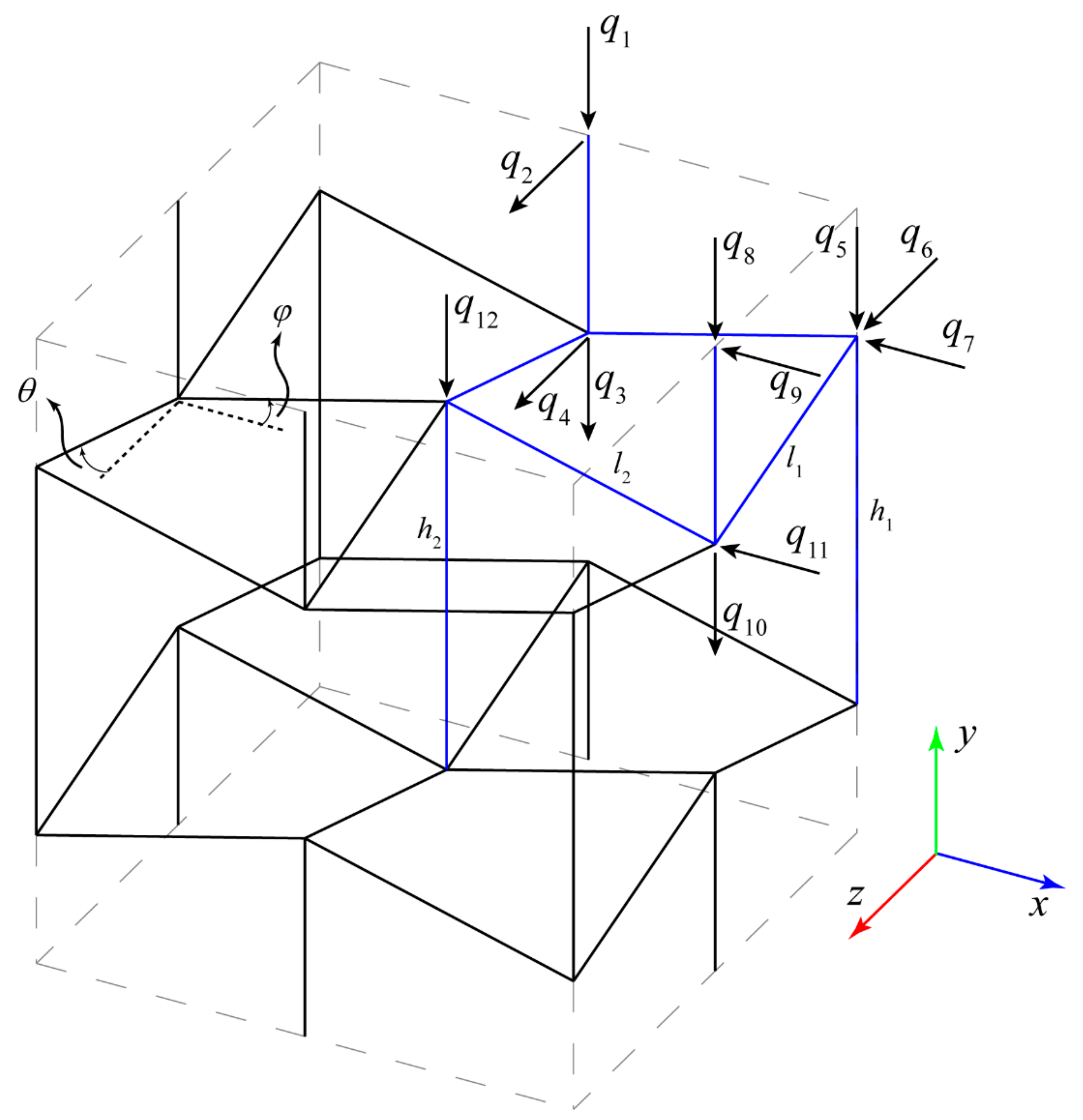
2.1.1. Stiffness Matrix Derivation
2.1.2. Elastic Properties Relationships
2.2. Numerical Analysis
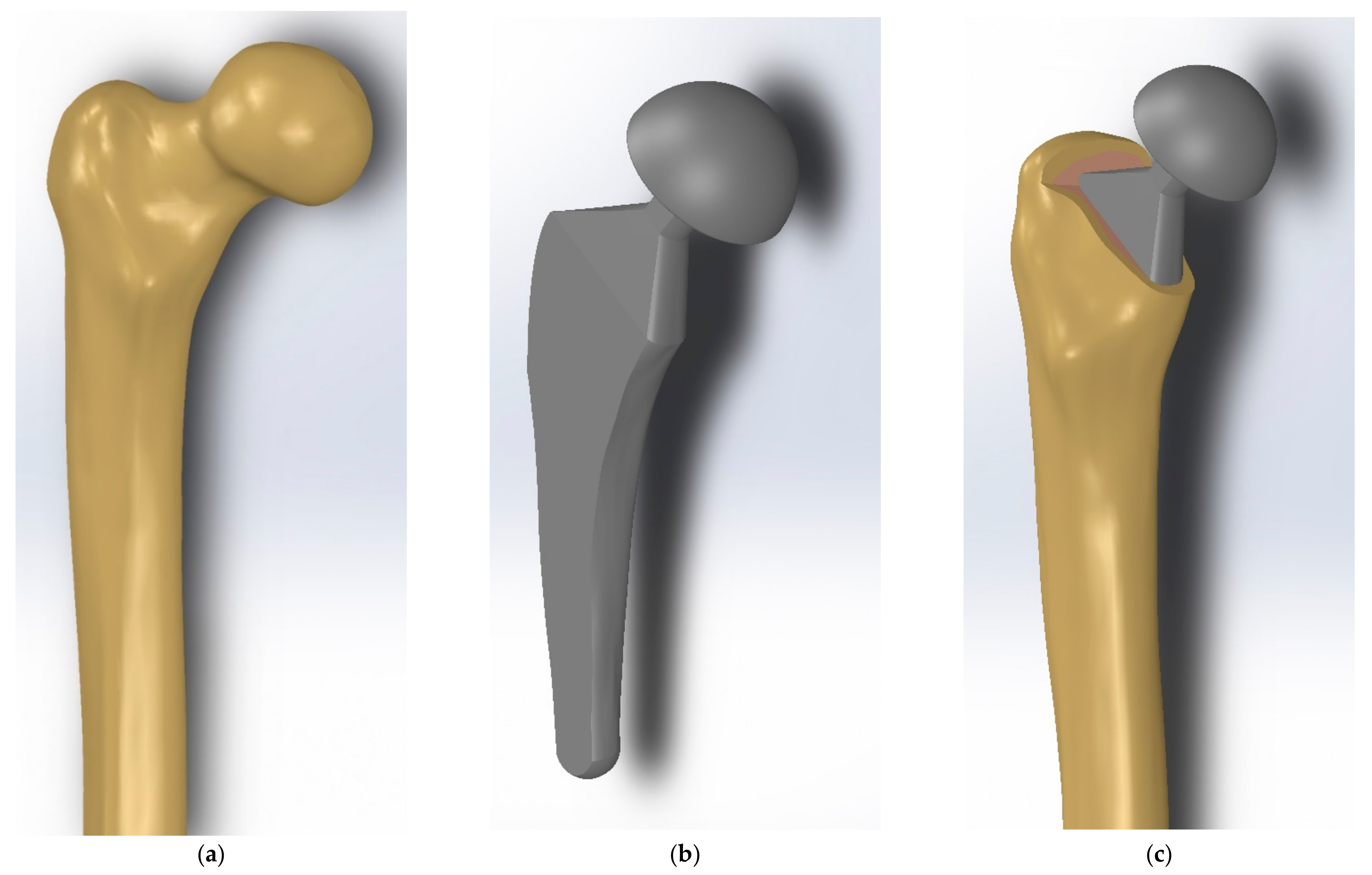
2.2.1. Methodology of the Implant Design
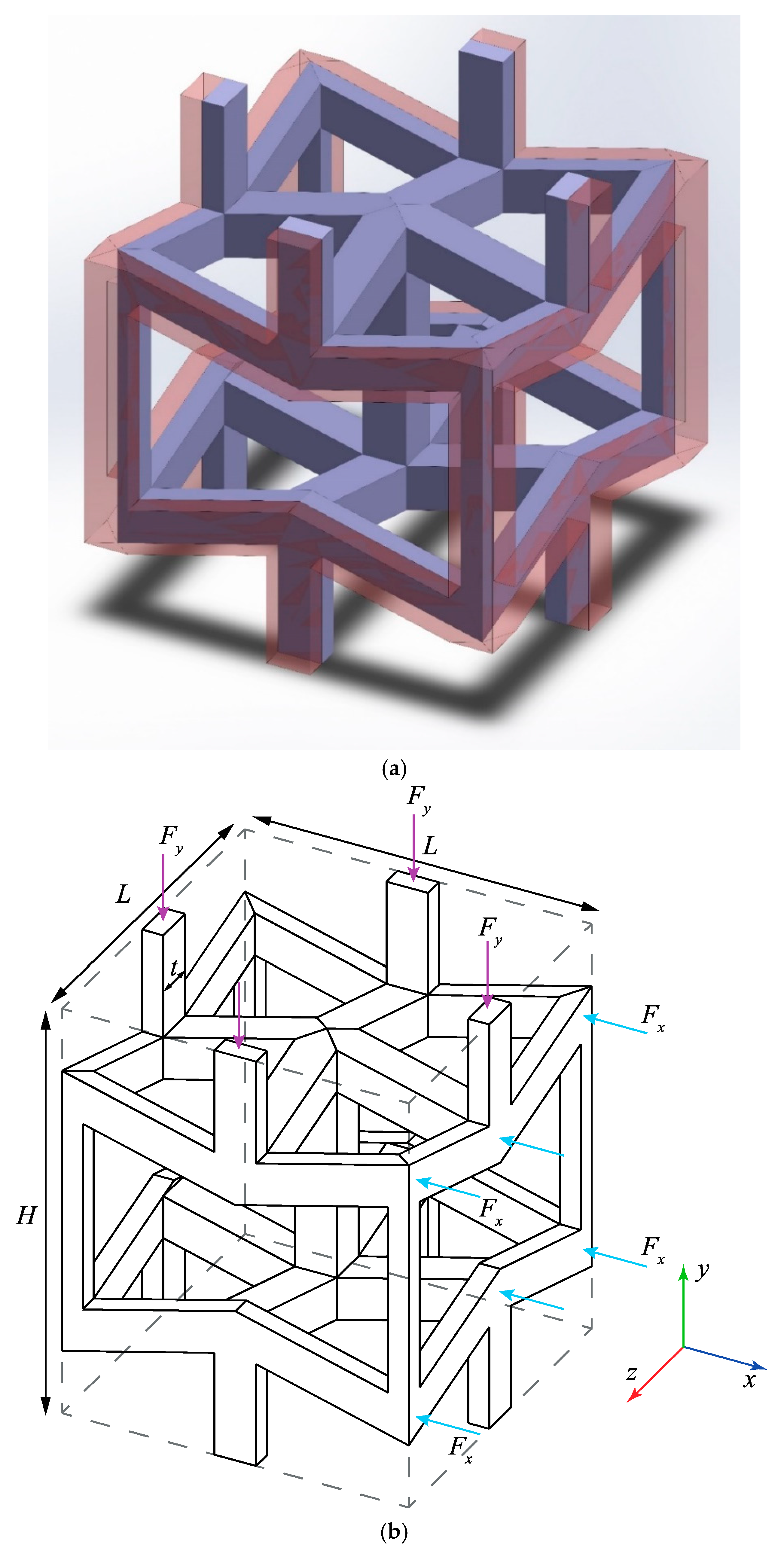
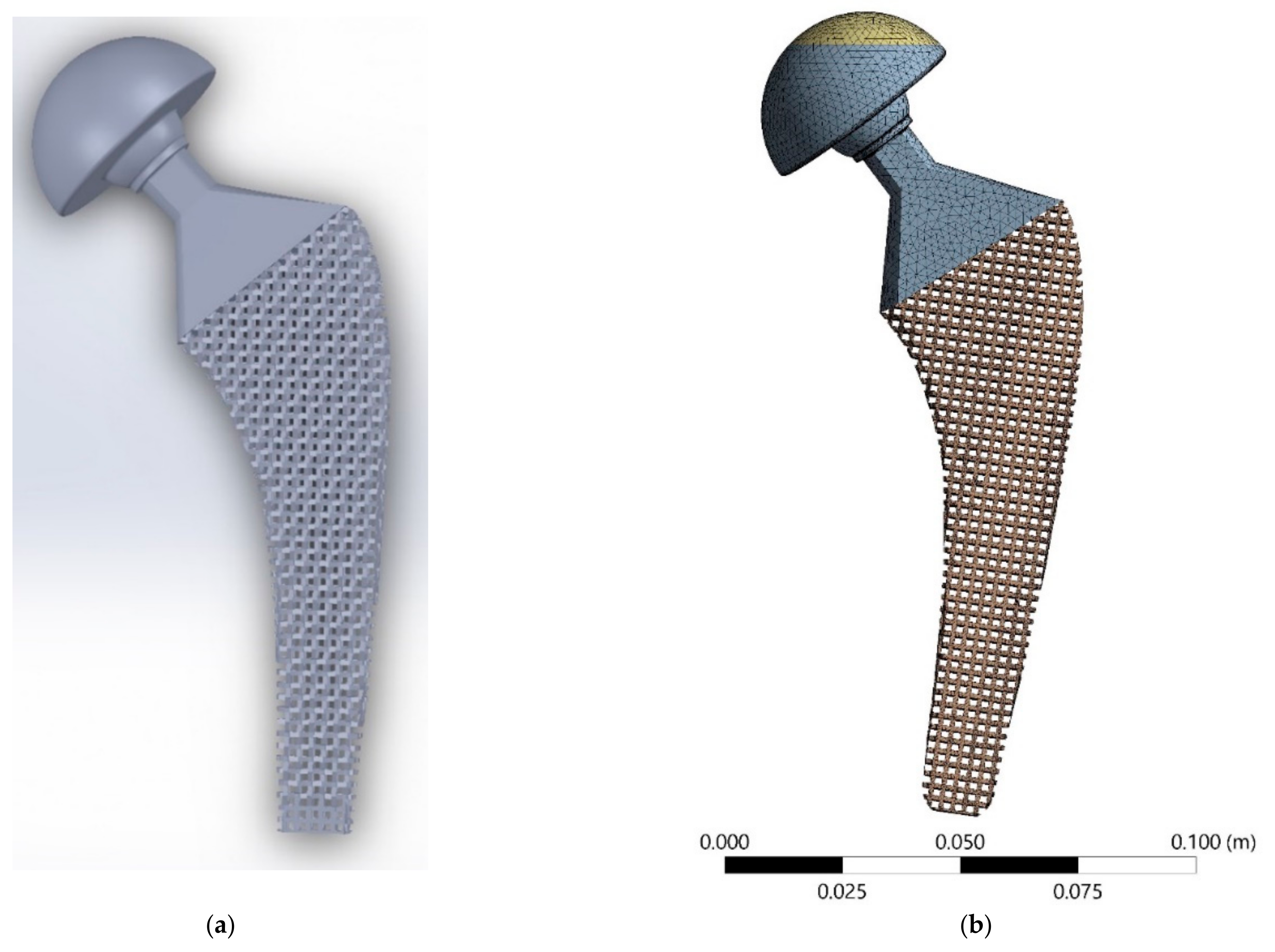
2.2.2. Meta-Implant Design
2.2.3. Graded Meta-Implant
2.2.4. FE Analysis
3. Results
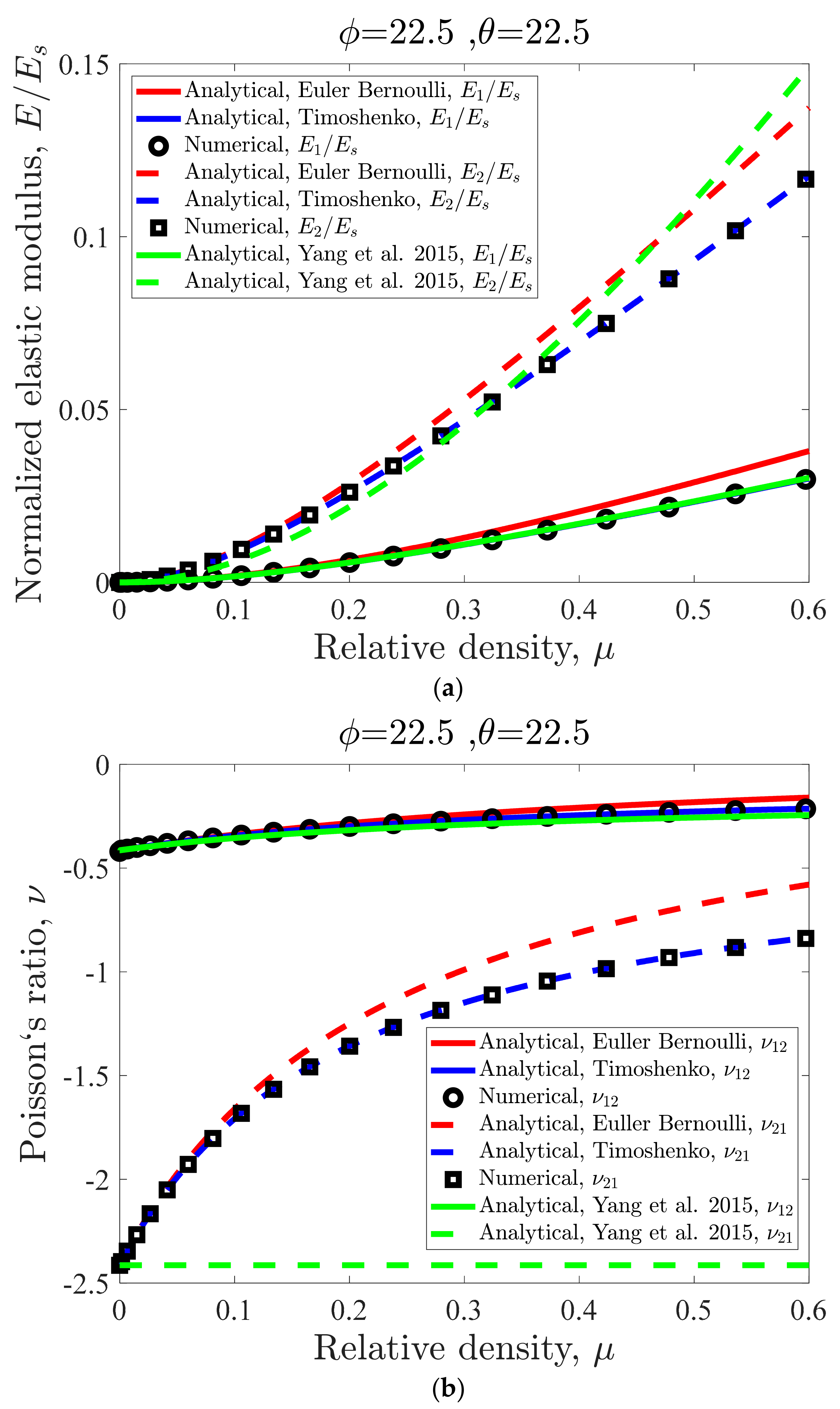
3.1. Effect of Relative Density on the Unit Cell Mechanical Properties
3.2. Effect of Internal Angle on the Unit Cell Mechanical Properties
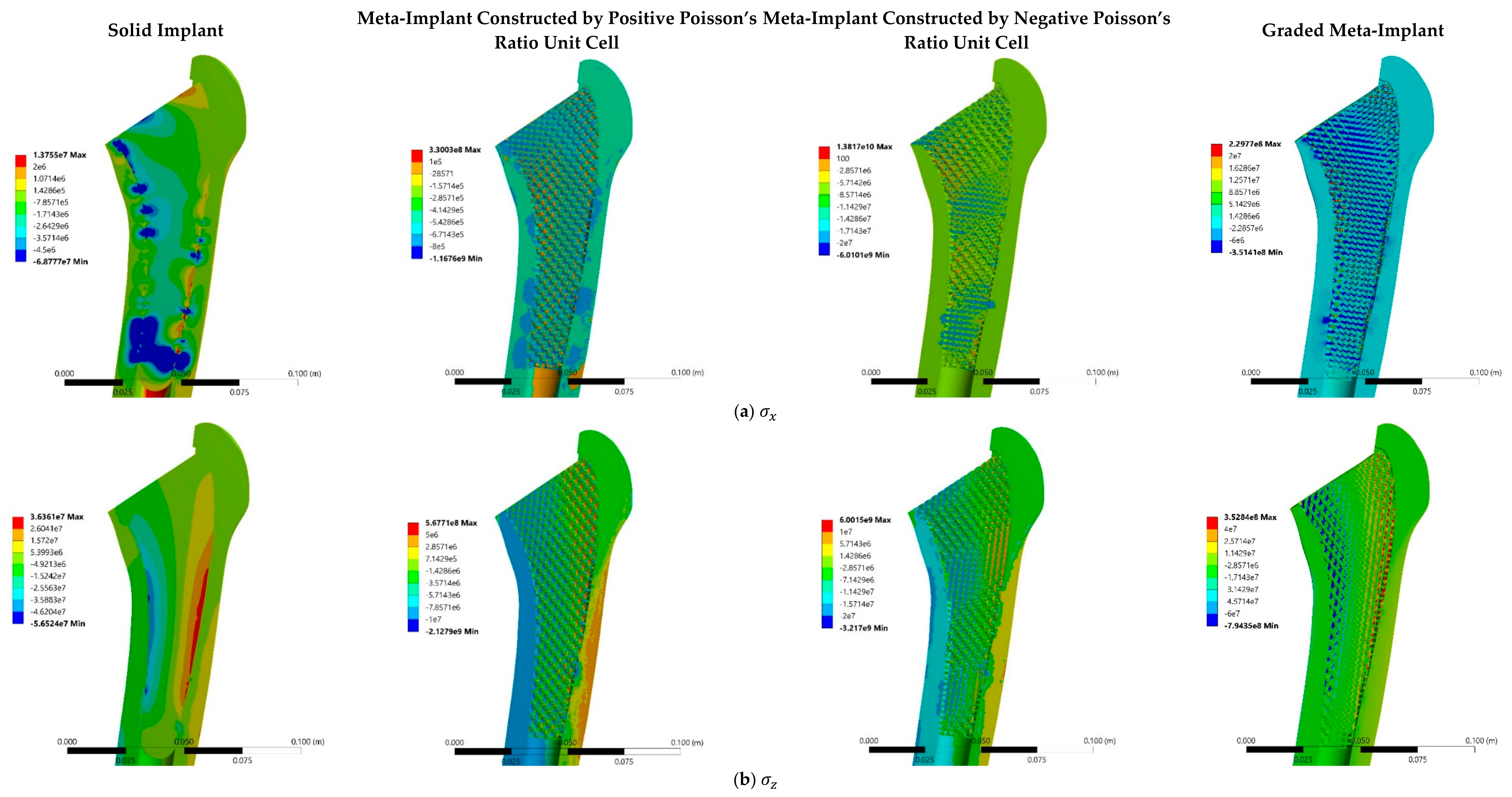
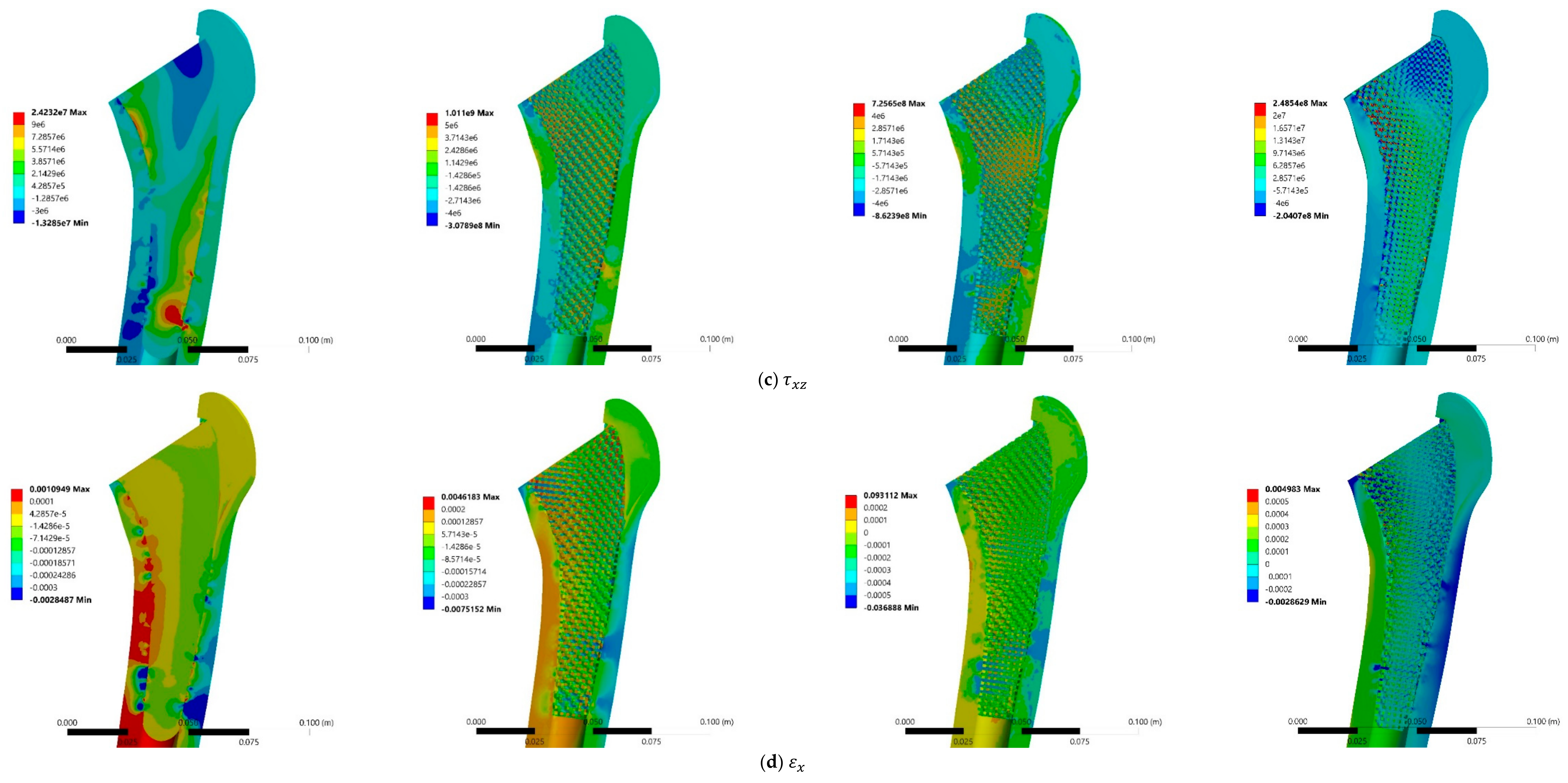
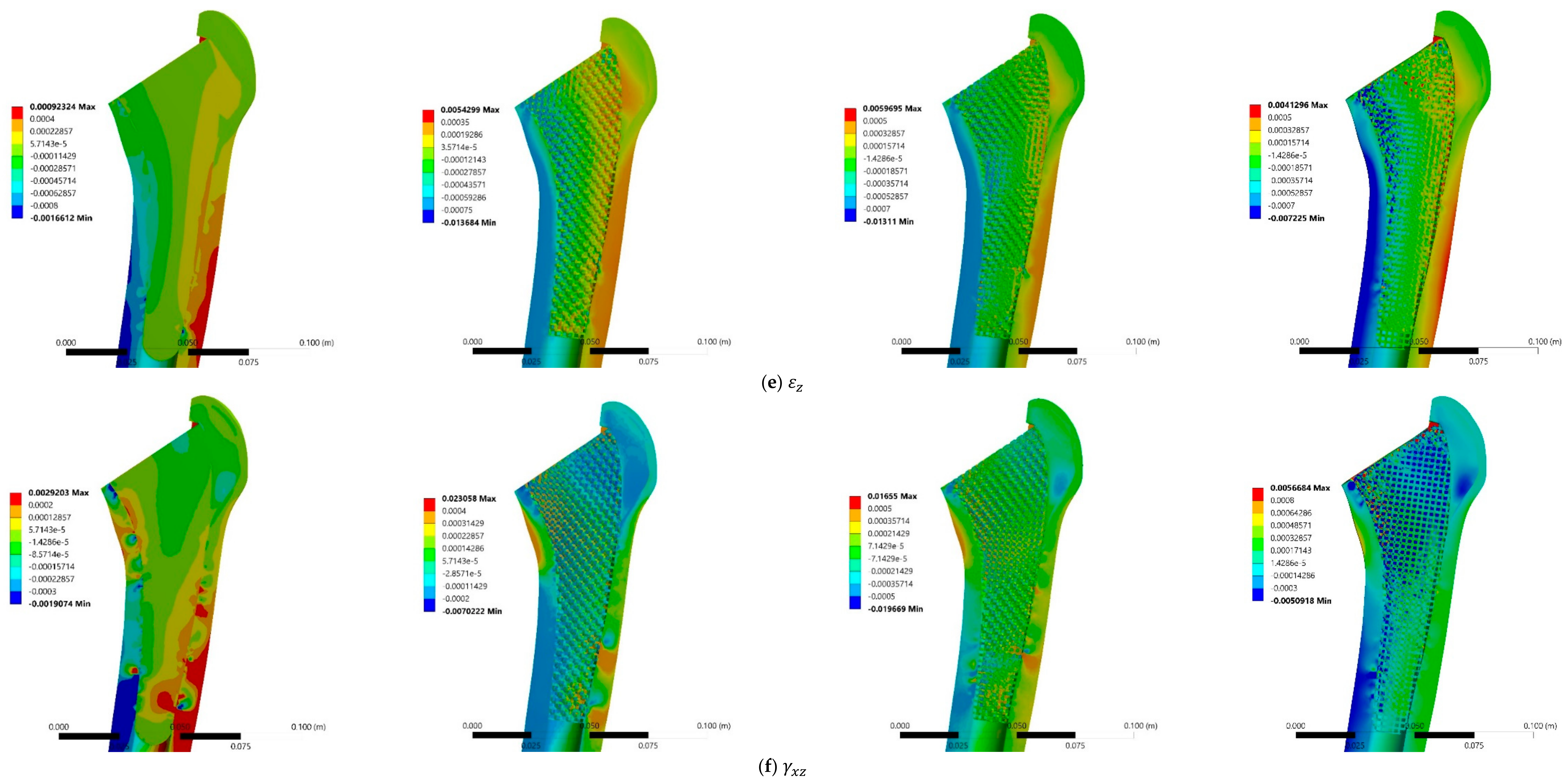
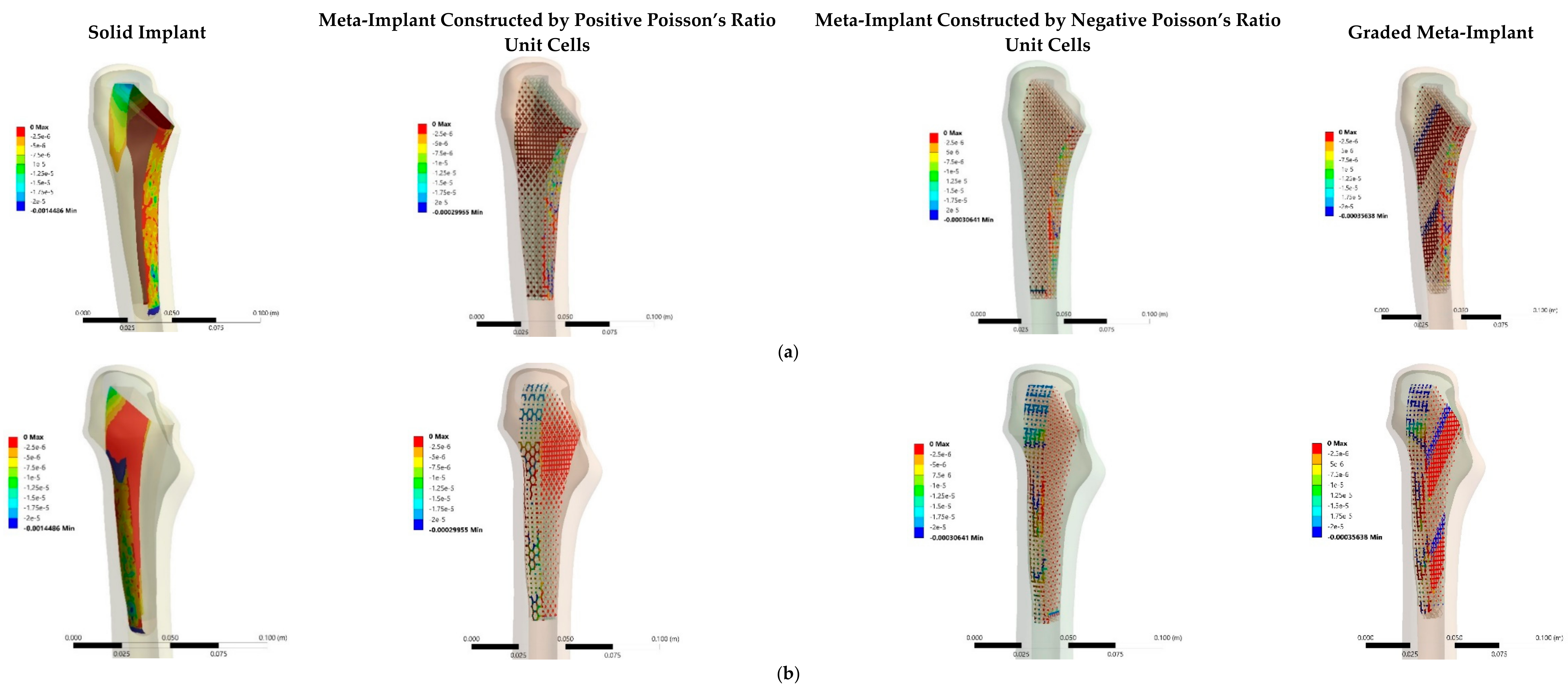
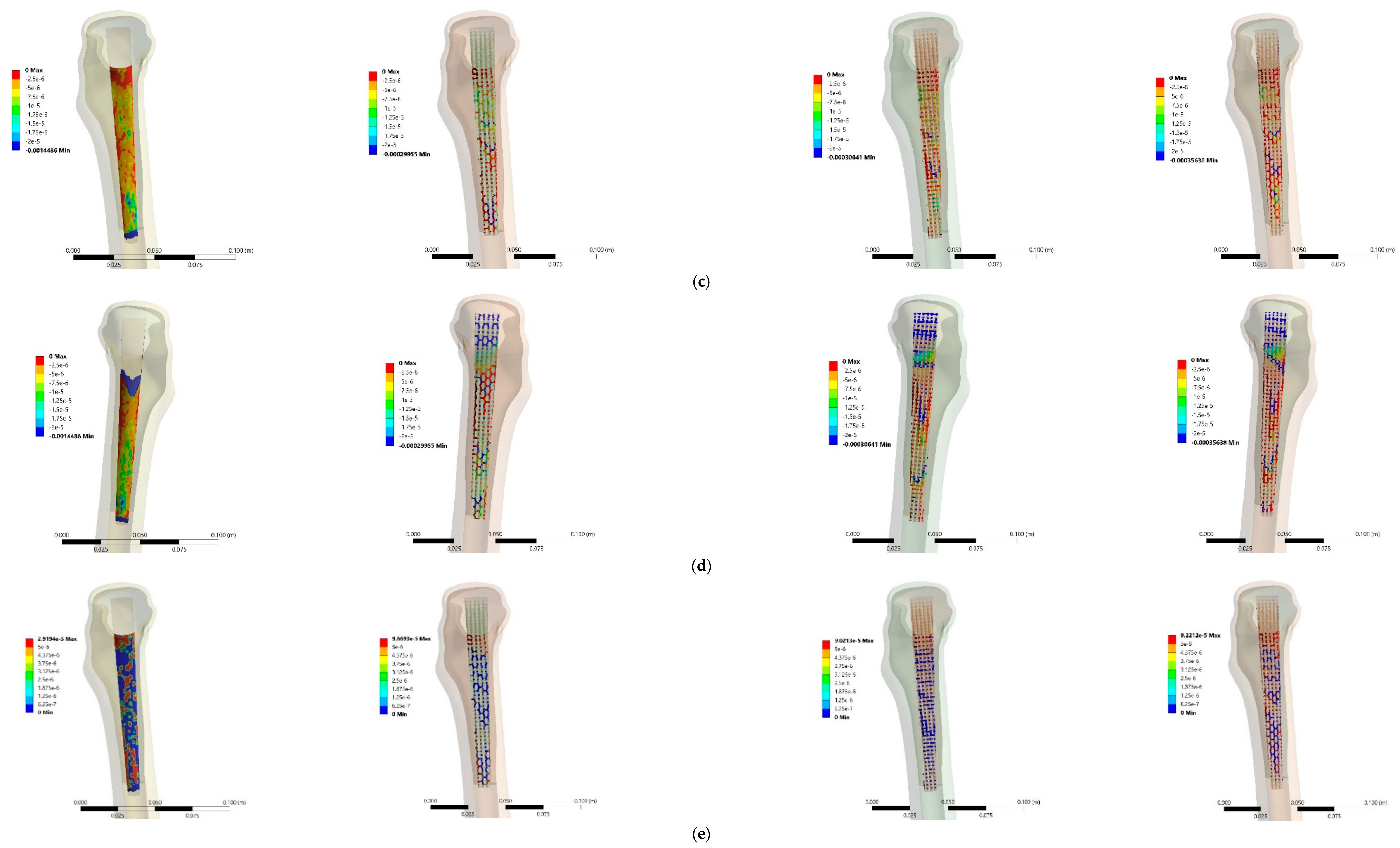
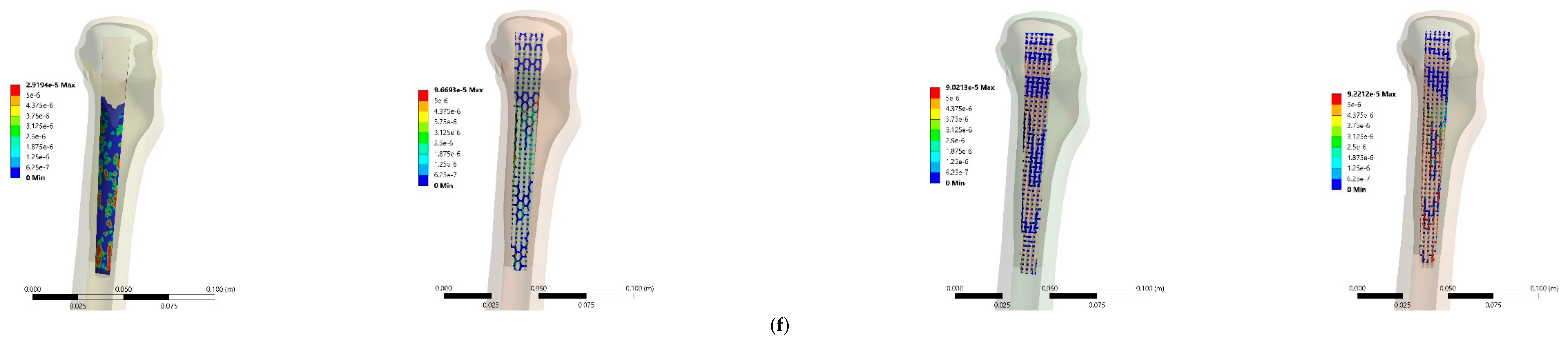
3.3. Stress and Strain Distributions
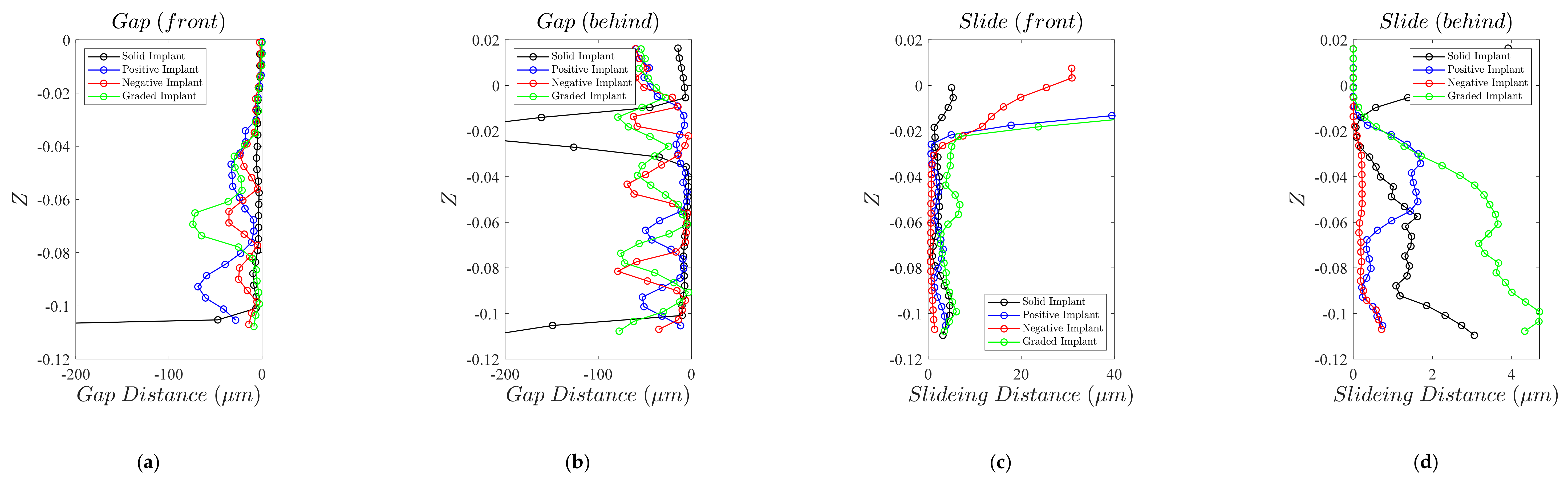
3.4. Micromotion
4. Discussions
4.1. Analytical Model
4.2. Implant Macro-Geometry
4.3. Stress and Strain Distributions
4.4. Micromotion
4.5. What Micro-Structure to Choose?
4.6. Further Improvements and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cue, G.; Ling, R.; Linder, L. Impacted Cancellous Allografts and Cement for Revision Total Hip Arthroplasty. J. Bone Jt. Surg. 1993, 7, 14–21. [Google Scholar]
- Beckenbaugh, R.D.; Ilstrup, D.M. Total Hip Arthroplasty. J. Bone Jt. Surg. Am. 1978, 60, 306–313. [Google Scholar] [CrossRef]
- Winter, W.; Klein, D.; Karl, M. Micromotion of Dental Implants: Basic Mechanical Considerations. J. Med. Eng. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, R.D.; Huo, M.H.; Rodrigues, D.C.; Kosmopoulos, V. Stem Geometry Changes Initial Femoral Fixation Stability of a Revised Press-Fit Hip Prosthesis: A Finite Ele-Ment Study. Technol. Health Care 2016, 1–8, Pre-print. [Google Scholar]
- Camine, V.M.; Rüdiger, H.; Pioletti, D.P.; Terrier, A. Distribution of Gap and Micromotion during Compressive Loading around a Cementless Femoral Stem. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1896–1897. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Kadir, M.R.; Hansen, U.; Klabunde, R.; Lucas, D.; Amis, A. Finite Element Modelling of Primary Hip Stem Stability: The Effect of Interference Fit. J. Biomech. 2008, 41, 587–594. [Google Scholar] [CrossRef]
- Pilliar, R. Quantitative Evaluation of the Effect of Movement at a Porous Coated Implant-Bone Interface. Bone-Biomater. Interface 1991, 380–387. [Google Scholar] [CrossRef]
- Engh, A.C.; O’Connor, D.; Jasty, M.; McGovern, T.F.; Bobyn, J.D.; Harris, W.H. Quantification of Implant Micromotion, Strain Shielding, and Bone Resorption with Porous-Coated Anatomic Medullary Locking Femoral Prostheses. Clin. Orthop. Relat. Res. 1992, 285, 13–29. [Google Scholar] [CrossRef]
- Pilliar, R.; Deporter, D.; Watson, P. Tissue-Implant Interface: Micromovement Effects. Adv. Sci. Technol. 1995, 12, 569–579. [Google Scholar]
- Vandamme, K.; Naert, I.; Geris, L.; Sloten, J.V.; Puers, R.; Duyck, J. The Effect of Micro-Motion on the Tissue Response around Immediately Loaded Roughened Titanium Im-Plants in the Rabbit. Eur. J. Oral Sci. 2007, 115, 21–29. [Google Scholar]
- Brunski, J.B. Avoid Pitfalls of Overloading and Micromotion of Intraosseous Implants. Dent. Implant. Updat. 1993, 4, 77–81. [Google Scholar]
- Weinans, H.; Huiskes, H.R.; Grootenboer, H.J. Quantitative Analysis of Bone Reactions to Relative Motions at Implant-Bone Interfaces. J. Biomech. 1993, 26, 12711279–12771281. [Google Scholar] [CrossRef] [Green Version]
- Søballe, K.; Brockstedt-Rasmussen, H.; Hansen, E.S.; Bünger, C. Hydroxyapatite Coating Modifies Implant Membrane Formation. Controlled Micromotion Studied in Dogs. Acta Orthop. Scand. 1992, 63, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. Validation of Value of Actual Micromotion as a Direct Measure of Implant Micromobility after Healing (Second-Ary Implant Sta-Bility). an in Vivo Histologic and Biomechanical Study. Clin. Oral Implant. Res. 2016, 27, 1423–1430. [Google Scholar] [CrossRef]
- Cameron, H.; Macnab, I.; Pilliar, R. Porous Surfaced Vitallium Staples. S. Afr. J. Surg. Suid-Afrik. Tydskr. Vir Chir. 1972, 10, 63–70. [Google Scholar]
- Cameron, H.U.; Pilliar, R.M.; Macnab, I. The Effect of Movement on the Bonding of Porous Metal to Bone. J. Biomed. Mater. Res. 1973, 7, 301–311. [Google Scholar] [CrossRef]
- Cristofolini, L.; Varini, E.; Viceconti, M. In-Vitro Method for Assessing Femoral Implant—Bone Micromotions in Resurfacing Hip Implants under Different Loading Conditions. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2007, 221, 943–950. [Google Scholar] [CrossRef]
- Hedayati, R.; Lakshmanan, S. Pneumatically-Actuated Acoustic Metamaterials Based on Helmholtz Resonators. Materials 2020, 13, 1456. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Su, Y.; Shi, J. Perspective of Additive Manufacturing for Metamaterials Development. Smart Mater. Struct. 2019, 28, 093001. [Google Scholar] [CrossRef]
- Hedayati, R.; Hosseini-Toudeshky, H.; Sadighi, M.; Mohammadi-Aghdam, M.; Zadpoor, A. Multiscale Modeling of Fatigue Crack Propagation in Additively Manufactured Porous Biomaterials. Int. J. Fatigue 2018, 113, 416–427. [Google Scholar] [CrossRef]
- Hedayati, R.; Janbaz, S.; Sadighi, M.; Mohammadi-Aghdam, M.; Zadpoor, A.A. How Does Tissue Regeneration Influence the Mechanical Behavior of Additively Manufactured Porous Bio-Materials? J. Mech. Behav. Biomed. Mater. 2017, 65, 831–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amendola, A.; Smith, C.J.; Godall, R.; Auricchio, F.; Feo, L.; Benzoni, G.; Fraternali, F. Experimental Response of Additively Manufactured Metallic Pentamode Materials Confined between Stiffening Plates. Compos. Struct. 2016, 142, 254–262. [Google Scholar] [CrossRef]
- Kadic, M.; Bückmann, T.; Stenger, N.; Thiel, M.; Wegener, M. On the Practicability of Pentamode Mechanical Metamaterials. Appl. Phys. Lett. 2012, 100, 191901. [Google Scholar] [CrossRef] [Green Version]
- Hosseinabadi, H.G.; Bagheri, R.; Altstädt, V. A Numerical Approach to Study the Post-yield Softening in Cellular Solids: Role of Microstructural Ordering and Cell Size Distribution. Acta Mech. 2017, 228, 2005–2016. [Google Scholar] [CrossRef]
- Karaji, Z.G.; Hedayati, R.; Pouran, B.; Apachitei, I.; Zadpoor, A.A. Effects of Plasma Electrolytic Oxidation Process on the Mechanical Properties of Additively Manufactured Porous Biomaterials. Mater. Sci. Eng. C 2017, 76, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, R.; Yavari, S.A.; Zadpoor, A. Fatigue Crack Propagation in Additively Manufactured Porous Biomaterials. Mater. Sci. Eng. C 2017, 76, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Hedayati, R.; Salami, S.J.; Li, Y.; Sadighi, M.; Zadpoor, A. Semianalytical Geometry-Property Relationships for Some Generalized Classes of Pentamodelike Additively Manufactured Mechanical Metamaterials. Phys. Rev. Appl. 2019, 11, 034057. [Google Scholar] [CrossRef] [Green Version]
- Hedayati, R.; Leeflang, A.M.; Zadpoor, A.A. Additively Manufactured Metallic Pentamode Meta-Materials. Appl. Phys. Lett. 2017, 110, 091905. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, K.; Movahhedy, M.R.; Shishovsky, I.; Hedayati, R. Hybrid Anisotropic Pentamode Mechanical Metamaterial Produced by Additive Manufacturing Technique. Appl. Phys. Lett. 2020, 117, 61901. [Google Scholar] [CrossRef]
- Mirzaali, M.; Hedayati, R.; Vena, P.; Vergani, L.; Strano, M.; Zadpoor, A.A. Rational Design of Soft Mechanical Metamaterials: Independent Tailoring of Elastic Properties with Ran-Domness. Appl. Phys. Lett. 2017, 111, 51903. [Google Scholar] [CrossRef] [Green Version]
- Babaee, S.; Shim, J.; Weaver, J.C.; Chen, E.R.; Patel, N.; Bertoldi, K. 3D Soft Metamaterials with Negative Poisson’s Ratio. Adv. Mater. 2013, 25, 5044–5049. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, Z.G.; Motter, A.E. Mechanical Metamaterials with Negative Compressibility Transitions. Nat. Mater. 2012, 11, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, R.; Mirzaali, M.J.; Vergani, L.; Zadpoor, A.A. Action-at-a-Distance Metamaterials: Distributed Local Actuation through Far-Field Global Forces. APL Mater. 2018, 6, 36101. [Google Scholar] [CrossRef] [Green Version]
- Rad, M.S.; Hatami, H.; Ahmad, Z.; Yasuri, A.K. Analytical Solution and Finite Element Approach to the Dense Re-Entrant Unit Cells of Auxetic Structures. Acta Mech. 2019, 230, 2171–2185. [Google Scholar] [CrossRef]
- Yang, L.; Harrysson, O.; West, H.; Cormier, D. Compressive Properties of Ti–6Al–4V Auxetic Mesh Structures Made by Electron Beam Melting. Acta Mater. 2012, 60, 3370–3379. [Google Scholar] [CrossRef]
- Wang, X.-T.; Wang, B.; Li, X.-W.; Ma, L. Mechanical Properties of 3D Re-Entrant Auxetic Cellular Structures. Int. J. Mech. Sci. 2017, 131, 396–407. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, P.; Zhou, L.; Han, F. An Enhanced Three-Dimensional Auxetic Lattice Structure with Improved Property. Materials 2020, 13, 1008. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Harrysson, A.O.L.; West, A.H.; Cormier, D. Mechanical Properties of 3D Re-Entrant Honeycomb Auxetic Structures Realized via Additive Manufacturing. Int. J. Solids Struct. 2015, 69, 475–490. [Google Scholar] [CrossRef]
- Chen, C.-M.; Tsai, W.-C.; Lin, S.-C.; Tseng, C.-S. A Cementless, Proximally Fixed Anatomic Femoral Stem Induces High Micromotion with Nontraumatic Fem-Oral Avascular Necrosis: A Finite Element Study. J. Orthop. Transl. 2014, 2, 149–156. [Google Scholar]
- Bieger, R.; Ignatius, A.; Decking, R.; Claes, L.; Reichel, H.; Dürselen, L. Primary Stability and Strain Distribution of Cementless Hip Stems as a Function of Implant Design. Clin. Biomech. 2012, 27, 158–164. [Google Scholar] [CrossRef]
- Wang, Y.; Arabnejad, S.; Tanzer, M.; Pasini, D. Hip Implant Design with Three-Dimensional Porous Architecture of Optimized Graded Density. J. Mech. Des. 2018, 140. [Google Scholar] [CrossRef] [Green Version]
- Kolken, H.M.A.; Janbaz, S.; Leeflang, S.M.A.; Lietaert, K.; Weinans, H.; Zadpoor, A.A. Rationally Designed Meta-Implants: A Combination of Auxetic and Conventional Meta-Biomaterials. Mater. Horiz. 2018, 5, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Ghavidelnia, N.; Hedayati, R.; Sadighi, M.; Mohammadi-Aghdam, M. Development of Porous Implants with Non-uniform Mechanical Properties Distribution Based on CT Images. Appl. Math. Model. 2020, 83, 801–823. [Google Scholar] [CrossRef]
- Hedayati, R.; Sadighi, M.; Mohammadi-Aghdam, M.; Zadpoor, A. Mechanical Properties of Regular Porous Biomaterials Made from Truncated Cube Repeating Unit Cells: Ana-Lytical Solutions and Computational Models. Mater. Sci. Eng. C 2016, 60, 163–183. [Google Scholar] [CrossRef]
- Hedayati, R.; Sadighi, M.; Aghdam, M.; Zadpoor, A. Analytical Relationships for the Mechanical Properties of Additively Manufactured Porous Biomaterials Based on Octahedral Unit Cells. Appl. Math. Model. 2017, 46, 408–422. [Google Scholar] [CrossRef] [Green Version]
- Hedayati, R.; Sadighi, M.; Mohammadi-Aghdam, M.; Zadpoor, A. Mechanics of Additively Manufactured Porous Biomaterials Based on the Rhombicuboctahedron Unit Cell. J. Mech. Behav. Biomed. Mater. 2016, 53, 272–294. [Google Scholar] [CrossRef]
- Hedayati, R.; Ghavidelnia, N.; Sadighi, M.; Bodaghi, M. Improving the Accuracy of Analytical Relationships for Mechanical Properties of Additively Manufactured Lattice Structures. EngrXiv 2020. [Google Scholar] [CrossRef]
- Charnley, J.; Kamangar, A.; Longfield, M.D. The Optimum Size of Prosthetic Heads in Relation to the Wear of Plastic Sockets in Total Replacement of the Hip. Med. Biol. Eng. Comput. 1969, 7, 31–39. [Google Scholar] [CrossRef]
- McKee, G.K. Paper 4: Developments in Total Hip Joint Replacement. In Proceedings of the Institution of Mechanical Engineers, Conference Proceedings; SAGE Publications: London, UK, 2010; Volume 181, pp. 85–89. [Google Scholar]
- Müller, M. 7 Total Hip Prostheses. Clin. Orthop. Relat. Res. 1970, 72, 46–68. [Google Scholar]
- Hybbinette, C.H. Long-Term Results of Wear of Plastic Hip Prostheses. Arch. Orthop. Trauma Surg. 1985, 104, 28–30. [Google Scholar] [CrossRef]
- El-Shiekh, H.E.-D.F. Finite Element Simulation of Hip Joint Replacement under Static and Dynamic Loading; Dublin City University: Dublin, Ireland, UK, 2002. [Google Scholar]
- Zysset, P.K.; Guo, X.E.; Hoffler, C.E.; Moore, E.K.; Goldstein, A.S. Elastic Modulus and Hardness of Cortical and Trabecular Bone Lamellae Measured by Nanoindentation in the Human Femur. J. Biomech. 1999, 32, 1005–1012. [Google Scholar] [CrossRef]
- Brown, T.D.; Ferguson, A.B. Mechanical Property Distributions in the Cancellous Bone of the Human Proximal Femur. Acta Orthop. Scand. 1980, 51, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Katsamanis, F.; Raftopoulos, D.D. Determination of Mechanical Properties of Human Femoral Cortical Bone by the Hop-Kinson Bar Stress Technique. J. Biomech. 1990, 23, 1173–1184. [Google Scholar] [CrossRef]
- Wirtz, D.C.; Schiffers, N.; Pandorf, T.; Radermacher, K.; Weichert, D.; Forst, R. Critical Evaluation of Known Bone Material Properties to Realize Anisotropic FE-Simulation of the Proximal Femur. J. Biomech. 2000, 33, 1325–1330. [Google Scholar] [CrossRef]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and Mechanobiology of Trabecular Bone: A Review. J. Biomech. Eng. 2015, 137. [Google Scholar] [CrossRef] [Green Version]
- Arabnejad, S.; Johnston, B.; Tanzer, M.; Pasini, D. Fully Porous 3D Printed Titanium Femoral Stem to Reduce Stress-Shielding Following Total Hip Arthroplas-Ty. J. Orthop. Res. 2017, 35, 1774–1783. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Chen, W.-C.; Huang, C.-H.; Cheng, C.-K.; Chan, K.-K.; Chang, T.-K. The Effect of Graft Strength on Knee Laxity and Graft in-Situ Forces after Posterior Cruciate Ligament Reconstruction. PLoS ONE 2015, 10, e0127293. [Google Scholar] [CrossRef] [Green Version]
- Izzawati, B.; Daud, R.; Afendi, M.; Majid, M.S.A.; Zain, N.A.M.; Bajuri, M.Y. Stress Analysis of Implant-Bone Fixation at Different Fracture Angle. J. Phys. Conf. Ser. 2017, 908, 12019. [Google Scholar] [CrossRef] [Green Version]
- Rudman, E.K.; Aspden, R.M.; Meakin, J.R. Compression or Tension? The Stress Distribution in the Proximal Femur. Biomed. Eng. Online 2006, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Demey, G.; Fary, C.; Lustig, S.; Neyret, P.; Selmi, T.A.S. Does a Collar Improve the Immediate Stability of Uncemented Femoral Hip Stems in Total Hip Arthroplasty? A Bilateral Comparative Cadaver Study. J. Arthroplast. 2011, 26, 1549–1555. [Google Scholar] [CrossRef]
- Moazen, M.; Jones, A.C.; Jin, Z.; Wilcox, R.K.; Tsiridis, E. Periprosthetic Fracture Fixation of the Femur Following Total Hip Arthroplasty: A Review of Biomechanical Testing. Clin. Biomech. 2011, 26, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Muccini, R.; Bernakiewicz, M.; Baleani, M.; Cristofolini, L. Large-Sliding Contact Elements Accurately Predict Levels of Bone–Implant Micromotion Relevant to Osseointegration. J. Biomech. 2000, 33, 1611–1618. [Google Scholar] [CrossRef]
- Wan, H.; Ohtaki, H.; Kotosaka, S.; Hu, G. A Study of Negative Poisson’s Ratios in Auxetic Honeycombs Based on a Large Deflection Model. Eur. J. Mech.—A/Solids 2004, 23, 95–106. [Google Scholar] [CrossRef]
- Østbyhaug, P.; Klasvik, J.; Romunstad, P.; Aadmond, A. An In Vitro Study of the Strain Distribution in Human Femora with Anatomical and Customised Femoral Stems. J. Bone Jt. Surg. Br. 2009, 91, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Nysted, M.; Benum, P.; Klasvik, J.; Foss, O.; Aamodt, A. Periprosthetic Bone Loss after Insertion of an Uncemented, Customized Femoral Stem and an Uncemented Anatomical Stem: A Randomized DXA Study with 5-Year Follow-Up. Acta Orthop. Scand. 2011, 82, 410–416. [Google Scholar] [CrossRef]
- Karrholm, J.; Malchau, H.; Snorrason, F.; Herberts, P. Micromotion of Femoral Stems in Total Hip Arthroplasty. A Randomized Study of Cemented, Hydroxyapatite-Coated, and Po-Rous-Coated Stems with Roentgen Stereophotogrammetric Analysis. J. Bone Jt. Surg. 1994, 76, 1692–1705. [Google Scholar] [CrossRef]
- Engh, A.C.; Hooten, J.P.; Zettl-Schaffer, K.F.; Ghaffarpour, M.; McGovern, T.F.; Macalino, E.G.; Zicat, A.B. Porous-Coated Total Hip Replacement. Clin. Orthop. Relat. Res. 1994, 1994, 89–96. [Google Scholar] [CrossRef]
- Haddad, R.; Cook, S. Brinker a Comparison of Three Varieties of Noncemented Porous-Coated Hip Replacement. J. Bone Jt. Surg. Br. 1990, 72, 2–8. [Google Scholar] [CrossRef]
- Burke, D. Dynamic Measurement of Interface Mechanics in Vivo and the Effect of Micromotion on Bone Ingrowth into Porous Surface Device under Controlled Loads in Vivo. Trans. ORS 1991, 16, 103. [Google Scholar]
- Ghavidelnia, N.; Hedayati, R.; Bodaghi, M. 3D Auxetic Mechanical Metamaterials: An Analytical, Numerical, and Experimental Study. EngrXiv 2020. [Google Scholar] [CrossRef]
- Hedayati, R.; Sadighi, M.; Aghdam, M.; Zadpoor, A.A. Effect of Mass Multiple Counting on the Elastic Properties of Open-Cell Regular Porous Biomaterials. Mater. Des. 2016, 89, 9–20. [Google Scholar] [CrossRef]
- Gao, X.; Fraulob, M.; Haïat, G. Biomechanical Behaviours of the Bone–Implant Interface: A Review. J. R. Soc. Interface 2019, 16, 20190259. [Google Scholar] [CrossRef] [PubMed]
- Henyš, P.; Čapek, L. Impact Force, Polar Gap and Modal Parameters Predict Acetabular Cup Fixation: A Study on a Compo-Site Bone. Ann. Biomed. Eng. 2018, 46, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, R.; Sadighi, M. Finite Element Investigation of Mechanical Behavior of Rhombic Dodecahedron Micro-structures under Static Loading. In Proceedings of the First International Conference on MEMS and Microfabrication-ICMEMS2014, Tehran, Iran, 18–19 February 2014. [Google Scholar]
- Henyš, P.; Čapek, L.; Březina, J. Comparison of Current Methods for Implementing Periodic Boundary Conditions in Multi-Scale Homogenisation. Eur. J. Mech.—A/Solids 2019, 78, 103825. [Google Scholar] [CrossRef]
- Pal, B.; Gupta, S.; New, A.M.; Browne, M. Strain and Micromotion in Intact and Resurfaced Composite Femurs: Experimental and Numerical Investigations. J. Biomech. 2010, 43, 1923–1930. [Google Scholar] [CrossRef]
- Gortchacow, M.; Wettstein, M.; Pioletti, D.P.; Müller-Gerbl, M.; Terrier, A. Simultaneous and Multisite Measure of Micromotion, Subsidence and Gap to Evaluate Femoral Stem Stability. J. Biomech. 2012, 45, 1232–1238. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghavidelnia, N.; Bodaghi, M.; Hedayati, R. Femur Auxetic Meta-Implants with Tuned Micromotion Distribution. Materials 2021, 14, 114. https://doi.org/10.3390/ma14010114
Ghavidelnia N, Bodaghi M, Hedayati R. Femur Auxetic Meta-Implants with Tuned Micromotion Distribution. Materials. 2021; 14(1):114. https://doi.org/10.3390/ma14010114
Chicago/Turabian StyleGhavidelnia, Naeim, Mahdi Bodaghi, and Reza Hedayati. 2021. "Femur Auxetic Meta-Implants with Tuned Micromotion Distribution" Materials 14, no. 1: 114. https://doi.org/10.3390/ma14010114





