Corrosion Resistance of the CpTi G2 Cellular Lattice with TPMS Architecture for Gas Diffusion Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation of TPMS Mesh Surface
+ cos(2·z)·cos(2·x)) − k3
+ cos(x)·cos(y)·sin(z)) − k2·(cos(4·x) + cos(4·y) + cos(4·z)) − k3
− k2·(cos(2·x)·cos(2·y) + cos(2·y)·cos(2·z) + cos(2·z)·cos(2·x)) − k3
2.2. Selective Laser Melting of the Cellular Lattice Specimens
2.3. Topographic Studies
2.4. Corrosion Resistance Studies
3. Results and Discussion
3.1. Surface Morphology
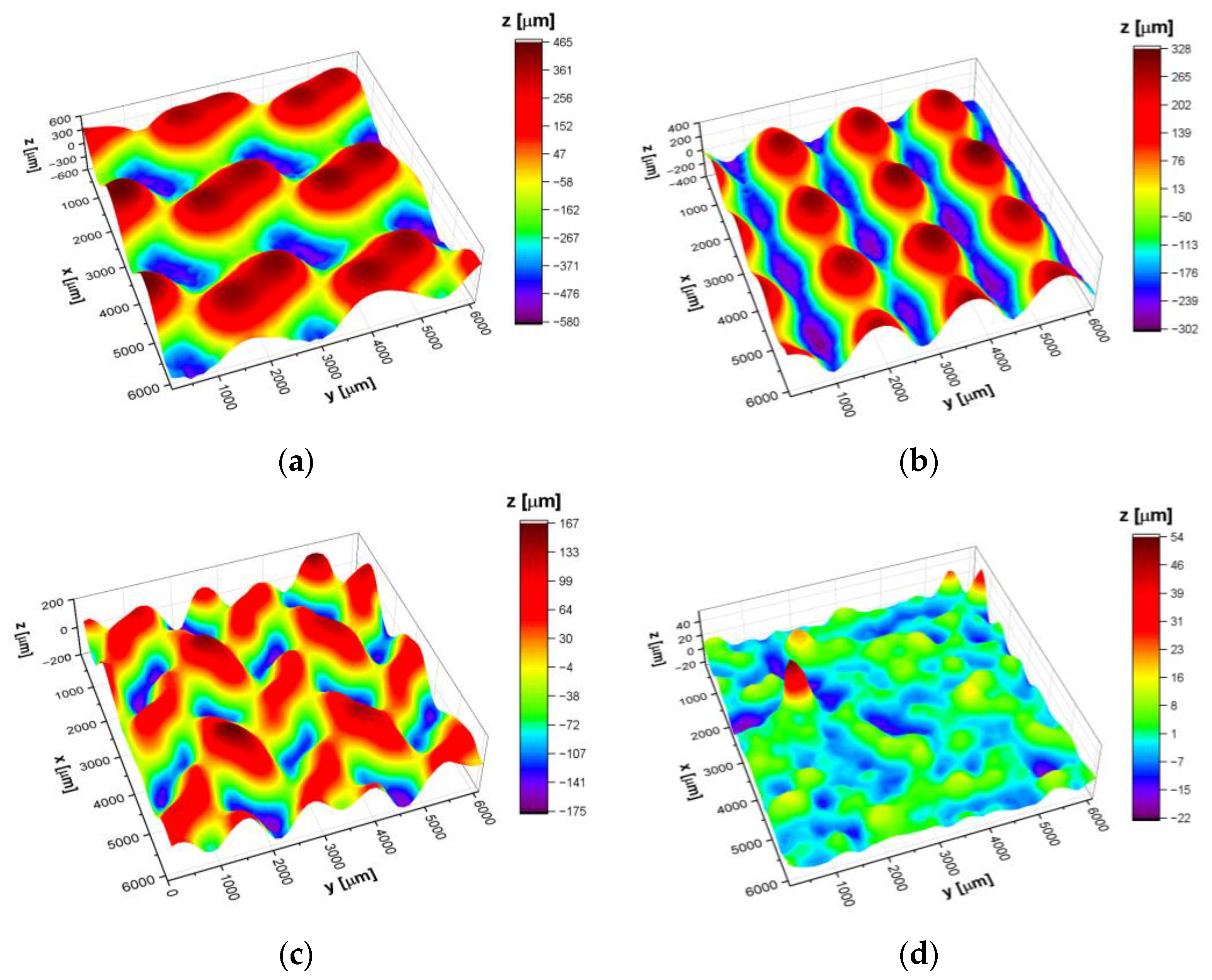
3.2. Surface Topography
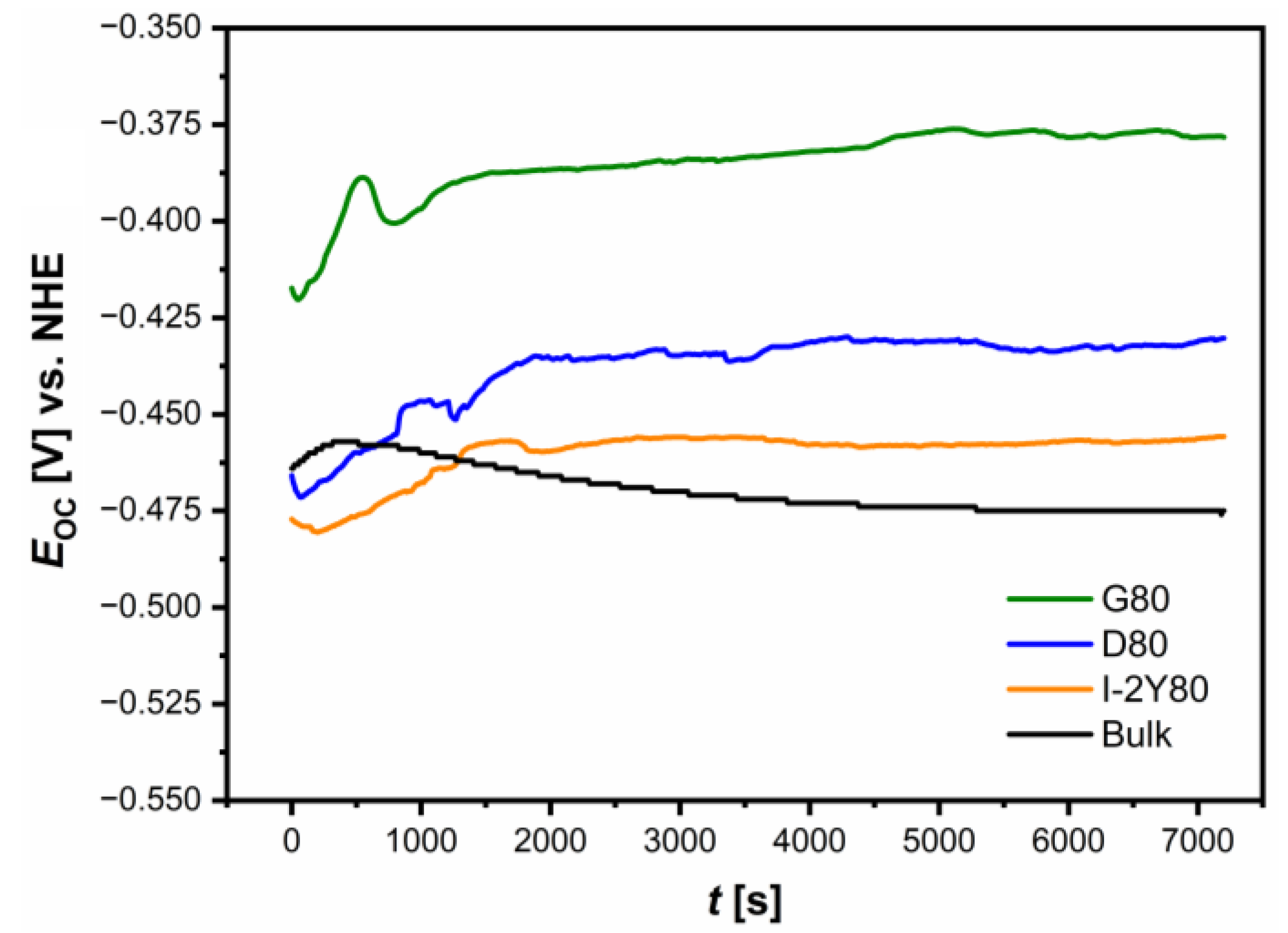
3.3. Open Circuit Potential Studies
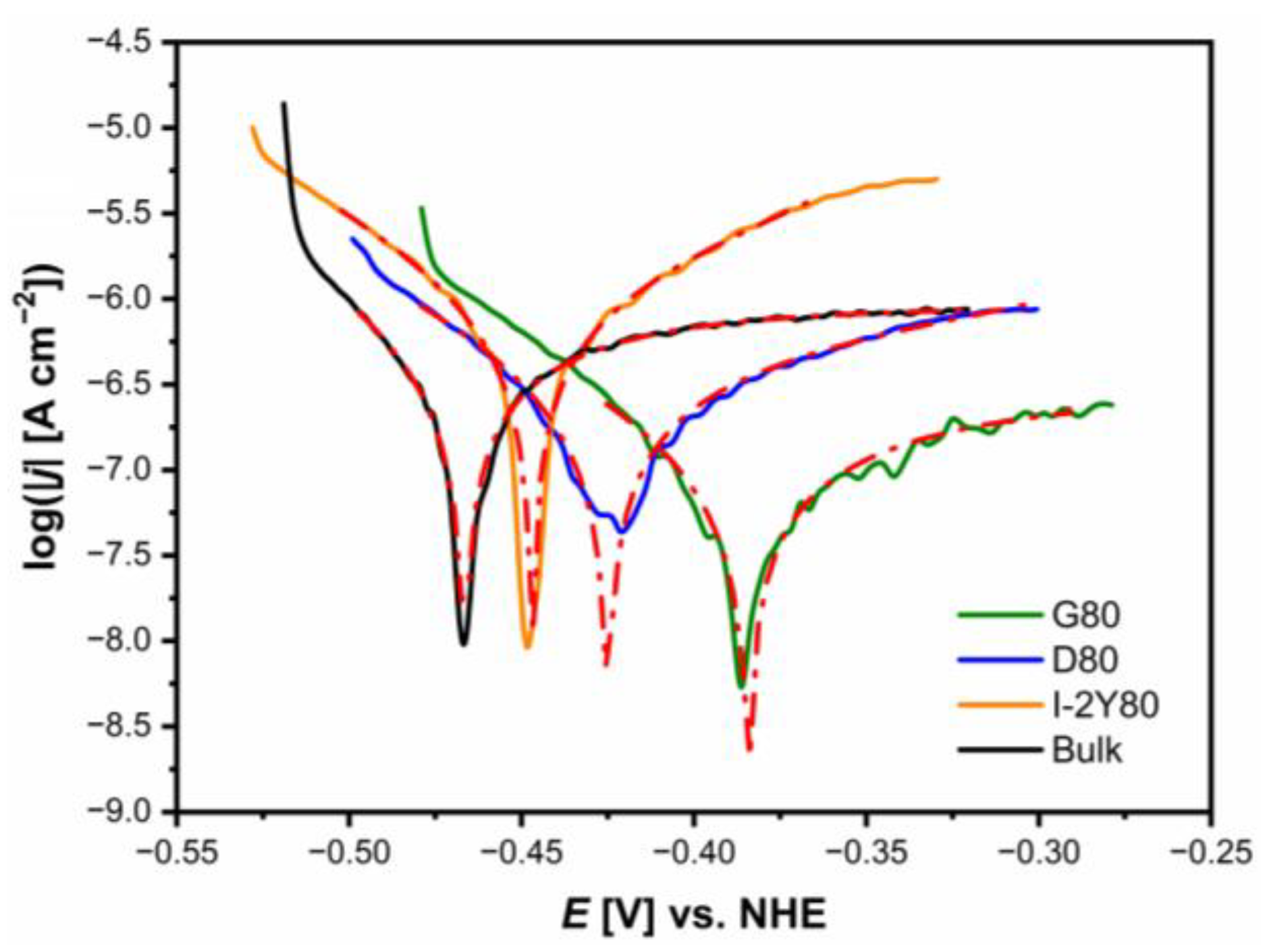
3.4. Tafel Curves
3.5. Electrochemical Impedance Spectroscopy
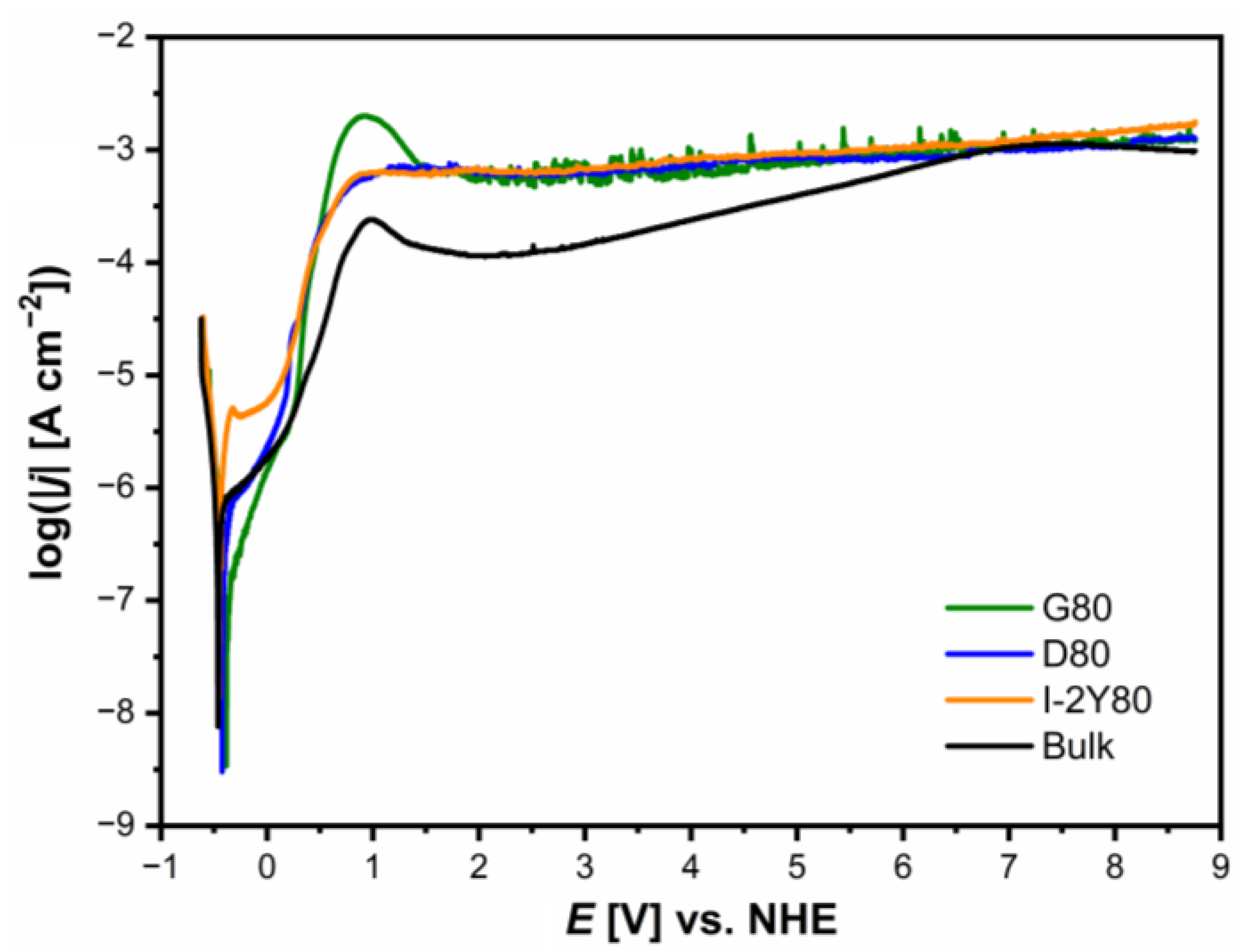
3.6. Anodic Polarization Curves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Yu, Y.; Niu, J.; Liu, Y.; Bridges, D.; Liu, X.; Pooran, J.; Zhang, Y.; Hu, A. Recent Progress of Metal–Air Batteries—A Mini Review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z.; Zhang, X. Introduction to Metal-Air Batteries: Theory and Basic Principles. Metal-Air Batter. 2018, 1–9. [Google Scholar] [CrossRef]
- Wang, H.-F.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Zhang, X. Metal-Air Batteries: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2018; ISBN 978-3-527-34279-2. [Google Scholar]
- Liu, Q.; Pan, Z.; Wang, E.; An, L.; Sun, G. Aqueous metal-air batteries: Fundamentals and applications. Energy Storage Mater. 2020, 27, 478–505. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc-Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; Von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lu, J. Metal–Air Batteries: Will They Be the Future Electrochemical Energy Storage Device of Choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Wang, H.-F.; Tang, C.; Zhang, Q. A Review of Precious-Metal-Free Bifunctional Oxygen Electrocatalysts: Rational Design and Applications in Zn−Air Batteries. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Danner, T.; Eswara, S.; Schulz, V.P.; Latz, A. Characterization of gas diffusion electrodes for metal-air batteries. J. Power Sources 2016, 324, 646–656. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.F.; Chervin, C.N.; Nelson, E.S.; Rolison, D.R.; Long, J.W. Wiring zinc in three dimensions re-writes battery performance—dendrite-free cycling. Energy Environ. Sci. 2014, 7, 1117–1124. [Google Scholar] [CrossRef]
- Bonnick, P.; Dahn, J.R. A Simple Coin Cell Design for Testing Rechargeable Zinc-Air or Alkaline Battery Systems. J. Electrochem. Soc. 2012, 159, A981–A989. [Google Scholar] [CrossRef]
- Fu, J.; Lee, D.U.; Hassan, F.M.; Yanga, L.; Baia, Z.; Park, M.G.; Chen, Z. Flexible High-Energy Polymer-Electrolyte-Based Rechargeable Zinc-Air Batteries. Adv. Mater. 2015, 27, 5617–5622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Z.; Huang, J.; Feng, Z.; Xie, X. Enhancement of electrochemical performance with Zn-Al-Bi layered hydrotalcites as anode material for Zn/Ni secondary battery. Electrochim. Acta 2015, 155, 61–68. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Z.; Yang, B.; Wang, T.; Chu, Z. Superior cycle stability and high rate capability of Zn–Al–In-hydrotalcite as negative electrode materials for Ni–Zn secondary batteries. J. Power Sources 2014, 251, 344–350. [Google Scholar] [CrossRef]
- Renuka, R.; Srinivasan, L.; Ramamurthy, S.; Veluchamy, A.; Venkatakrishnan, N. Cyclic voltammetric study of zinc and zinc oxide electrodes in 5.3 M KOH. J. Appl. Electrochem. 2001, 31, 655–661. [Google Scholar] [CrossRef]
- Caramia, V.; Bozzini, B. Materials science aspects of zinc–air batteries: A review. Mater. Renew. Sustain. Energy 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yang, Z.; Wang, R.; Feng, Z.; Xie, X.; Liao, Q. Electrochemical performance of ZnO/SnO2 composites as anode materials for Zn/Ni secondary batteries. Electrochim. Acta 2014, 134, 287–292. [Google Scholar] [CrossRef]
- Gagnon, E.G. Effect of Ten Weight Percent KOH Electrolyte on the Durability of Zinc/Nickel Oxide Cells Containing Zinc Electrodes with Calcium Hydroxide. J. Electrochem. Soc. 1991, 138, 3173–3176. [Google Scholar] [CrossRef]
- Rani, J.V.; Trivedi, D.C.; Ragavendran, K.; Warrier, P.C. Effect of Polyaniline Coating on “Shape Change” Phenomenon of Porous Zinc Electrode. J. Electrochem. Soc. 2005, 152, A1974–A1978. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.U.; Choi, J.-Y.; Feng, K.; Park, H.W.; Chen, Z. Advanced Extremely Durable 3D Bifunctional Air Electrodes for Rechargeable Zinc-Air Batteries. Adv. Energy Mater. 2014, 4, 1301389. [Google Scholar] [CrossRef]
- Xu, J.-J.; Chang, Z.-W.; Yin, Y.-B.; Zhang, X. Nanoengineered Ultralight and Robust All-Metal Cathode for High-Capacity, Stable Lithium–Oxygen Batteries. ACS Central Sci. 2017, 3, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Feng, N.; Shen, Z.; Li, F.; He, P.; Zhang, H.; Zhou, H. Carbon-Free O2 Cathode with Three-Dimensional Ultralight Nickel Foam-Supported Ruthenium Electrocatalysts for Li-O2 Batteries. ChemSusChem 2017, 10, 2714–2719. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Dong, S.; Ju, Y.; Xiao, D.; Zhou, X.; Zhang, L.; Chen, X.; Shang, C.; Gu, L.; Peng, Z.; et al. A Carbon- and Binder-Free Nanostructured Cathode for High-Performance Nonaqueous Li-O2 Battery. Adv. Sci. 2015, 2, 1500092. [Google Scholar] [CrossRef]
- Van Der Ven, A.; Puchala, B.; Nagase, T. Ti- and Zr-based metal-air batteries. J. Power Sources 2013, 242, 400–404. [Google Scholar] [CrossRef]
- Boyer, R.; Collings, E.W.; Welsch, G. Materials Properties Handbook: Titanium Alloys; ASM International: Materials Park, OH, USA, 1994; ISBN 978-0-87170-481-8. [Google Scholar]
- Browne, M.P.; Redondo, E.; Pumera, M. 3D Printing for Electrochemical Energy Applications. Chem. Rev. 2020, 120, 2783–2810. [Google Scholar] [CrossRef]
- Kou, S.T.; Tan, S.T. An Approach of Irregular Porous Structure Modeling Based on Subdivision and NURBS. Comput. Des. Appl. 2013, 10, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Song, G.-H.; Jing, S.; Zhao, F.-L.; Wang, Y.-D.; Xing, H.; Zhou, J.-T. Design Optimization of Irregular Cellular Structure for Additive Manufacturing. Chin. J. Mech. Eng. 2017, 30, 1184–1192. [Google Scholar] [CrossRef]
- Maszybrocka, J.; Gapiński, B.; Dworak, M.; Skrabalak, G.; Stwora, A. Modelling, manufacturability and compression proper-ties of the CpTi grade 2 cellular lattice with radial gradient TPMS architecture. Bull. Pol. Acad. Sci. Tech. Sci. 2019, 67, 719–727. [Google Scholar] [CrossRef]
- Maszybrocka, J.; Gapiński, B.; Dworak, M.; Skrabalak, G.; Stwora, A. The manufacturability and compression properties of the Schwarz Diamond type Ti6Al4V cellular lattice fabricated by selective laser melting. Int. J. Adv. Manuf. Technol. 2019, 105, 3411–3425. [Google Scholar] [CrossRef] [Green Version]
- Maszybrocka, J.; Stwora, A.; Gapinski, B.; Skrabalak, G.; Karolus, M. Morphology and surface topography of Ti6Al4V lattice structure fabricated by selective laser sintering. Bull. Pol. Acad. Sci. Tech. Sci. 2017, 65, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Schoen, A.H. Infinite periodic minimal surfaces without self-intersections. In NASA TN D-5541, Work of the US Gov. Public Use Permitted; National Aeronautics and Space Administration: Washington, DC, USA, 1970. [Google Scholar]
- Gandy, P.J.; Klinowski, J. Exact computation of the triply periodic G (‘Gyroid’) minimal surface. Chem. Phys. Lett. 2000, 321, 363–371. [Google Scholar] [CrossRef]
- Gandy, P.J.; Cvijović, D.; Mackay, A.L.; Klinowski, J. Exact computation of the triply periodic D (‘diamond’) minimal surface. Chem. Phys. Lett. 1999, 314, 543–551. [Google Scholar] [CrossRef]
- Gandy, P.J.; Klinowski, J. Exact computation of the triply periodic Schwarz P minimal surface. Chem. Phys. Lett. 2000, 322, 579–586. [Google Scholar] [CrossRef]
- Gandy, P.J.; Bardhan, S.; Mackay, A.L.; Klinowski, J. Nodal surface approximations to the P,G,D and I-WP triply periodic minimal surfaces. Chem. Phys. Lett. 2001, 336, 187–195. [Google Scholar] [CrossRef]
- Yoo, D.J. Advanced porous scaffold design using multi-void triply periodic minimal surface models with high surface area to volume ratios. Int. J. Precis. Eng. Manuf. 2014, 15, 1657–1666. [Google Scholar] [CrossRef]
- ASTM F67-13(2017): Standard Specification for Unalloyed Titanium, for Surgical Implant Applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700); ASTM International: West Conshohocken, PA, USA, 2017.
- Kubisztal, J.; Haneczok, G. Quantitative characterization of material surface—Application to Ni + Mo electrolytic composite coatings. Mater. Charact. 2016, 122, 45–53. [Google Scholar] [CrossRef]
- Kubisztal, J.; Stach, S.; Haneczok, G. Corrosion resistance of anodic coatings studied by scanning microscopy and electrochemical methods. Surf. Coat. Technol. 2018, 350, 419–427. [Google Scholar] [CrossRef]
- Bak, A.; Łosiewicz, B.; Kozik, V.; Kubisztal, J.; Dybal, P.; Swietlicka, A.; Barbusiński, K.; Kus, S.; Howaniec, N.; Jampilek, J. Real-Time Corrosion Monitoring of AISI 1010 Carbon Steel with Metal Surface Mapping in Sulfolane. Materials 2019, 12, 3276. [Google Scholar] [CrossRef] [Green Version]
- Kubisztal, J.; Kubisztal, M.; Haneczok, G. New scaling procedure for quantitative determination of surface anisotropy–application to plastic deformation of AISI 316 L stainless steel. Surf. Topogr. Metrol. Prop. 2020, 8, 015003. [Google Scholar] [CrossRef]
- Kubisztal, J.; Kubisztal, M.; Haneczok, G. Corrosion damage of 316L steel surface examined using statistical methods and artificial neural network. Mater. Corros. 2020, 71, 1842–1855. [Google Scholar] [CrossRef]
- Kubisztal, J.; Łosiewicz, B.; Dybal, P.; Kozik, V.; Bak, A. Temperature-Related Corrosion Resistance of AISI 1010 Carbon Steel in Sulfolane. Materials 2020, 13, 2563. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Reference Electrodes; Springer Science & Business Media: New York, NY, USA, 2013; ISBN 3642361889. [CrossRef]
- Boukamp, B.A. A Linear Kronig-Kramers Transform Test for Immittance Data Validation. J. Electrochem. Soc. 1995, 142, 1885–1894. [Google Scholar] [CrossRef]
- User Manual for Frequency Response Analysis (FRA) for Windows Version 4.9; Eco Chemie B.V.: Utrecht, The Netherlands, 2001.
- Han, X.; Zhu, H.; Nie, X.; Wang, G.; Zeng, X. Investigation on Selective Laser Melting AlSi10Mg Cellular Lattice Strut: Molten Pool Morphology, Surface Roughness and Dimensional Accuracy. Materials 2018, 11, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karolus, M.; Maszybrocka, J.; Stwora, A.; Skrabalak, G. Residual stresses of AlSi10Mg fabricated by selective laser melting (SLM). Arch. Metall. Mater. 2019, 64, 1011–1016. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Stach, S. Effect of Autoclaving Time on Corrosion Resistance of Sandblasted Ti G4 in Artificial Saliva. Materials 2020, 13, 4154. [Google Scholar] [CrossRef]
- Mathis, A.; Rocca, E.; Veys-Renaux, D.; Tardelli, J. Electrochemical behaviour of titanium in KOH at high potential. Electrochim. Acta 2016, 202, 253–261. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Rupprecht, S.; Brunette, D.M.; Tengvall, P.; Textor, M.; Thomson, P. (Hrsg): Titanium in medicine: Materialscience, surface science, engineering, biological responses and medical applications. Mund Kiefer und Gesichtschirurgie 2002, 6, 203. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer Science & Business Media: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J. An X-ray photoelectron spectroscopy sputter profile study of the native air-formed oxide film on titanium. Appl. Surf. Sci. 1999, 143, 92–100. [Google Scholar] [CrossRef]
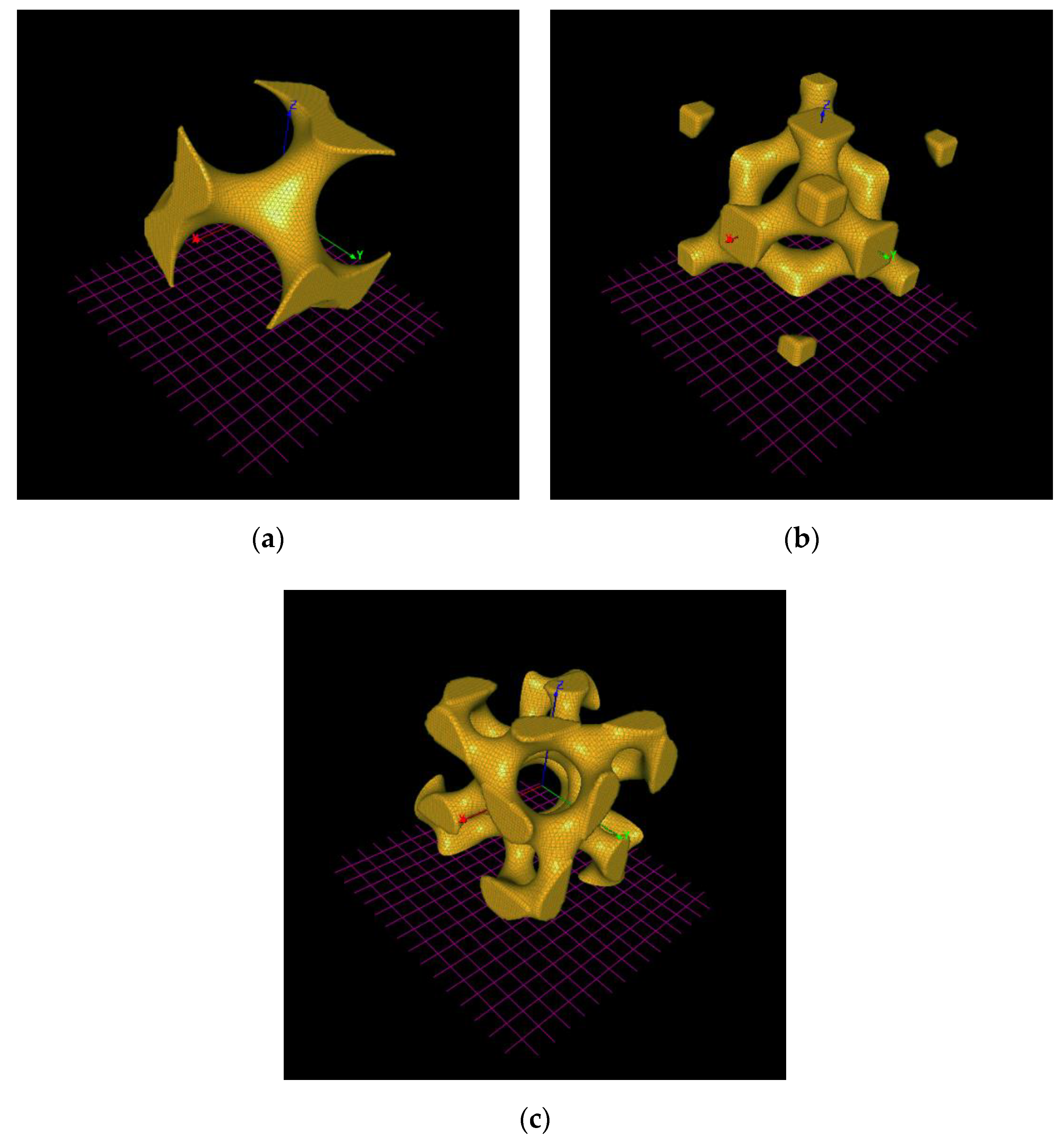


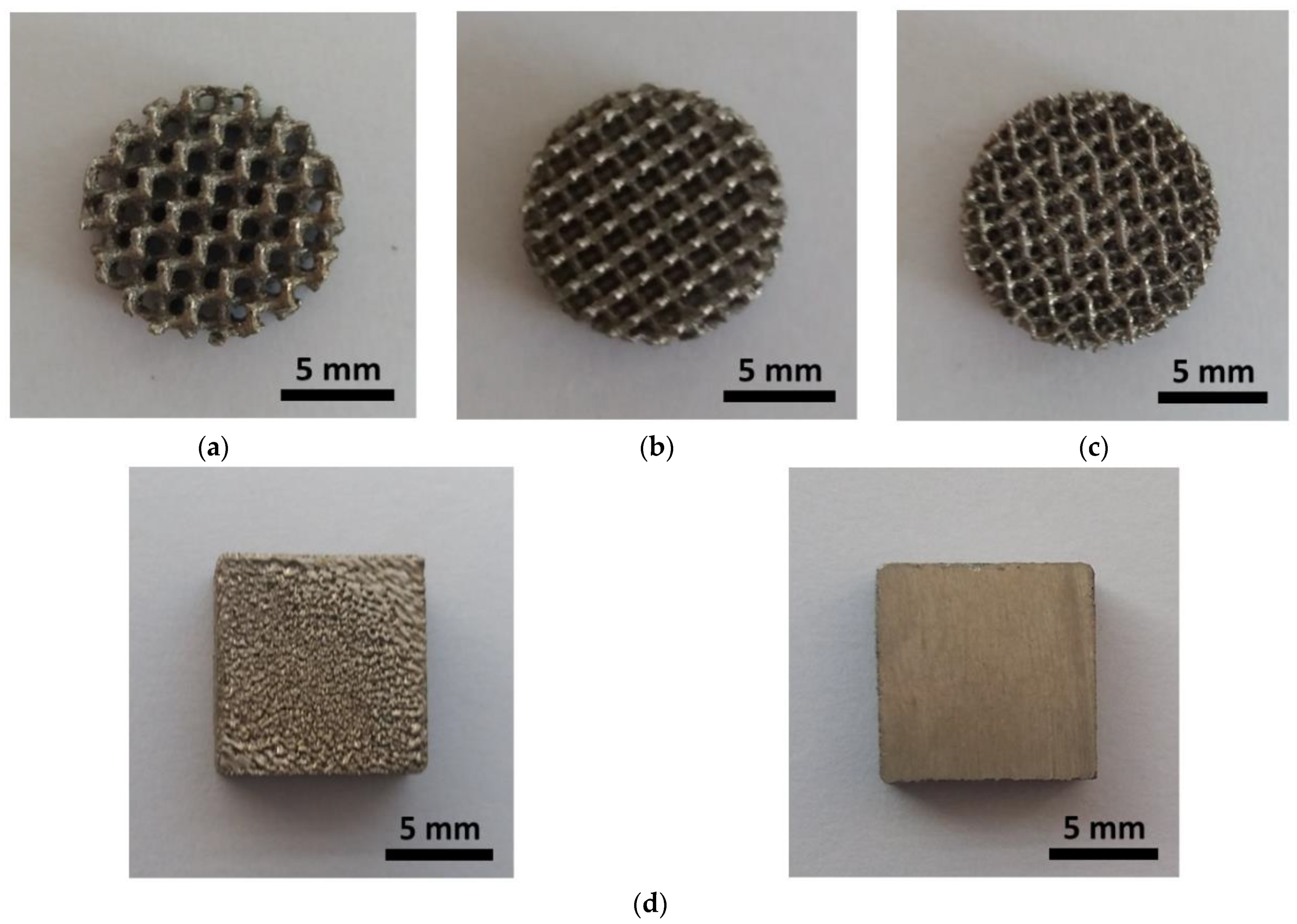



| Type of the TPMS | S [cm2] | V [cm3] | Vv [%] | S/V [cm2 cm−3] |
|---|---|---|---|---|
| G80 | 7.99 | 0.14 | 81.2 | 57.0 |
| D80 | 10.03 | 0.15 | 79.8 | 66.9 |
| I-2Y80 | 13.46 | 0.13 | 82.5 | 103.5 |
| CpTi G2 | Sa [μm] | Sp [μm] | Sv [μm] | Ssk | Sku |
|---|---|---|---|---|---|
| G80 cellular lattice | 232.2 | 463.2 | 577.3 | −0.08 | −1.19 |
| D80 cellular lattice | 145.8 | 327.3 | 301.1 | 0.10 | −1.22 |
| I-2Y80 cellular lattice | 59.7 | 166.2 | 174.9 | −0.11 | −0.81 |
| Bulk | 4.8 | 53.9 | 22.4 | 1.44 | 8.09 |
| CpTi G2 | Ecor [V] | jcor [A cm−2] | bc [V dec−1] | ba [V dec−1] | Rp [Ω cm2] | CR at Ecor [mm yr−1] |
|---|---|---|---|---|---|---|
| G80 cellular lattice | −0.384(8) | 2.85(14) × 10−7 | 0.153(10) | 0.291(6) | 1.53 × 105 | 2.48 × 10−3 |
| D80 cellular lattice | −0.425(9) | 3.58(39) × 10−7 | 0.108(10) | 0.286(32) | 9.51 × 104 | 3.11 × 10−3 |
| I-2Y cellular lattice | −0.447(3) | 7.75(25) × 10−7 | 0.094(2) | 0.277(2) | 3.93 × 104 | 6.74 × 10−3 |
| Bulk | −0.467(2) | 8.96(5) × 10−7 | 0.107(1) | 0.317(11) | 3.88 × 104 | 7.79 × 10−3 |
| CpTi G2 | Tdl [F cm−2 s ϕ−1] | ϕdl | Rox [Ω cm2] |
|---|---|---|---|
| G80 cellular lattice | 2.61(8) × 10−6 | 0.896(1) | 3.21(6) × 105 |
| D80 cellular lattice | 1.09(8) × 10−6 | 0.872(3) | 1.41(9) × 105 |
| I-2Y80 cellular lattice | 2.18(2) × 10−4 | 0.809(5) | 3.03(7) × 104 |
| CpTi G2 | Tout [F cm−2 s ϕ−1] | ϕout | Rout [Ω cm2] | Tin [F cm−2 s ϕ−1] | ϕin | Rin [Ω cm2] |
|---|---|---|---|---|---|---|
| Bulk | 2.34(7) × 10−5 | 0.877(5) | 2.15(4) × 103 | 2.75(4) × 10−4 | 0.893(6) | 2.13(6) × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łosiewicz, B.; Maszybrocka, J.; Kubisztal, J.; Skrabalak, G.; Stwora, A. Corrosion Resistance of the CpTi G2 Cellular Lattice with TPMS Architecture for Gas Diffusion Electrodes. Materials 2021, 14, 81. https://doi.org/10.3390/ma14010081
Łosiewicz B, Maszybrocka J, Kubisztal J, Skrabalak G, Stwora A. Corrosion Resistance of the CpTi G2 Cellular Lattice with TPMS Architecture for Gas Diffusion Electrodes. Materials. 2021; 14(1):81. https://doi.org/10.3390/ma14010081
Chicago/Turabian StyleŁosiewicz, Bożena, Joanna Maszybrocka, Julian Kubisztal, Grzegorz Skrabalak, and Andrzej Stwora. 2021. "Corrosion Resistance of the CpTi G2 Cellular Lattice with TPMS Architecture for Gas Diffusion Electrodes" Materials 14, no. 1: 81. https://doi.org/10.3390/ma14010081





