Analysis of the In Situ Crack Evolution Behavior in a Solid Solution Mg-13Gd-5Y-3Zn-0.3Zr Alloy
Abstract
:1. Introduction
2. Materials and Experimental Methods
3. Results
3.1. Microstructure Analysis
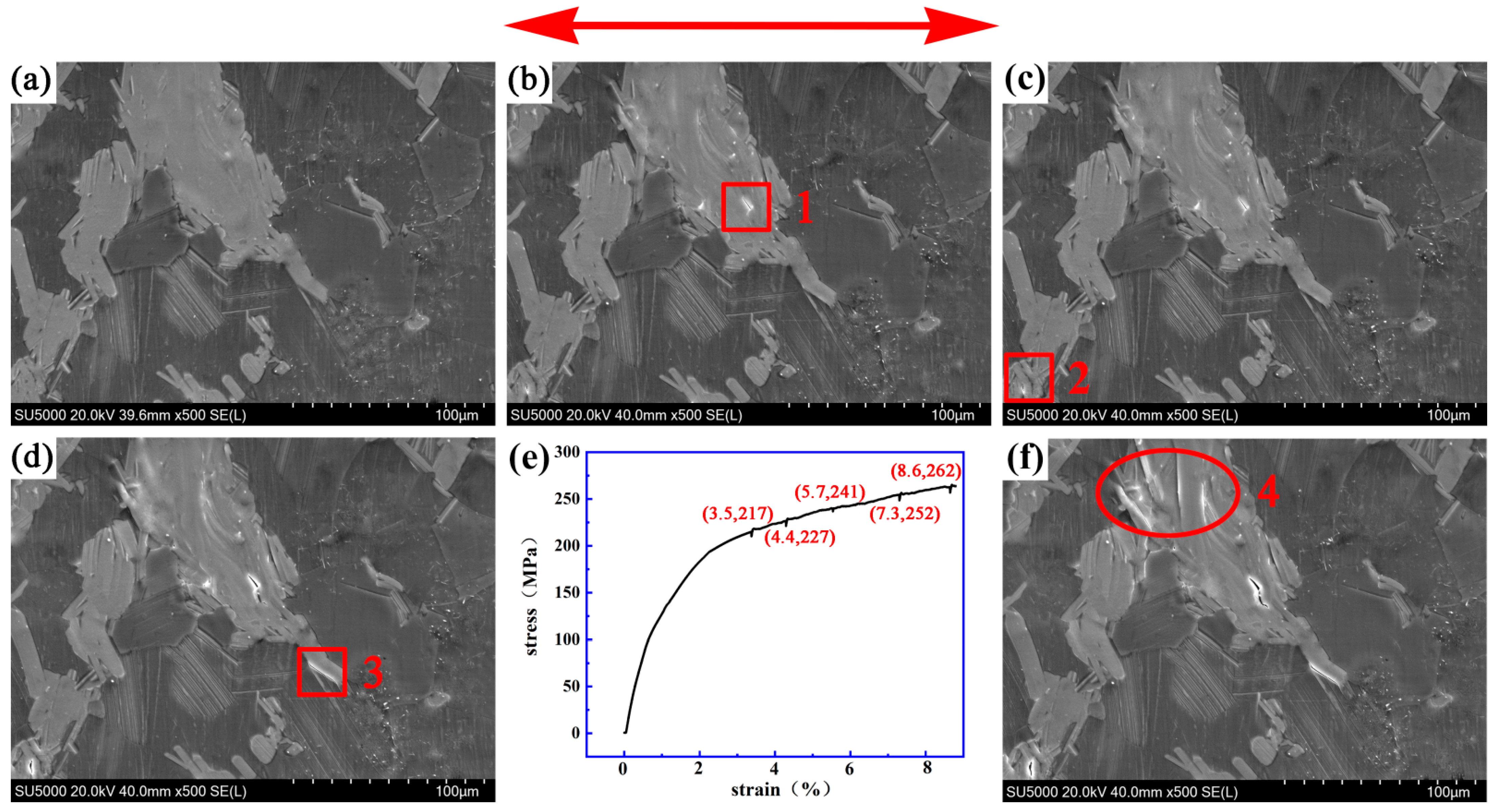
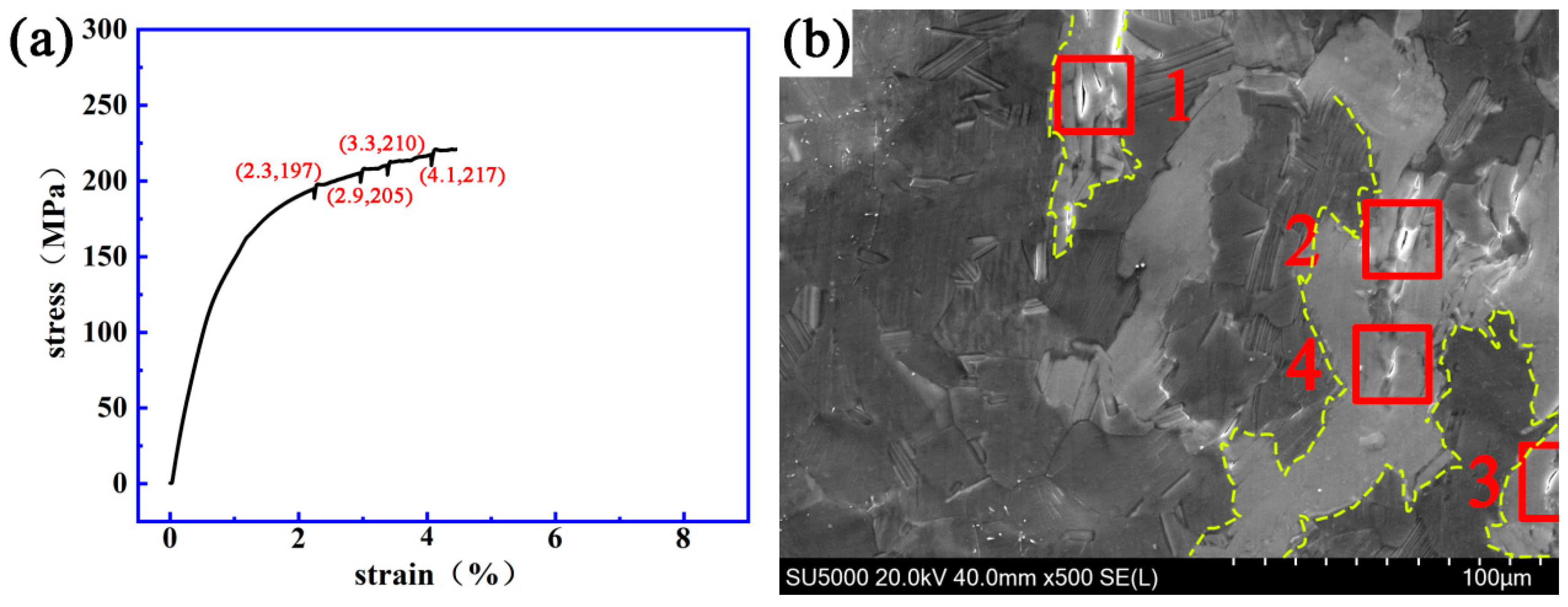
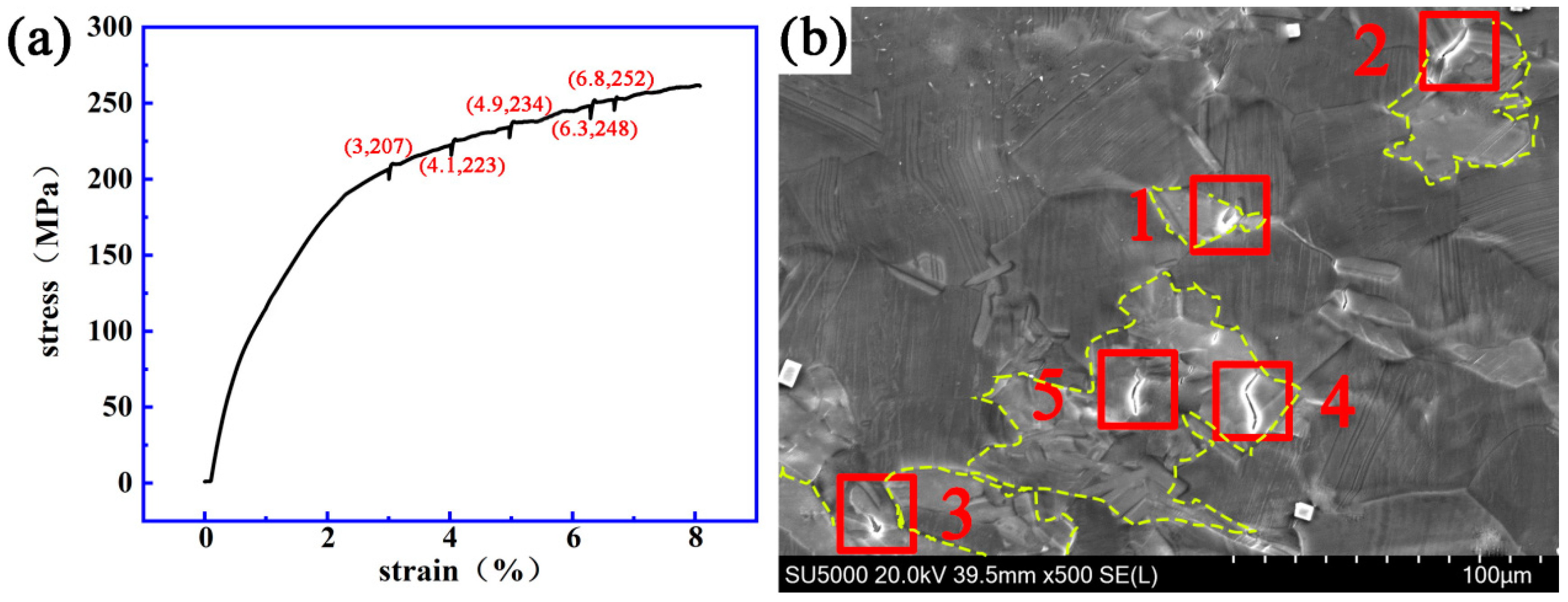
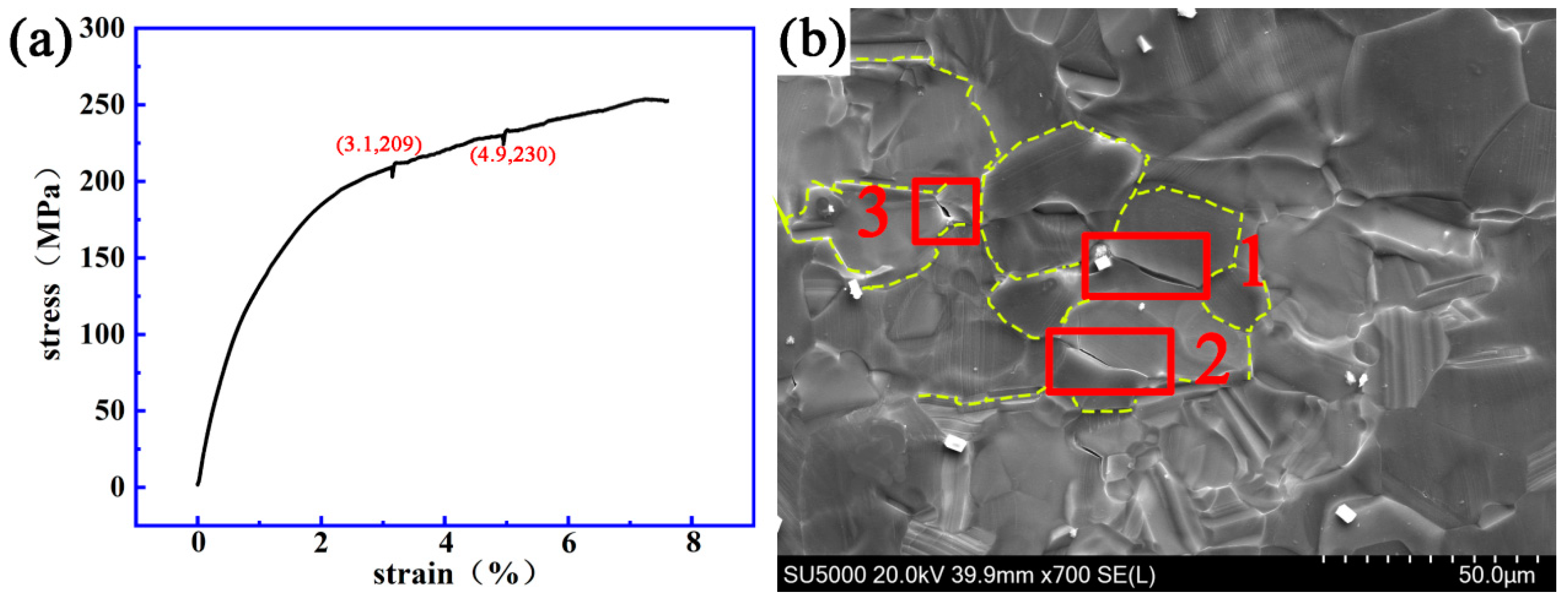
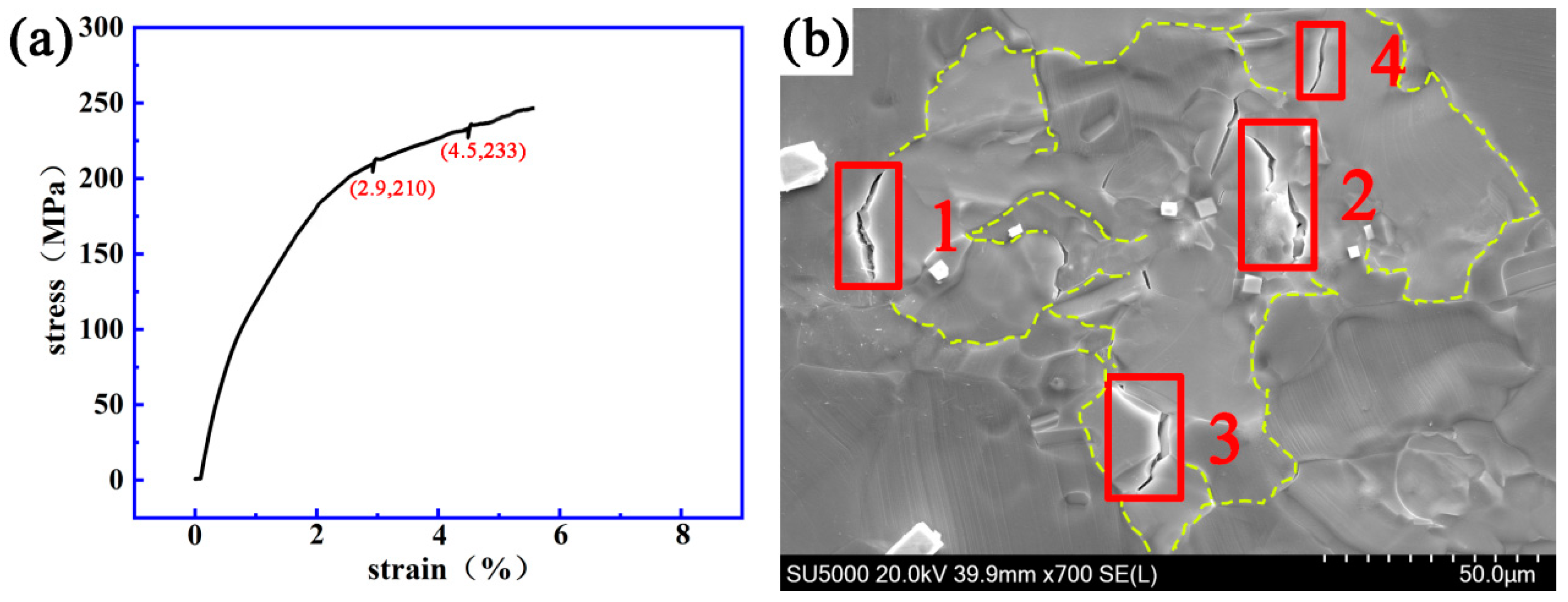
3.2. Dynamic Crack Initiating and Extending Behavior
4. Discussion
4.1. Initiation and Propagation Behaviur of Cracks in the Bulk LPSO Phase
4.2. Initiation and Propagation Behavior of the Cracks at the LPSO/α-Mg Interface
4.3. Initiation and Propagation Behavior of Cracks on the Grain Boundary
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.X.; Li, B.; Tang, H.X.; Xu, Y.C.; Gao, Y.P.; Huang, L.H.; Guan, J.Y. Effect of long period stacking ordered structure on mechanical and damping properties of as-cast Mg-Zn-Y-Zr alloy. Mater. Sci. Eng. A 2015, 640, 287–294. [Google Scholar] [CrossRef]
- Lan, A.; Huo, L.F. Effect of substitution of minor Nd for Y on mechanical and damping properties of heat-treated Mg-Zn-Y-Zr alloy. Mater. Sci. Eng. A 2016, 615, 646–656. [Google Scholar] [CrossRef]
- Yu, J.M.; Zhang, Z.M.; Zhang, X.; Ren, F.L.; Wu, Y.J. Study on microstructure evolution of deformed Mg-Gd-Y-Nd-Zr heat-resistant magnesium alloys after solid solution and ageing. Sci. Sinter. 2016, 48, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.M.; Li, X.B.; Zhang, Z.M.; Wang, Q.; Wu, Y.J.; Wang, J. Mechanical behavior and microstructure evolution of Mg-Gd-Y-Zn-Zr alloy during multipass hot compression deformation. Mater. Sci. Forum 2016, 849, 186–195. [Google Scholar] [CrossRef]
- Yu, J.M.; Zhang, Z.M.; Wang, Q.; Hao, H.Y.; Cui, J.Y.; Li, L.L. Rotary extrusion as a novel severe plastic deformation method for cylindrical tubes. Mater. Lett. 2018, 215, 195–199. [Google Scholar] [CrossRef]
- Liu, X.L.; Chu, M.L.; Wen, Y.Z.; Hui, Z.L.; Su, M.Z. Effects of Homogenization Treatment on Microstructure and Properties of Mg-Zn-Nd-Cd-Zr Alloy. Adv. Mater. Res. 2011, 11, 76–79. [Google Scholar] [CrossRef]
- Tian, Z.K.; Zhang, Z.M.; Yu, J.M.; Yu, H.T.; Yang, Y.Q. Influence of Heat Treatment on Microstructure and Mechanical Properties of Large Deforming and Heat-resisting Magnesium Alloys. New Technol. New Process 2016, 4, 76–78. [Google Scholar] [CrossRef]
- Wang, X.S.; Liang, F.; Fan, J.H.; Zhang, F.H. Low-cycle fatigue small crack initiation and propagation behaviour of cast magnesium alloys based on in-situ SEM observations. Philos. Mag. A 2006, 86, 1581–1596. [Google Scholar] [CrossRef]
- Cui, X.M.; Bai, P.C.; Liu, F.; Hou, X.; Zhang, X.; Du, Z. Microstructure and Crack Evolution Behavior of Mg-8.07Al-0.53Zn-1.36Nd Magnesium Alloy. Rare Met. Mater. Eng. 2017, 46, 728–734. [Google Scholar]
- Okuda, H.; Horiuchi, T.; Yamasaki, M.; Kawamura, Y.; Kohara, S. In situ measurements on stability of long-period stacking-ordered structures in Mg85Y9Zn6 alloys during heating examined by multicolor synchrotron radiation small-angle scattering. Scr. Mater. 2014, 75, 66–69. [Google Scholar] [CrossRef]
- Garces, G.; Morris, D.G.; Muñoz-Morris, M.A.; Perez, P.; Tolnai, D.; Mendis, C.; Stark, A.; Lim, H.K.; Kim, S.; Shelld, N.; et al. Plasticity analysis by synchrotron radiation in a Mg97Y2Zn1 alloy with bimodal grain structure and containing LPSO phase. Acta Mater. 2015, 94, 78–86. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, M.Y.; Xu, S.W.; Wu, K.; Wang, E.D.; Fan, G.H.; Kamado, S. Improving strength and ductility of Mg-Gd-Y-Zn-Zr alloy simultaneously via extrusion, hot rolling and ageing. Mater. Sci. Eng. A 2015, 643, 137–141. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xu, C.; Jing, Y.B.; Lv, S.H.; Liu, S.J.; Fang, D.Q.; Zhuang, J.P.; Zhang, M.L.; Wu, R.Z. New horizon for high performance Mg-based biomaterial with uniform degradation behavior: Formation of stacking faults. Sci. Rep. 2015, 5, 13933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, X.H.; Yang, Z.Q.; Ma, X.L. Strengthening and toughening mechanisms in Mg-Zn-Y alloy with a long period stacking ordered structure. Acta Mater. 2010, 58, 4760–4771. [Google Scholar] [CrossRef]
- Zhang, J.; Kou, Z.; Yang, Y.; Li, B.; Li, X.; Yi, M.; Han, Z. Optimisation of Heat Treatment Process for Damping Properties of Mg-13Gd-5Y-3Zn-0.3Zr Magnesium Alloy Using Box–Behnken Design Method. Metals 2019, 9, 157. [Google Scholar] [CrossRef] [Green Version]
- Tahreen, N.; Zhang, D.F.; Pan, F.S.; Jiang, X.Q.; Li, C.; Li, D.Y.; Chen, D.L. Characterization of hot deformation behavior of an extruded Mg-Zn-Mn-Y alloy containing LPSO phase. J. Alloys Compd. 2015, 644, 814–823. [Google Scholar] [CrossRef]
- Yu, J.M.; Zhang, Z.M.; Wang, Q.; Yin, X.Y.; Cui, J.Y. Dynamic recrystallization behavior of magnesium alloys with LPSO during hot deformation. J. Alloys Compd. 2017, 704, 382–389. [Google Scholar] [CrossRef]
- Oñorbe, E.; Garces, G.; Pérez, P.; Cabezas, S.; Klauß, M.; Genzel, C.; Frutos, E.; Adeva, P.; Pérez, P. The evolution of internal strain in Mg-Y-Zn alloys with a long period stacking ordered structure. Scr. Mater. 2011, 65, 719–722. [Google Scholar] [CrossRef]
- Lv, B.J.; Peng, J.; Zhu, L.L.; Wang, Y.J.; Tang, A.T. The effect of 14H LPSO phase on dynamic recrystallization behavior and hot workability of Mg-2.0Zn-0.3Zr-5.8Y alloy. Mater. Sci. Eng. A 2014, 599, 150–159. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, X.; Lin, D.L.; Peng, L.; Ding, W. The microstructure evolution with lamellar 14H-type LPSO structure in an Mg96.5-Gd2,5Zn1 alloy during solid solution heat treatment at 773K. J. Alloys Compd. 2009, 447, 193. [Google Scholar] [CrossRef]
- Wu, Y.J.; Lin, D.; Zeng, X.; Peng, L.; Ding, W. Formation of a lamellar 14H-type long period stacking ordered structure in an as-cast Mg-Gd-Zn-Zr alloy. J. Mater. Sci. 2009, 44, 1607–1612. [Google Scholar] [CrossRef]
- Zeng, X.; Wu, Y.; Peng, L.; Lin, D.; Ding, W.; Peng, Y. LPSO Structure and aging phases in Mg-Gd-Zn-Zr alloy. Acta Met. Sin. 2010, 46, 1041–1046. [Google Scholar] [CrossRef]
- Yamasaki, M.; Anan, T.; Yoshimoto, S.; Kawamura, Y. Mechanical properties of warm-extruded Mg-Zn-Gd alloy with coherent 14H long periodic stacking bordered structure precipitate. Scr. Mater. 2005, 53, 799–803. [Google Scholar] [CrossRef]
- Yamasaki, M.; Sasaki, M.; Nishijima, M.; Hiraga, K.; Kawamura, Y. Formation of 14H long period stacking ordered structure and profuse stacking faults in Mg-Zn-Gd alloys during isothermal aging at high temperature. Acta Mater. 2007, 55, 6798. [Google Scholar] [CrossRef]
- Nishida, M.; Kawamura, Y.; Yamamuro, T. Formation process of unique microstructure in rapidly solidified Mg97Zn1Y2 alloy. Mat. Sci. Eng. A 2004, 375–377, 1217–1223. [Google Scholar] [CrossRef]
- Liu, H. Investigarion on Microstructures and Properties of Mg-Y-Zn Alloys Strengthened by Long Period Stacking Ordered Structures. Ph.D. Thesis, Southeast University, Nanjing, China, 2014. [Google Scholar]
- Pugh, S.F. The fracture of brittle materials. Br. J. Appl. Phys. 1967, 18, 129–162. [Google Scholar] [CrossRef]
- Zener, C. The micro-mechanism of fracture. In Fracturing of Metals; American Society for Metals: Cleveland, OH, USA, 1948; pp. 3–31. [Google Scholar]
- Stroh, A.N. A theory of the fracture of metals. Adv. Phys. 1957, 6, 418–465. [Google Scholar] [CrossRef]
- Sansour, C.; Skatulla, S. Generalized Continua and Dislocation Theory: Theoretical Concepts, Computational Methods and Experimental Verification; Springer: Vienna, Austria, 2012; Volume 1. [Google Scholar] [CrossRef]
- He, S.M. Properties and Fracture Behavior of Mg-Gd-Y-Zr(-Ca) Alloys. Ph.D. Thesis, Shanghai Jiaotong University, Shanghai, China, 2007. [Google Scholar]
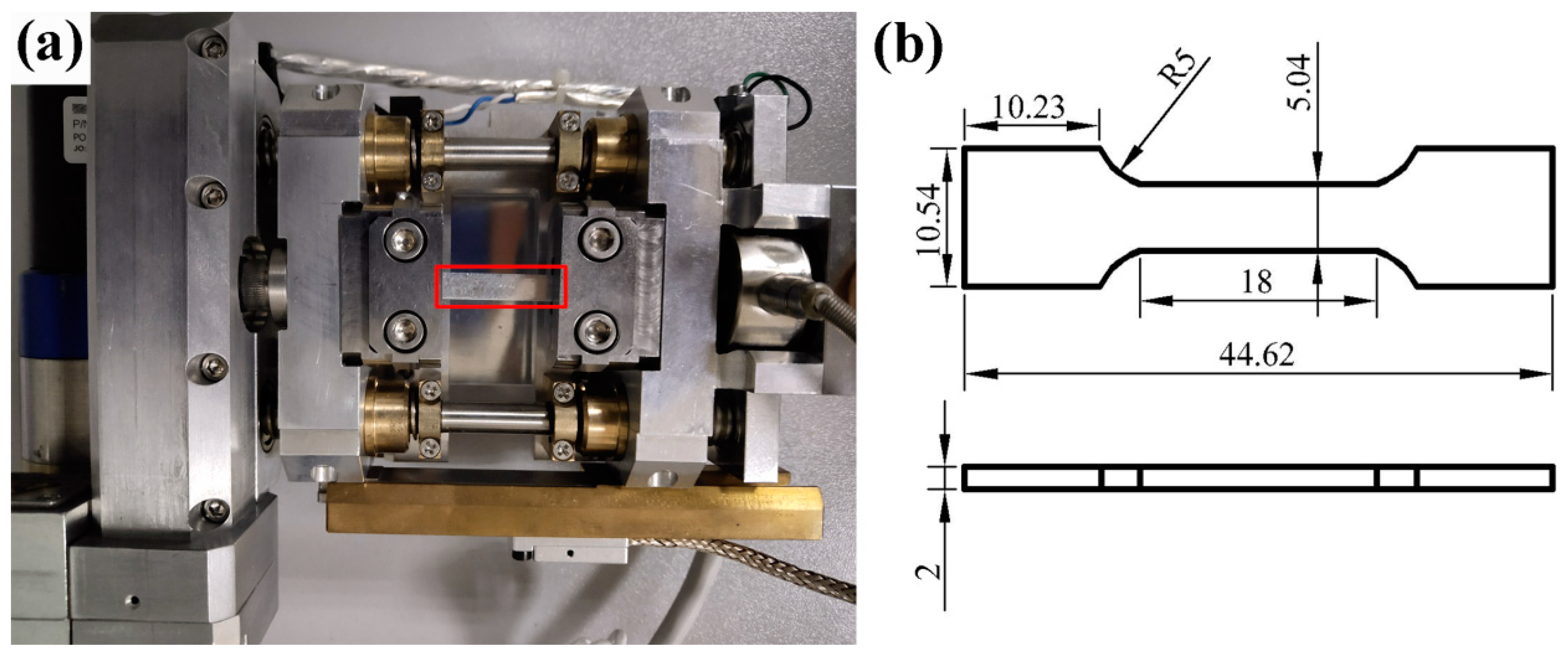
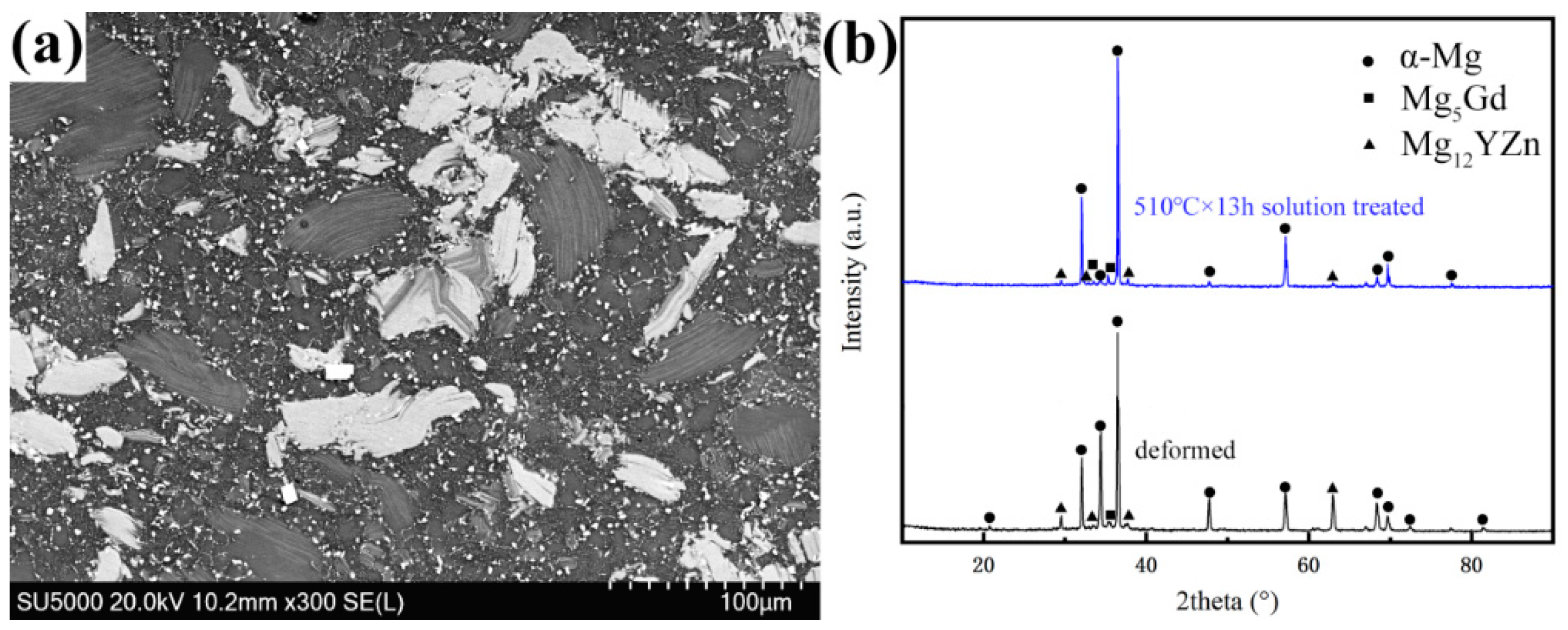
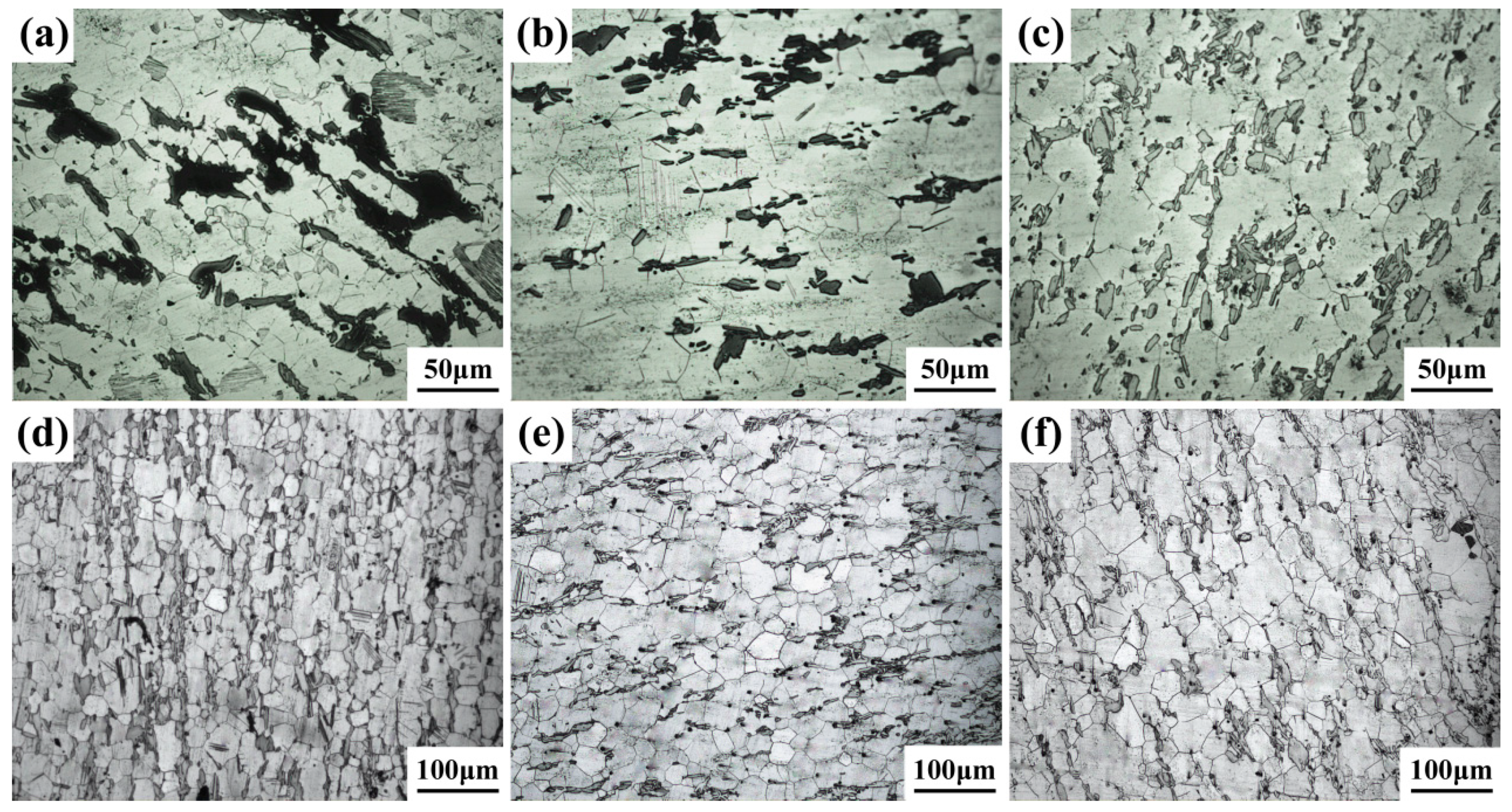
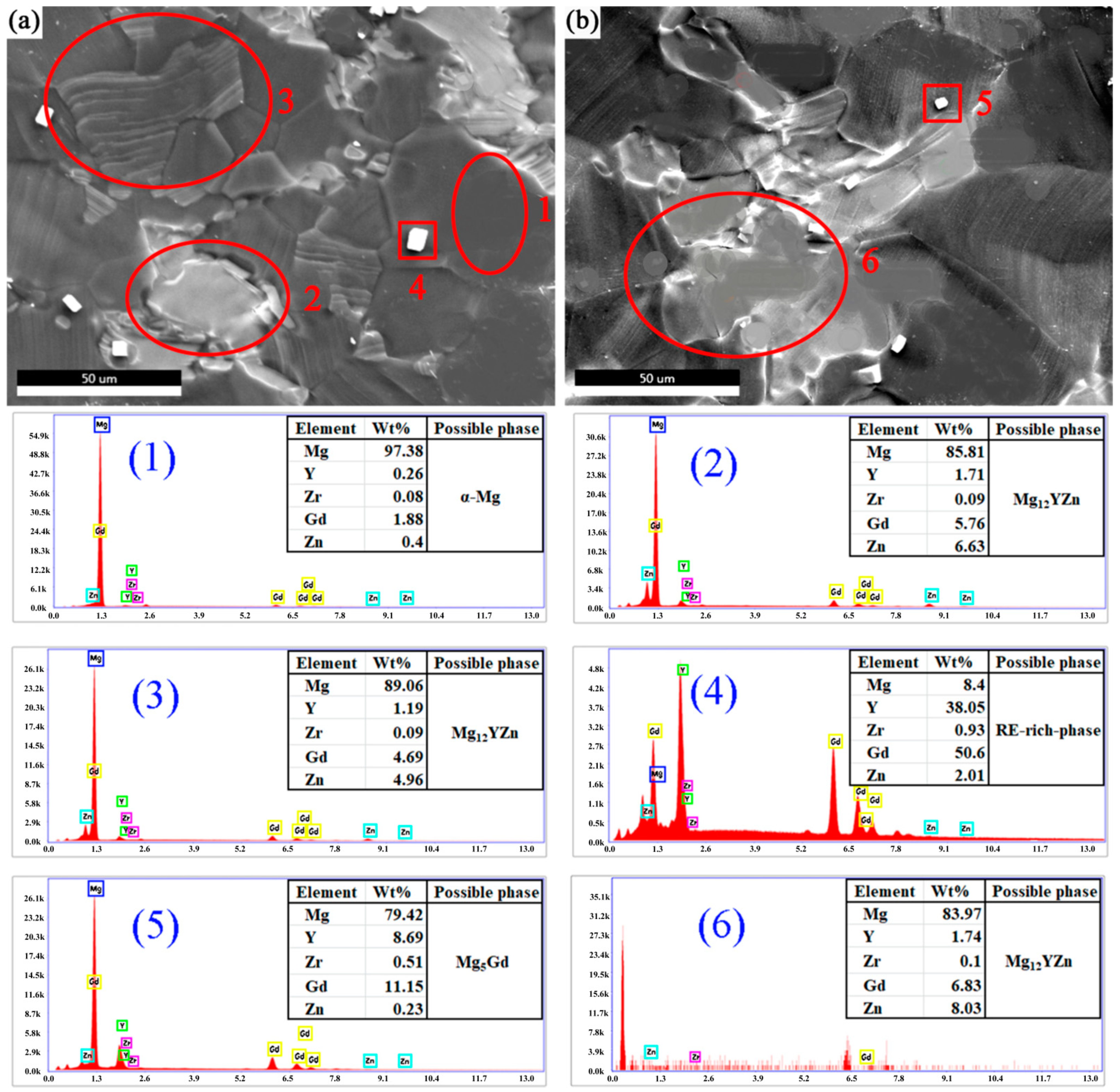

| Mg | Gd | Y | Zn | Zr |
|---|---|---|---|---|
| Bal | 12.77 | 4.5 | 2.65 | 0.34 |
| Solution Treatment | 480 °C × 10 h | 510 °C × 10 h | 510 °C × 13 h | 510 °C × 16 h | 510 °C × 19 h | 510 °C × 22 h |
|---|---|---|---|---|---|---|
| Grain size (µm) | 17 | 24 | 26 | 31 | 32 | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Mu, C.; Han, Z.; Xu, J.; Li, B. Analysis of the In Situ Crack Evolution Behavior in a Solid Solution Mg-13Gd-5Y-3Zn-0.3Zr Alloy. Materials 2021, 14, 36. https://doi.org/10.3390/ma14010036
Yang Y, Mu C, Han Z, Xu J, Li B. Analysis of the In Situ Crack Evolution Behavior in a Solid Solution Mg-13Gd-5Y-3Zn-0.3Zr Alloy. Materials. 2021; 14(1):36. https://doi.org/10.3390/ma14010036
Chicago/Turabian StyleYang, Yaqin, Chongli Mu, Zhongjian Han, Jian Xu, and Baocheng Li. 2021. "Analysis of the In Situ Crack Evolution Behavior in a Solid Solution Mg-13Gd-5Y-3Zn-0.3Zr Alloy" Materials 14, no. 1: 36. https://doi.org/10.3390/ma14010036




