Microstructure and Properties of As-Cast and Heat-Treated 2017A Aluminium Alloy Obtained from Scrap Recycling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microscopic Tests
2.2. XRD Examination
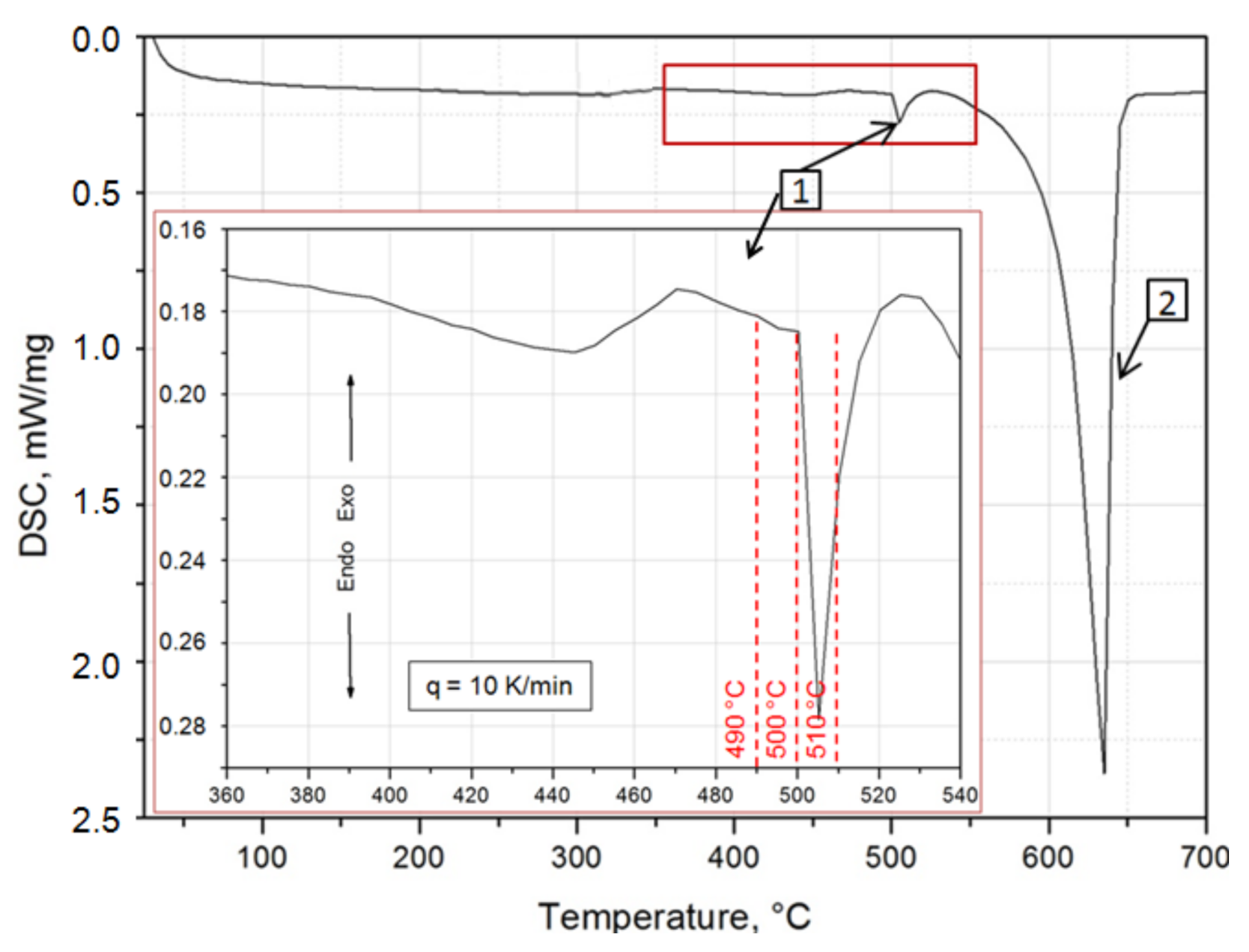
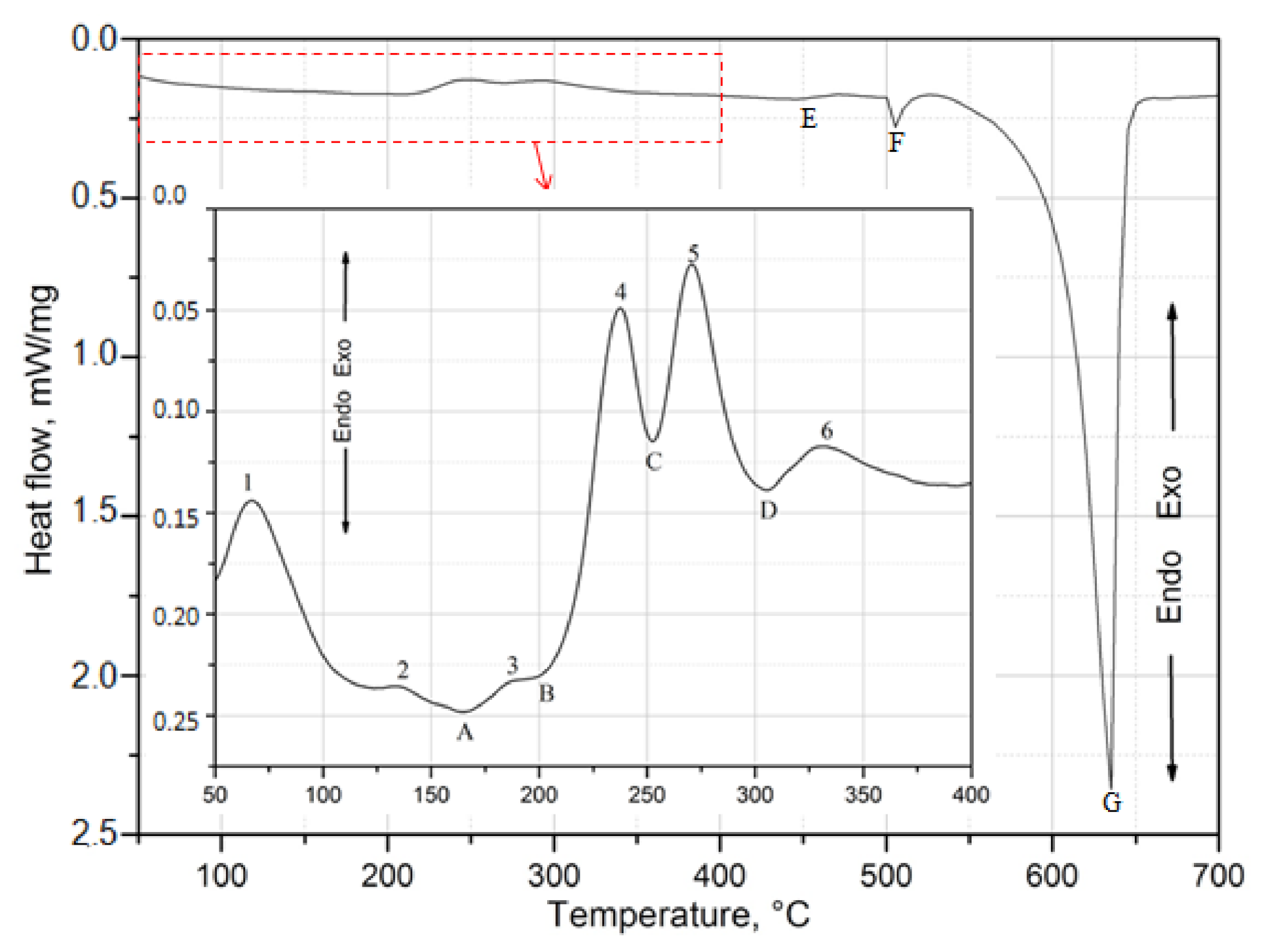
2.3. Calorimetric Testing
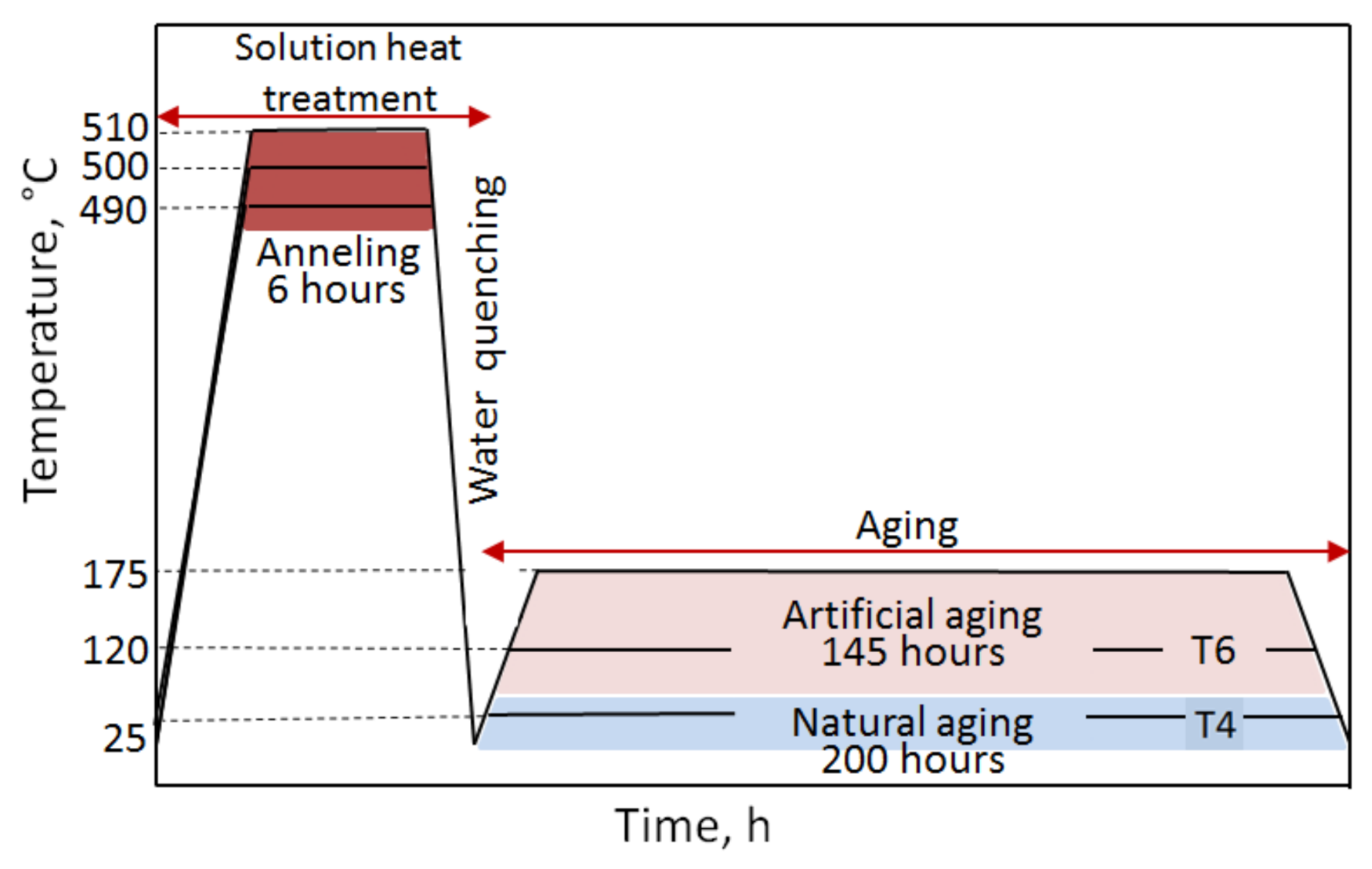
2.4. Heat Treatment
2.5. Mechanical Properties
3. Results and Discussion
- -
- HBmax—maximum hardness value obtained during aging.
- -
- HBw—starting hardness of the alloy (directly after homogenization and solution heat treatment).
- -
- Δt—aging time after which the alloy reached maximum hardness value HBmax.
4. Conclusions
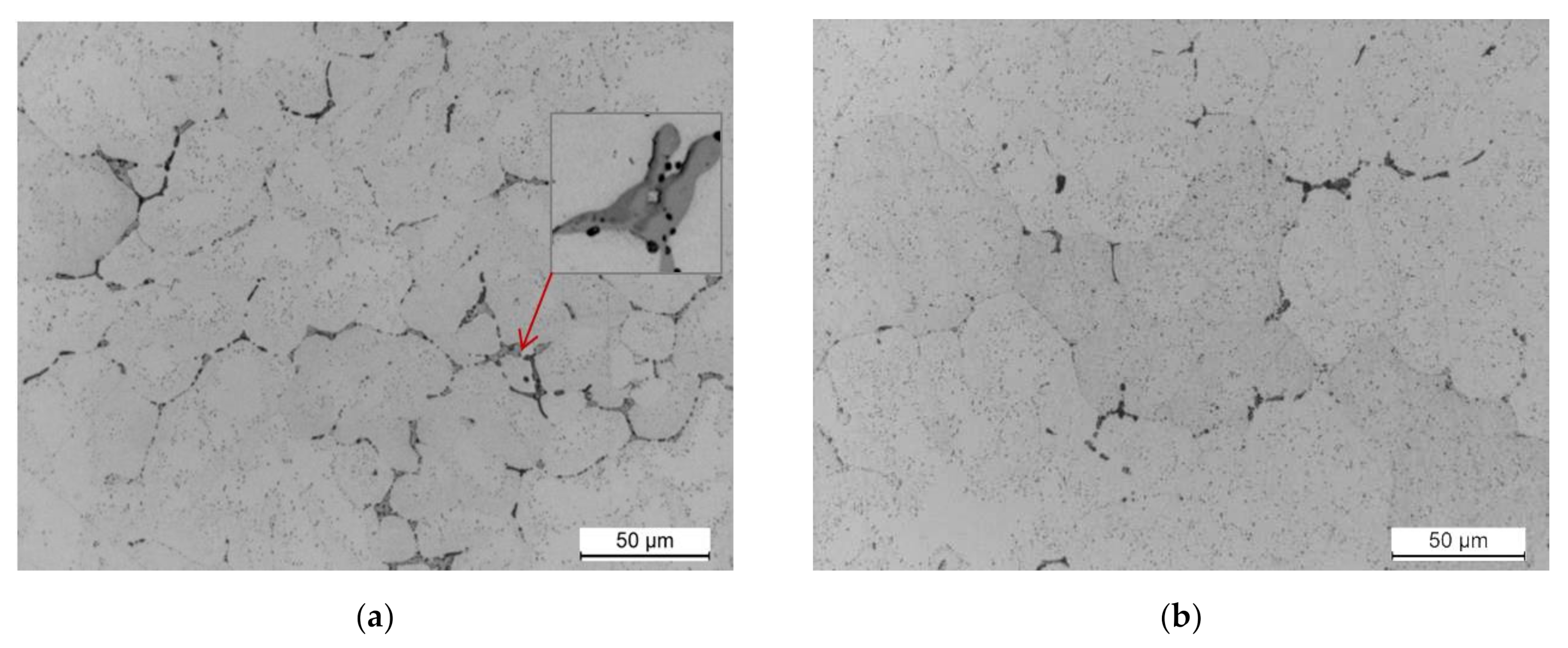
- The 2017 alloy, obtained in the process of scrap recycling, is characterized by homogeneous and fine grains throughout the entire volume of the ingot. No discontinuities or casting defects were observed on the cross section of the ingot. The mechanical properties of the as-cast ingot obtained using the continuous casting method are as follows; tensile strength Rm = 315.7 MPa, yield strength R0.2 = 165.2 MPa, and relative elongation A5 = 8.8%.
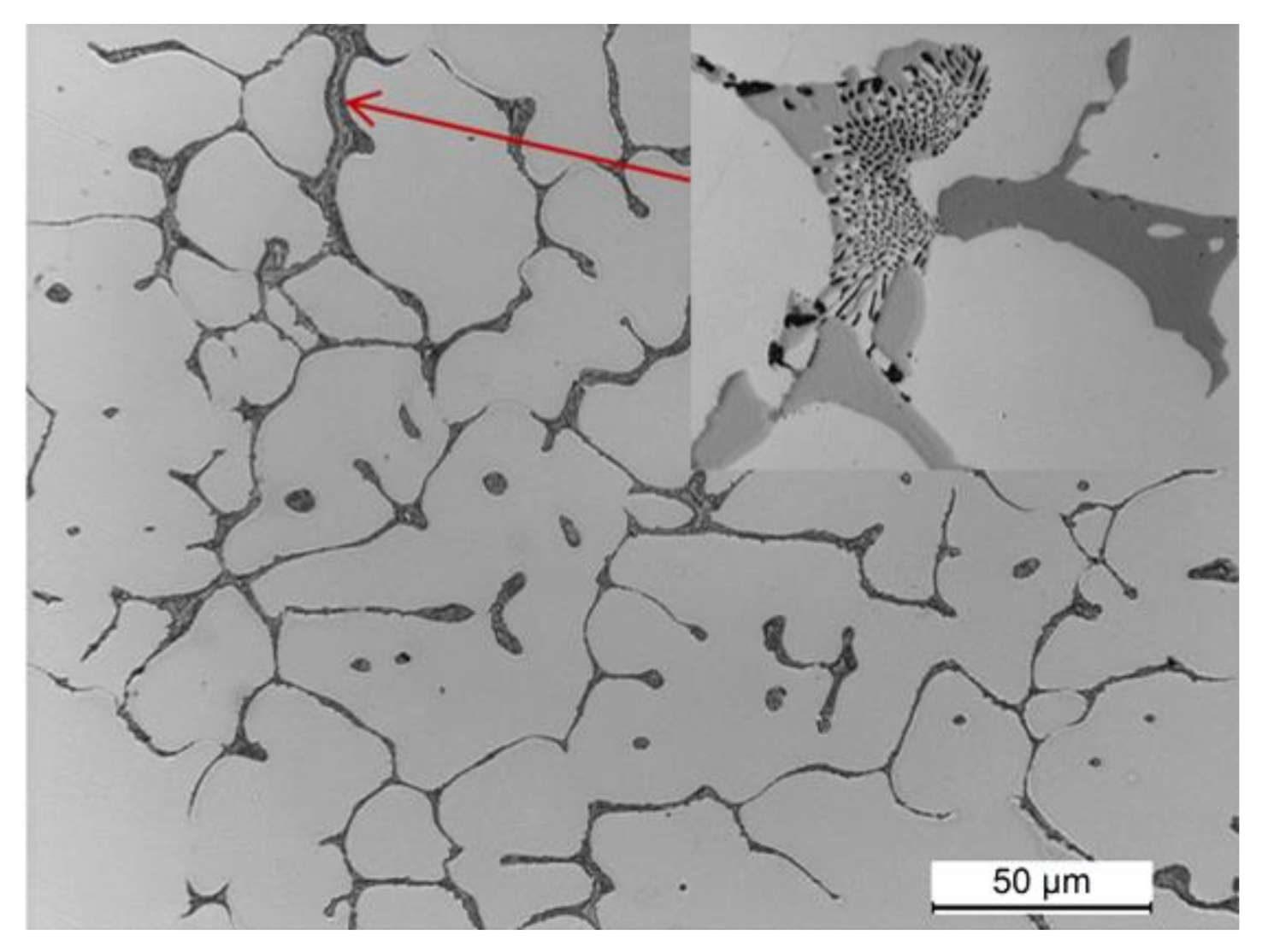
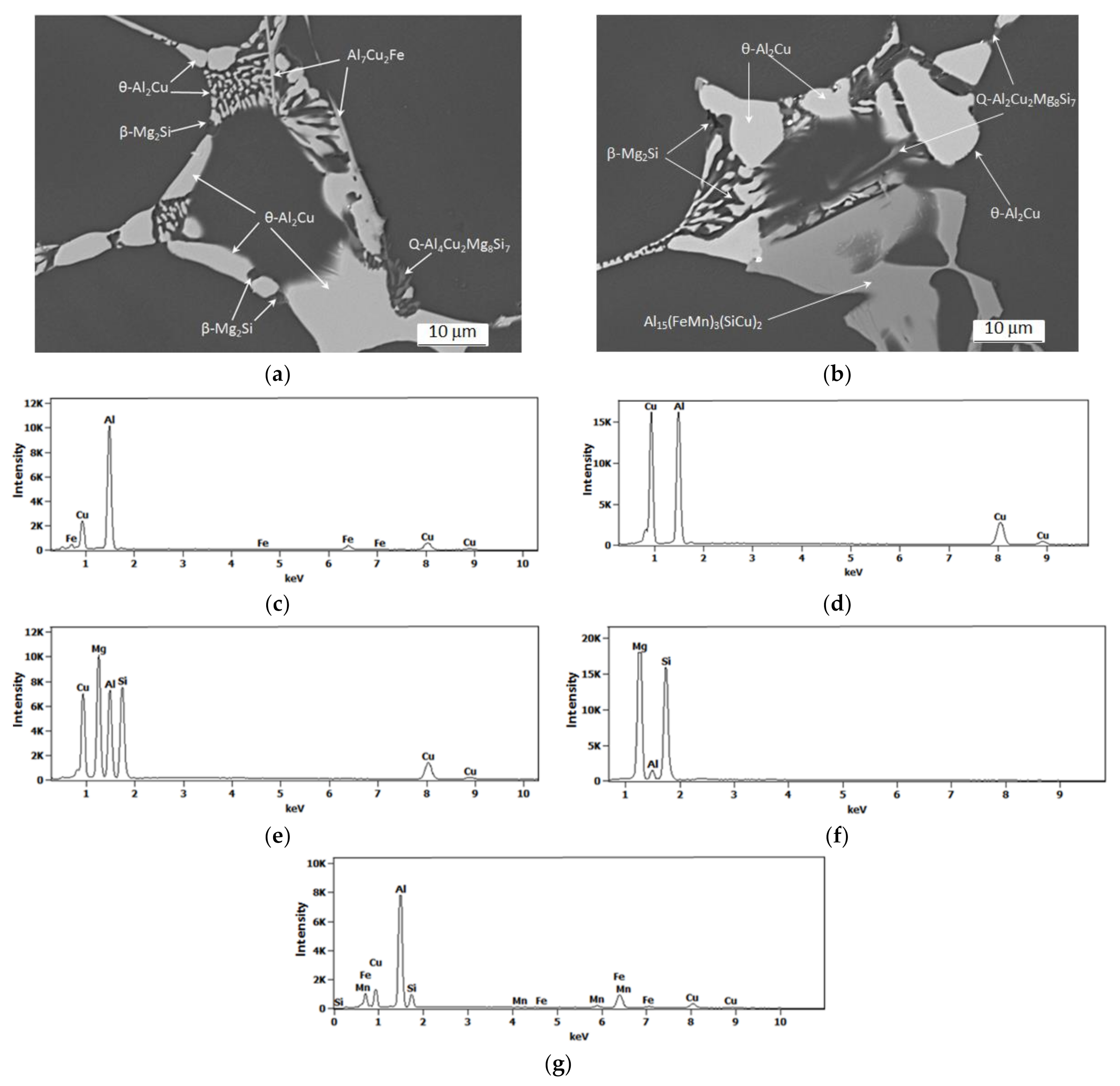
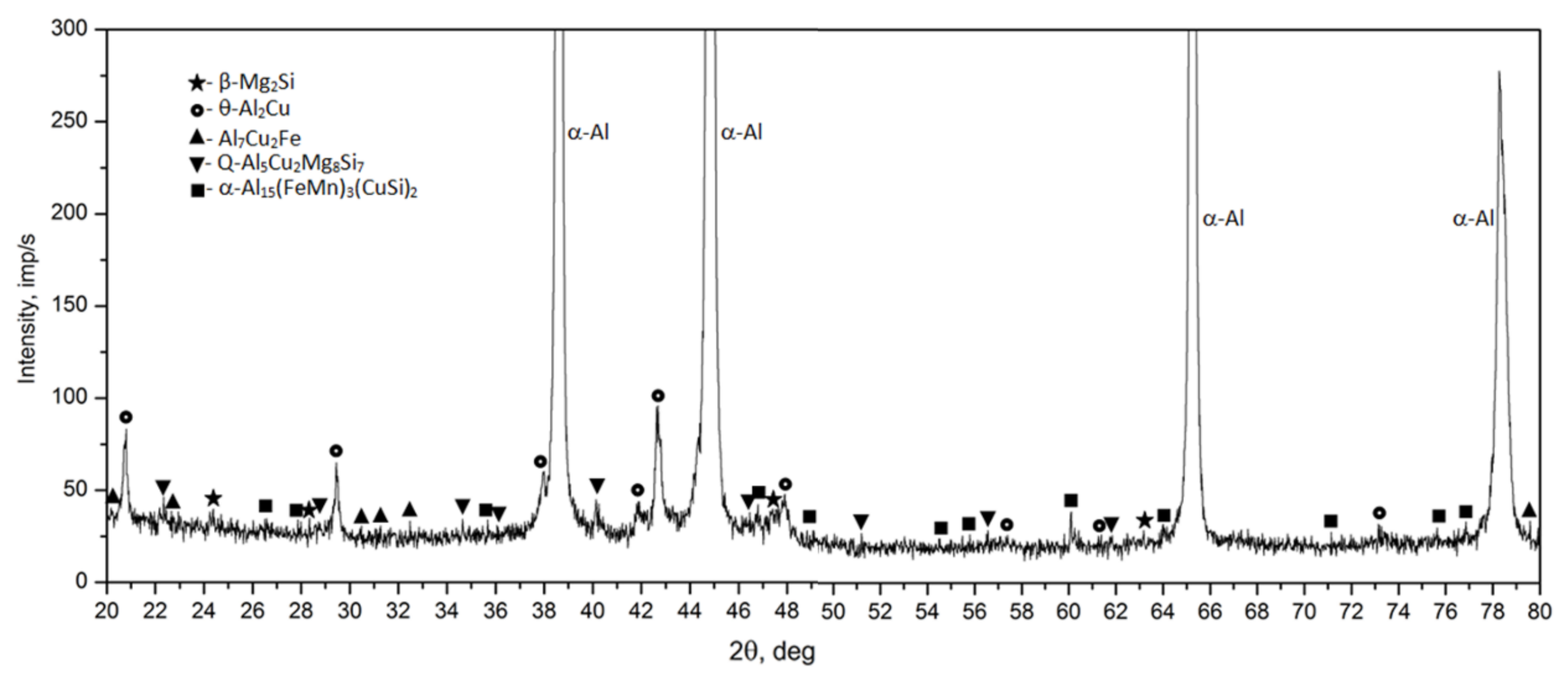
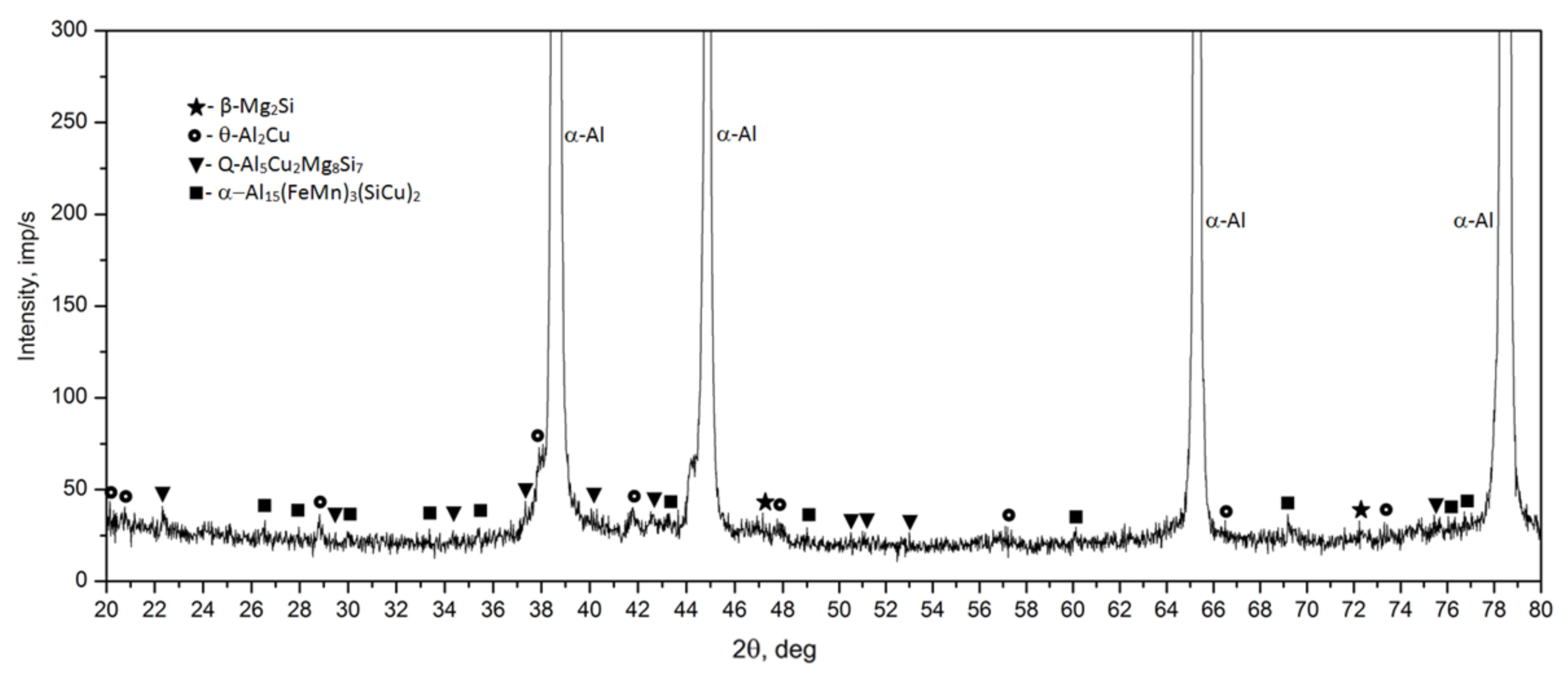
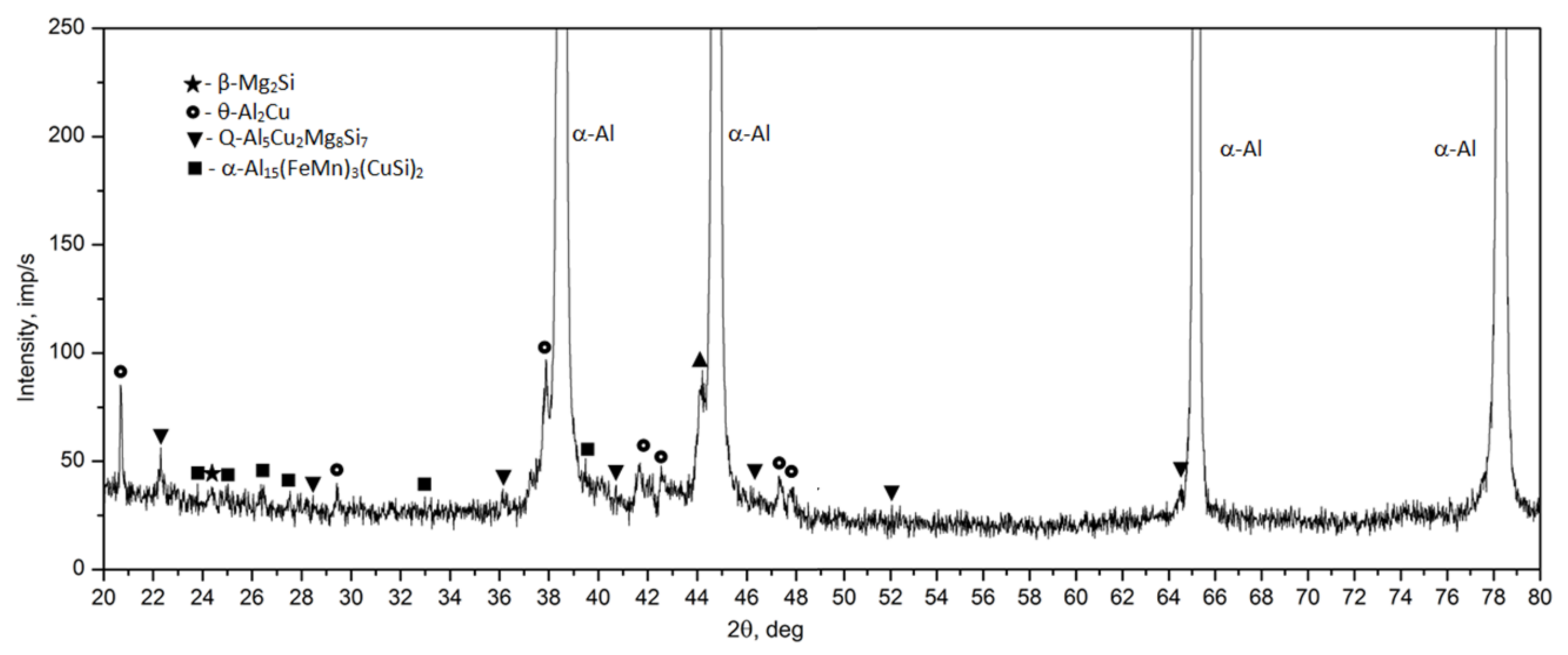
- The microstructure of the as-cast 2017 alloy consists of precipitates of intermetallic phases crystallizing mainly in the form of eutectics in the interdendritic regions of the solid α-Al solution. The precipitates of the following phases were identified based on the results of LM, SEM + EDS and XRD studies: θ-Al2Cu, β-Mg2Si, Al7Cu2Fe, Q-Al4Cu2Mg8Si7, and α-Al15(FeMn)3(SiCu)2.
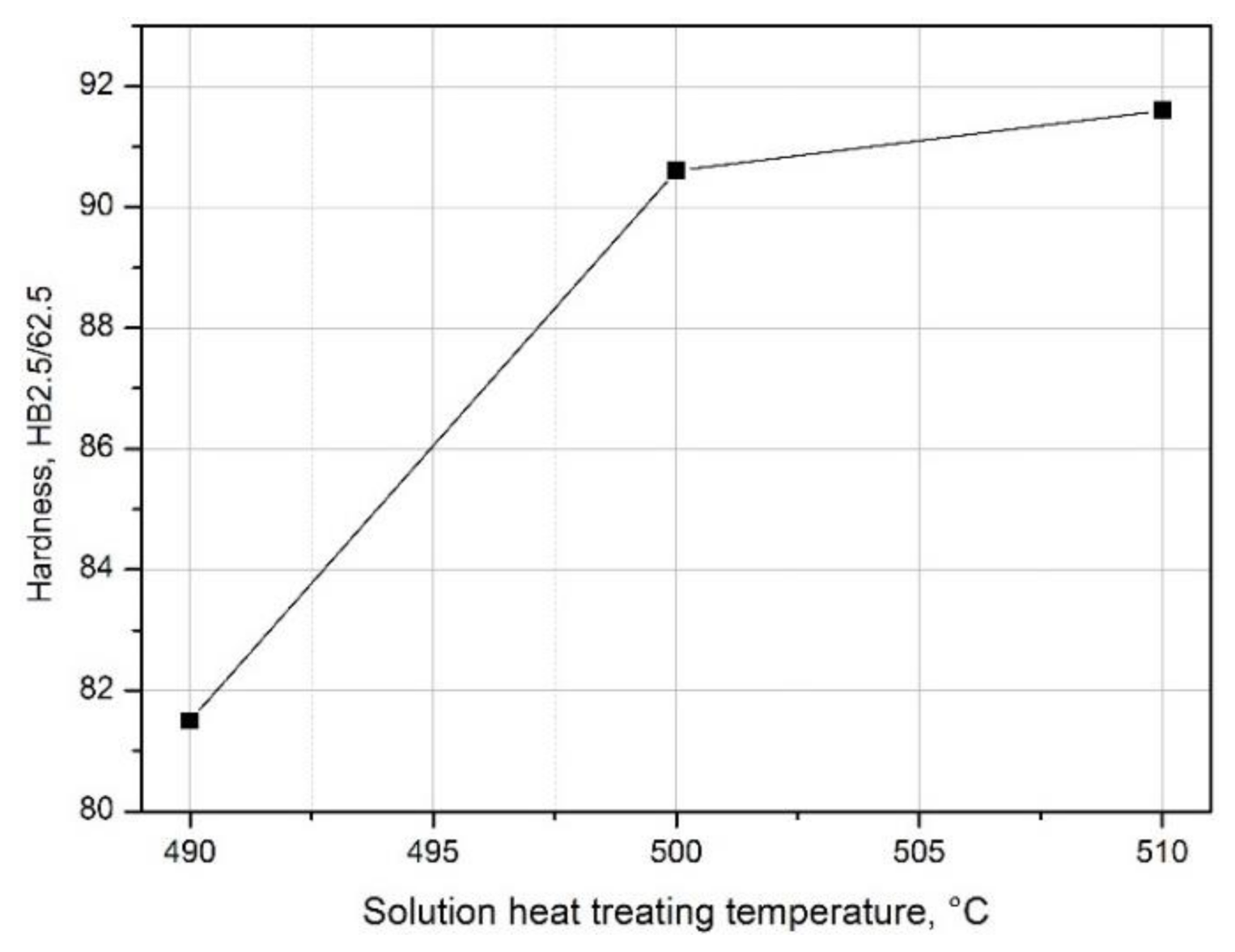
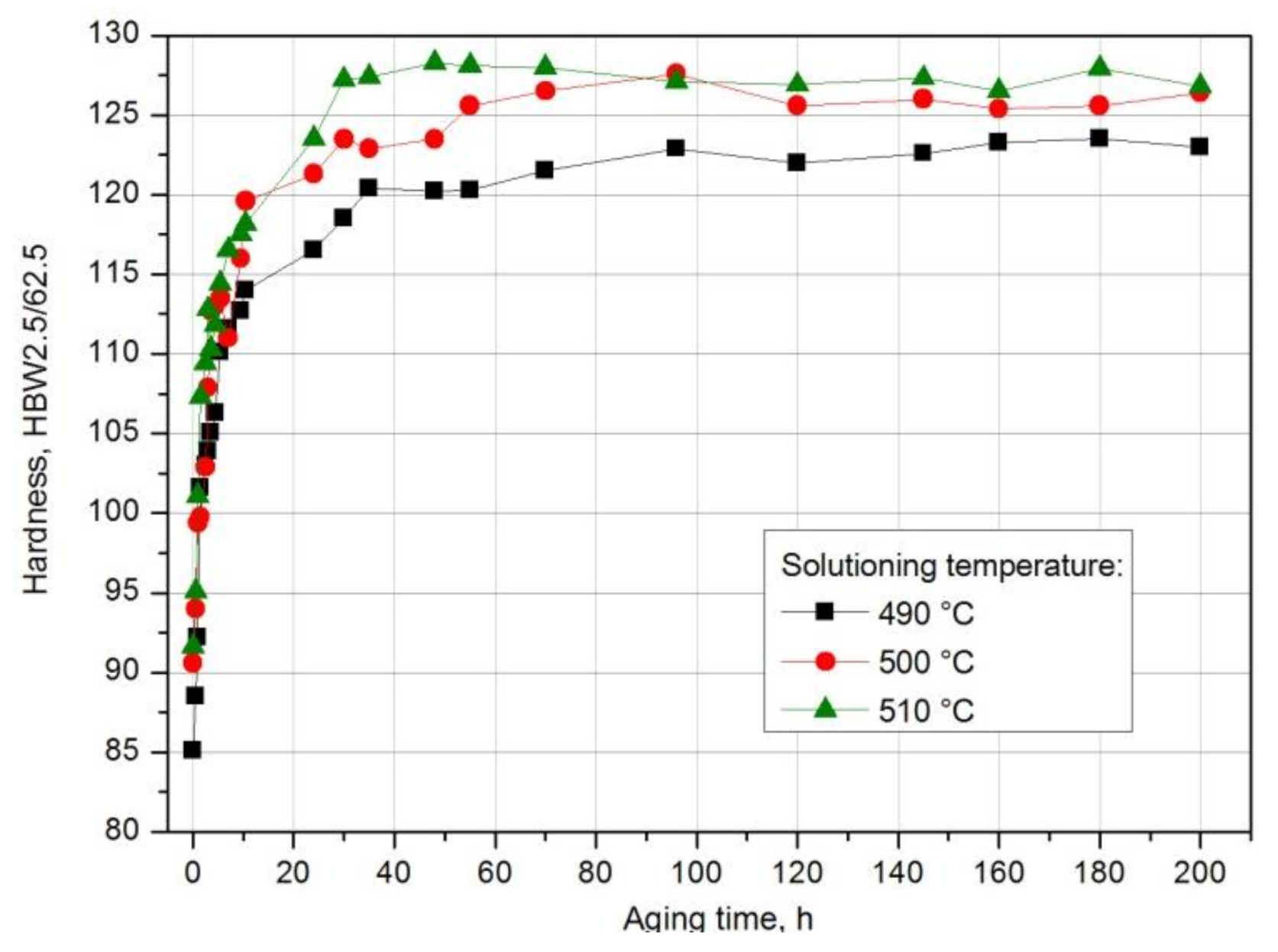
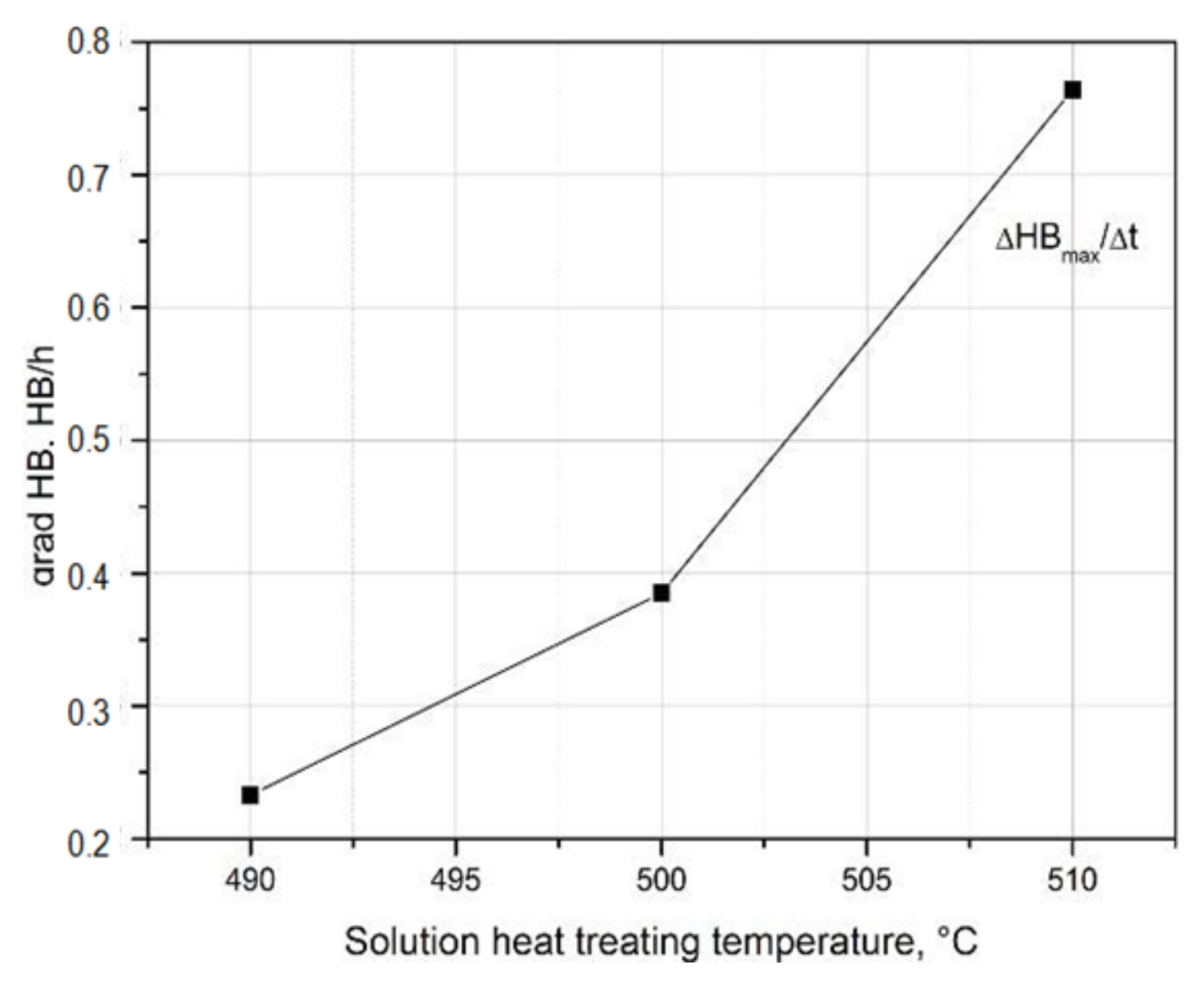
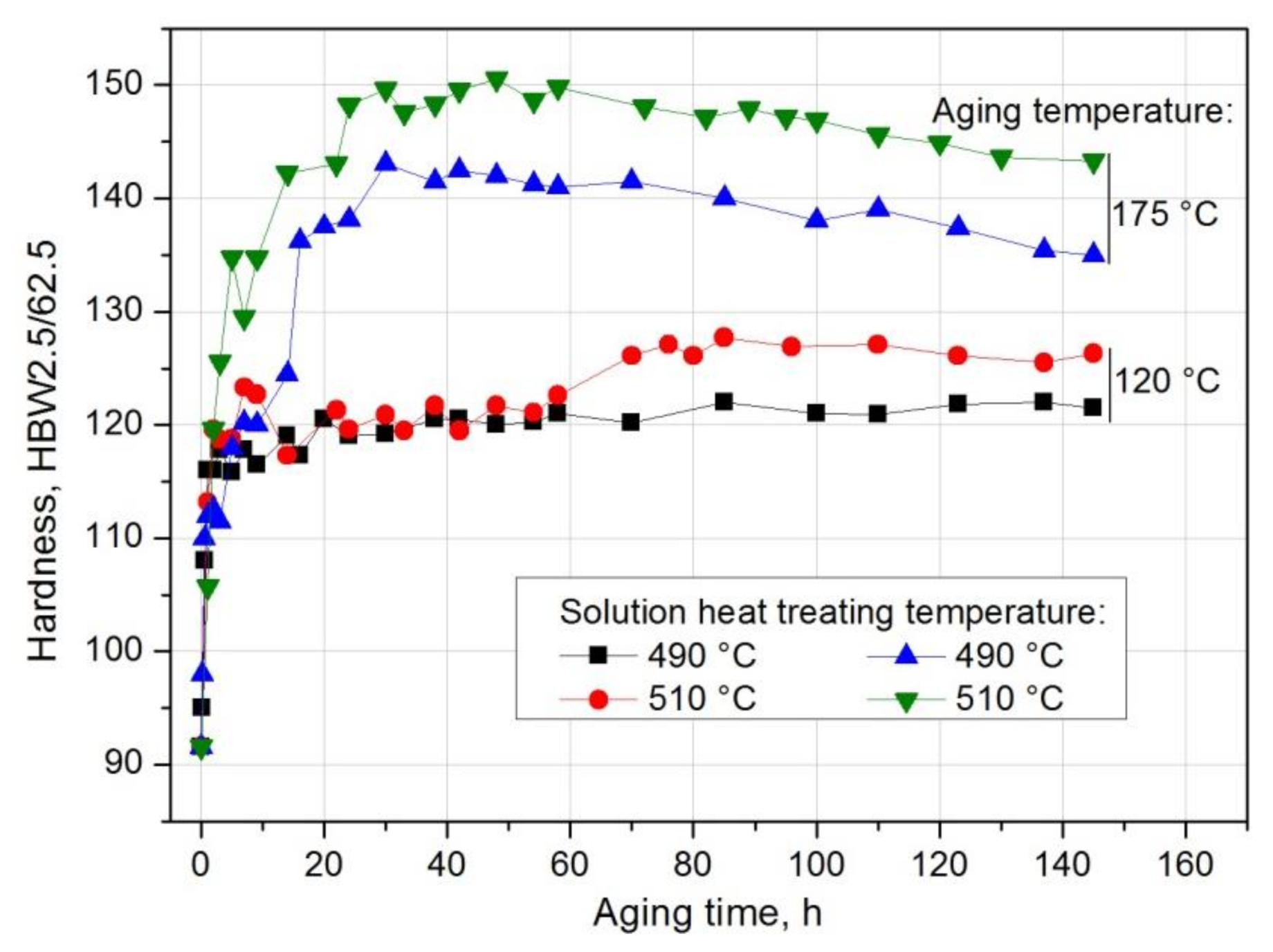
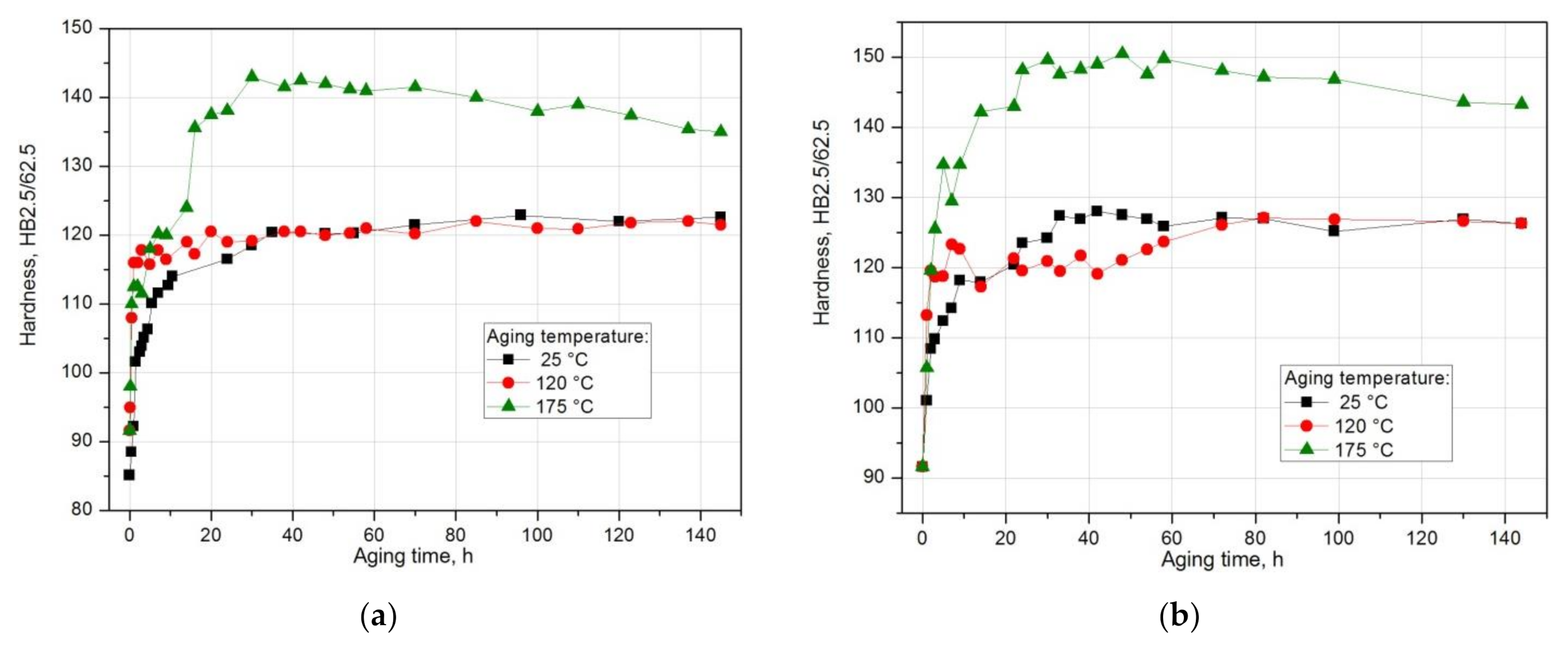
- The value of solution heat treatment temperature affects the mechanical properties and kinetics of precipitation during the natural aging of the 2017 alloy. The highest hardness and the rate of hardness increase to the maximum value were obtained for the 2017 aluminium alloy solution heat treated at 510 °C and aged naturally.
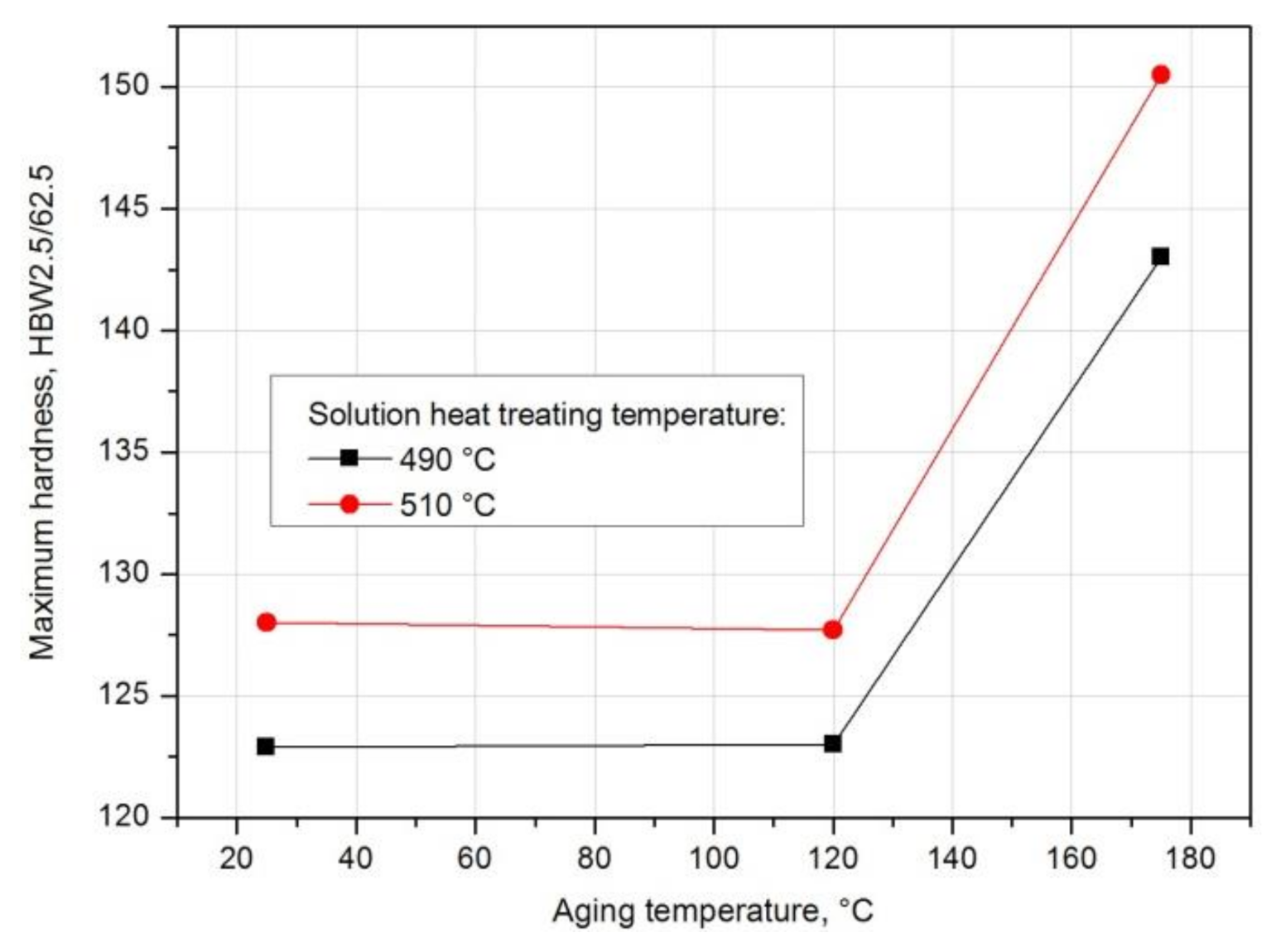
- The solution heat treatment temperature also affects the hardness of the studied alloy during artificial aging. It was found that artificial aging allows for achieving higher hardness values compared to natural aging. Alloy 2017 solution heat treated from 510 °C and aged at 175 °C shows the highest hardness. During aging at 120 °C, the hardness is close to the value achieved during natural aging. It was found that during artificial aging, the solution heat treatment temperature does not affect the rate of hardness increase to the maximum value.
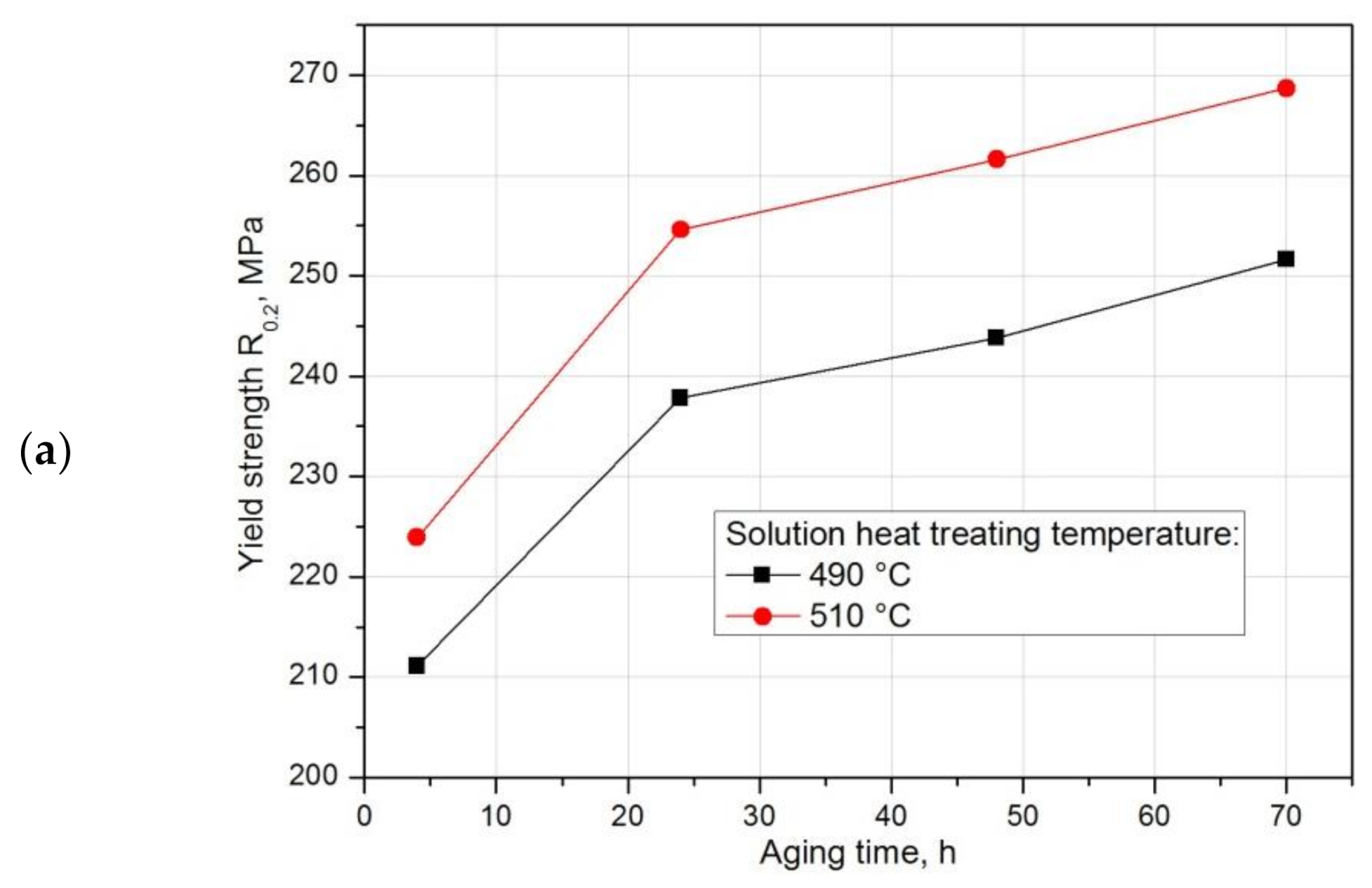
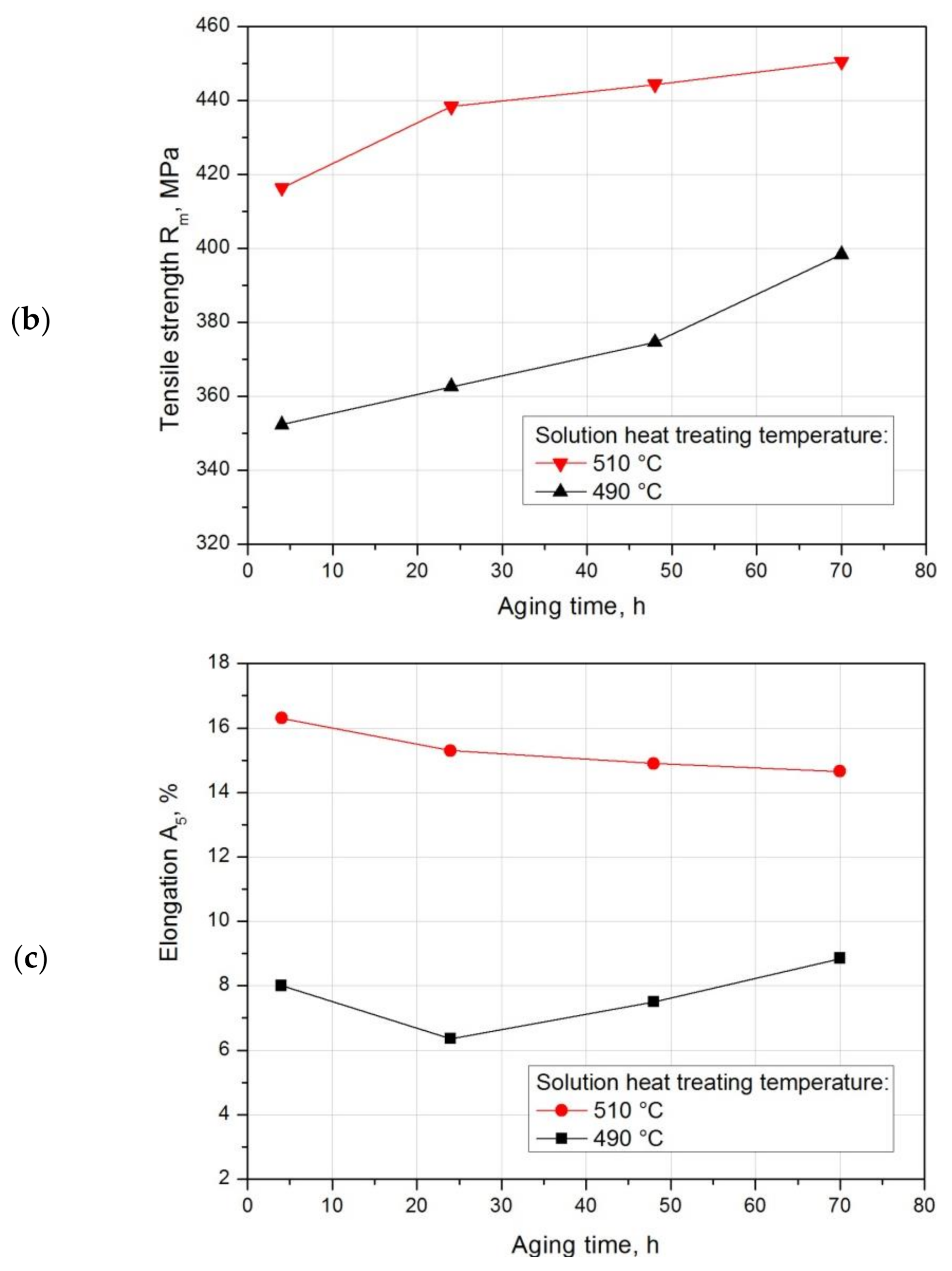
- Strength properties and hardness for both solution heat treatment temperatures—500 °C and 510 °C—increase with the increase in aging time. The highest strength properties—tensile strength Rm = 450.5 MPa and yield strength R0.2 = 268.7 MPa—while maintaining good plastic properties, relative elongation A5 = 14.65%, were obtained after annealing at 510 °C/6h and solution heat treatment in water and natural aging at 25 °C for 70 h.
- Based on calorimetric and diffractometric studies, it was established that the process of precipitation of strengthening phases from a solution heat treated alloy proceeds according to the following pattern: GP/GPB zones→ θ/Q” → θ/Q” → θ-Al2Cu/Q-Al4Cu2Mg8Si7. The maximum strengthening of the alloy is the results of the precipitation of metastable, transitional phases θ” and θ’ and Q” and Q’.
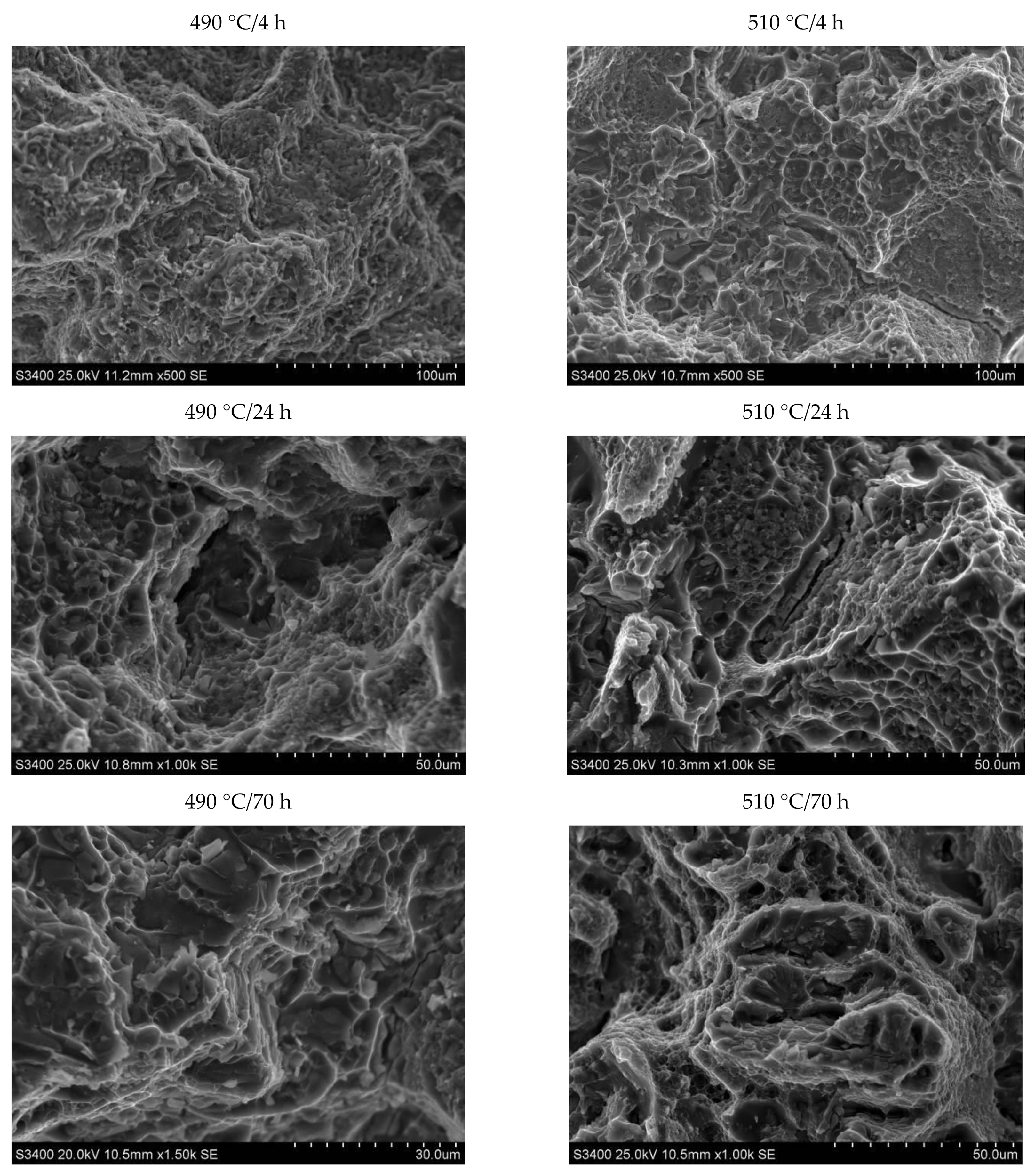
- Based on fractographic studies, it was established that in the tested alloy, regardless of the parameters of heat treatment, decohesion under tensile stress occurs through nucleation, growth, and void connection. It was also found that in the places where the primary undissolved precipitates of the θ-Al2Cu phase occurred, the decohesion process was initiated at the matrix/particle interface. The particles of the primary hard and brittle phases containing iron: Al7Cu2Feand Al15(FeMn)3(SiCu)2 are defragmented as a result of tensile loads.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polmear, I.J. Light Alloys: Metallurgy of Light Metals; Arnold: London, UK; New York, NY, USA; Sydney, NSW, Australia; Auckland, New Zealand, 1995. [Google Scholar]
- MacKenzie, S.D.; Totten, G.E. Analytical Characterization of Aluminium, Steel and Superalloys; Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- King, F. Aluminium and Its Alloys; John Willey and Sons: New York, NY, USA; Chichester, UK; Brisbane, Qld, Australia; Toronto, BC, Canada, 1987. [Google Scholar]
- Hatch, J.E. Aluminium: Properties and Physical Metallurgy; ASM Metals Park: Ohio, OH, USA, 1984. [Google Scholar]
- Kammer, C. Aluminium Handbook Vol. 1: Fundamentals and Materials; Aluminium Verlag Marketing & Kommunikation GmbH: Düsseldorf, Germany, 1999. [Google Scholar]
- Davis, J.R. ASM Specialty Handbook: Aluminium and Aluminium Alloys; International Materials Information Society: Materials Park, OH, USA, 1998. [Google Scholar]
- Mondolfo, L.F. Aluminium Alloys: Structure and Properties; Butterworths: London, UK; Boston, MA, USA, 1976. [Google Scholar]
- Grażina, M.-N. Influence of chemical composition variation and heat treatment on microstructure and mechanical properties of 6xxx alloys. Arch. Mater. Sci. Eng. 2010, 46, 98–107. [Google Scholar]
- Schlesinger, M.E. Aluminium Recycling; Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2014. [Google Scholar]
- Chino, Y.; Mabuchi, M.; Otsuka, S.; Shimojima, K.; Hosokawa, H.; Yamada, Y.; Wen, C.; Iwasaki, H. Corrosion and Mechanical Properties of Recycled 5083 Aluminum Alloy by Solid State Recycling. Mater. Trans. 2003, 44, 1284–1289. [Google Scholar] [CrossRef] [Green Version]
- Nur, K.Y.; Mohd, A.L.; Azlan, A. Hot Press as a Sustainable Direct Recycling Technique of Aluminium: Mechanical Properties and Surface Integrity. Materials 2017, 10, 902. [Google Scholar]
- Tenorio, J.A.S.; Espinosa, D.C. Encyclopedia of Aluminum and Its Alloys. Recycling of Aluminium; Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2019; Volume 2, pp. 2341–2360. [Google Scholar]
- Gronostajski, J.; Marciniak, H.; Matuszak, A. New methods of aluminium and aluminium-alloy chips recycling. J. Mater. Process. Technol. 2000, 106, 34–39. [Google Scholar] [CrossRef]
- Rahim, A.S.N.; Laji, M.A.; Ariffin, S. Review on Recycling Aluminum Chips by Hot Extrusion Process. Procedia CIRP 2015, 26, 761–766. [Google Scholar] [CrossRef] [Green Version]
- Samuel, M. A new technique for recycling aluminium scrap. J. Mater. Process. Technol. 2003, 135, 117–124. [Google Scholar] [CrossRef]
- Jirang, C.; Roven, H.J. Recycling of automotive aluminium. Trans. Nonferrous Met. Soc. China 2010, 20, 2057–2063. [Google Scholar]
- Fogagnolo, J.B.; Ruiz, N.E.M.; Simón, M.A.; Martinez, M.A. Recycling of aluminium alloy and aluminium matrix composite chips by pressing and hot extrusion. J. Mater. Process. Technol. 2003, 143, 792–795. [Google Scholar] [CrossRef]
- Gronostajski, J.Z.; Marciniak, H.; Matuszak, A. Production of composites on the base of AlCu4 alloy chips. J. Mater. Process. Technol. 1996, 60, 719–722. [Google Scholar] [CrossRef]
- Pawłowska, B.; Śliwa, R.E. Recycling aluminum chips by KoBo method. Met. Form. 2017, 28, 301–316. [Google Scholar]
- Das, S.K.; Kaufman, J.G. Recycling aluminum aerospace alloys. Ligth Met. 2007, 166, 1161–1165. [Google Scholar]
- Korbel, A.; Bochniak, W.; Śliwa, R.E.; Ostachowski, P.; Łagoda, M.; Kusion, Z.; Trzebuniak, B. Low-temperature consolidation of machining chips from hardly-deformable aluminum alloys. Met. Form. 2016, 27, 33–152. [Google Scholar]
- Vafaei, R.; Toroghinejad, M.R.; Pippan, R. Evaluation of mechanical behavior of nano-grained 2024 Al alloy during high pressure torsion (HPT) process at various temperatures. Mater. Sci. Eng. 2012, 536, 73–81. [Google Scholar] [CrossRef]
- Rosen, M.; Ives, L.; Biancaniello, F.; Mehrabian, R. Correlation between ultrasonic and hardness measurements in aged aluminum alloy 2024. Mater. Sci. Eng. 1985, 74, 1–10. [Google Scholar] [CrossRef]
- Fawad, T.; Nausheen, N.; Rasheed, A.; Baloch, F. Characterization of Material Properties of 2xxx Series Al-Alloys by Non Destructive Testing Techniques. J. Nondestruct. Eval. 2012, 3, 117–133. [Google Scholar]
- Xia, Q.K.; Liu, Z.Y.; Li, Y.T. Microstructure and properties of Al-Cu-Mg-Ag alloy exposed at 200 °C with and without stress. Trans. Nonferrous Met. Soc. China Engl. Ed. 2008, 18, 789–794. [Google Scholar] [CrossRef]
- Mrówka, N.G.; Sieniawski, J.; Nowotnik, A.; Gradzik, A. Analysis of precipitation strengthening process in 2xxx aluminium alloys. Inżynieria Mater. 2016, 37, 104–108. [Google Scholar]
- Xu, R.; Lin, B.; Li, H.; Xiao, H.; Zhao, Y.; Zhang, W. Microstructure evolution and mechanical properties of Al−6.5 Cu−0.6 Mn−0.5 Fe alloys with different Si additions. Trans. Nonferrous Met. Soc. China. 2019, 29, 1583–1591. [Google Scholar] [CrossRef]
- Belov, N.A.; Eskin, D.G.; Aksenov, A. Iron in Aluminium Alloys: Impurity and Alloying Element; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Mrówka, N.G.; Sieniawski, J. Analysis of intermetallic phases in 2024 aluminium alloy. Solid State Phenom. 2013, 197, 238–243. [Google Scholar] [CrossRef]
- Warmuzek, M.; Gazda, A.; Sieniawski, J.; Mrówka, G. Processes of the formation of the Fe(Mn)-bearing intermetallic phases in the Al-Fe(Mn)-Si alloys. Adv. Mater. Sci. 2003, 2, 81–91. [Google Scholar]
- Bäckerud, L.; Król, E.; Tamminen, J. Solidification Characteristic of Aluminium Alloys; Wrought Alloys; Skanaluminium: Oslo, Norway, 1968. [Google Scholar]
- Ghosh, K.S. Calorimetric studies of 2024 Al–Cu–Mg and 2014 Al–Cu–Mg–Si alloys of various tempers. J. Therm. Anal. Calorim. 2019, 136, 447–459. [Google Scholar] [CrossRef]
- Banerjee, S.; Robi, P.S.; Srinivasan, A. Calorimetric study of precipitation kinetics of Al–Cu–Mg and Al–Cu–Mg-0.06 wt. % Sn alloys. Met. Mater. Int. 2010, 16, 523–531. [Google Scholar] [CrossRef]
- Wang, S.C.; Starink, M.J.; Gao, N. Precipitation hardening in Al–Cu–Mg alloys revisited. Scr. Mater. 2006, 54, 287–291. [Google Scholar] [CrossRef]
- Bassani, P.; Gariboldi, E.; Vimercati, G. Calorimetric analysis on aged Al-4.4Cu-0.5 Mg-0.9Si-0.8Mn alloy (AA2014 grade). J. Therm. Anal. Calorim. 2007, 87, 247–253. [Google Scholar] [CrossRef]
- Kent, D.; Schaffer, G.B.; Drennan, J. Age hardening of a sintered Al–Cu–Mg–Si–(Sn) alloy. Mater. Sci. Eng. A 2005, 405, 65–73. [Google Scholar] [CrossRef]
- Eskin, D.G. Decomposition of supersaturated solid solutions in Al–Cu–Mg–Si Alloys. J. Mater. Sci. 2003, 38, 279–290. [Google Scholar] [CrossRef]
- Hutchuinson, C.R.; Ringer, S.P. Precipitation process in Al–Cu–Mg alloys microalloyed with Si. Metall. Mater. Trans. A 2000, 31, 2721–2733. [Google Scholar] [CrossRef]
- Ringer, S.P.; Hono, K.; Sakurai, T.; Polmear, I.J. Cluster hardening in an aged Al–Cu–Mg alloy. Scr. Mater. 1997, 31, 517–521. [Google Scholar] [CrossRef]
- Starink, M.J.; Wang, S.C. The thermodynamics of and strengthening due to co-clusters: General theory and application to the case of Al–Cu–Mg alloys. Acta Mater. 2009, 57, 2376–2389. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Nie, J.F.; Muddle, B.C. Effect on Si additions on precipitation hardening response in Al-Cu-Mg-(Ag) alloys. Mater. Sci. Forum 1996, 217, 1251–1256. [Google Scholar] [CrossRef]
- PN EN 573–581. Aluminium i Stopy Aluminium-Skład Chemiczny i Rodzaje Wyrobów Przerobionych Plastycznie-Część 1: System Oznaczeń Numerycznych; Polski Komitet Normalizacyjny: Warsaw, Poland, 2006. [Google Scholar]
- ICDD PDF-4+ Diffraction Data Base. International Centre for Diffraction Data: Newtown Square, PA, USA, 2020.
- Mroczka, K.; Wójcicka, A.; Kurtyka, P. 2017A aluminum alloy in different heat treatment conditions. Acta Metall. Slovaca 2012, 18, 82–91. [Google Scholar]
- PN-EN 10002–1:2004. Metale. Próba rozciągania. Część 1: Metoda Badania w Temperaturze Otoczenia; Polish Committee for Standardization: Warsaw, Poland, 2004. [Google Scholar]
- Mrówka, N.G.; Sieniawski, J.; Wierzbińska, M.; Nowotnik, A. Wpływ długotrwałego wyżarzania ujednorodniającego na mikrostrukturę i właściwości stopu aluminium 6066. Inżynieria Mater. 2015, 6, 333–337. [Google Scholar]





| Temperature of Metal in Furnace (°C) | Temperature of Metal in Classifier (°C) | Amount of Cooling Water (L/min) | Casting Rate (mm/s) | Comments |
|---|---|---|---|---|
| 740 | 690–700 | 30 | 3.5–4.0 | Amount of water per one crystallizer. A total of 120 L/m for four crystallizers |
| Alloy | Elements Content, wt % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| - | Si | Fe | Cu | Mn | Mg | Cr | Zn | Zr | Ti | Al |
| 2017A alloy * | 0.20–0.80 | 0.70 | 3.50–4.50 | 0.4–1.0 | 0.4–1.8 | 0.10 | 0.25 | 0.10 | 0.10 | balance |
| 2017A alloy ** | 0.49 | 0.22 | 4.01 | 0.56 | 0.72 | 0.063 | 0.20 | 0.17 | 0.077 | balance |
| Number of Measured Particles | Suggested Type of Phases | % | Al | Si | Mn | Fe | Cu | Mg |
|---|---|---|---|---|---|---|---|---|
| 35 | α-Al15(FeMn)3(SiCu)2 | wt | 56.8–60.1 | 5.8–6.7 | 9.7–12.4 | 17.6–19.7 | 4.0–7.2 | - |
| at | 70.6–73.2 | 6.9–8.0 | 5.9–7.4 | 10.3–11.8 | 2.7–3.8 | - | ||
| 24 | Q-Al5Cu2Mg8Si6 | wt | 33.6–56.5 | 16.4–26.8 | - | - | 11.4–18.8 | 15.7–23.4 |
| at | 37.0–59.7 | 16.7–27.8 | - | 5.1–8.8 | 18.5–28.1 | |||
| 25 | Al7Cu2Fe | wt | 61.5–64.4 | - | - | 8.9–9.4 | 25.0–29.1 | - |
| at | 77.5–79.6 | - | - | 5.3–5.8 | 13.1–14.6 | - | ||
| 35 | θ-Al2Cu | wt | 47.0–47.8 | - | - | - | 52.2–53.0 | - |
| at | 67.0–68.3 | - | - | - | 31.7–32.3 | - | ||
| 25 | β-Mg2Si | wt | 2.5–3.7 | 52.7–60.3 | - | - | - | 38.5–47.1 |
| at | 2.6–3.6 | 47.3–49.5 | - | - | - | 47.7–52.7 |
| Solution Heat Treatment Temperature, °C | HBw | HBmax | ΔHB | Δt, h | Grad HB = ΔHB/Δt, HB/h |
|---|---|---|---|---|---|
| 490 | 81.5 | 123.5 | 42 | 180 | 0.233 |
| 500 | 90.6 | 127.6 | 37 | 96 | 0.385 |
| 510 | 91.6 | 128.3 | 36.7 | 48 | 0.764 |
| Temperature, °C | Exothermal Peaks | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Top of peak | 70 | 138.6 | 184.3 | 238.0 | 269.9 | 329.6 |
| onset | 54.6 | 127.7 | 178.1 | 225.9 | 257.9 | 319.0 |
| offset | 81 | 141.3 | 188.4 | 247.6 | 282.3 | 345.9 |
| Solution Heat Treatment Temperature, °C | Aging Temperature, °C | HBw | HBmax | ΔHB | Δt, h | Grad HB = ΔHB/Δt, HB/h |
|---|---|---|---|---|---|---|
| 490 | 25 | 81.5 | 123.5 | 42.0 | 180 | 0.233 |
| 120 | 81.5 | 122 | 40.5 | 85 | 0.476 | |
| 175 | 81.5 | 143 | 61.5 | 30 | 2.05 | |
| 510 | 25 | 91.6 | 128.3 | 36.7 | 48 | 0.746 |
| 120 | 91.6 | 127.7 | 36.1 | 76 | 0.475 | |
| 175 | 91.6 | 150.0 | 58.4 | 30 | 1.946 |
| Aging Time, h | Solution Temperature, °C | Yield Strength R0.2, MPa | Tensile Strength Rm, MPa | Elongation A5, % | Reduction Z, % |
|---|---|---|---|---|---|
| 4 | 490 | 211.1 | 352.3 | 8.0 | 4.85 |
| 510 | 223.9 | 416.4 | 16.3 | 15.7 | |
| 24 | 490 | 237.8 | 362.6 | 6.35 | 4.45 |
| 510 | 254.6 | 438.4 | 15.3 | 14.25 | |
| 48 | 490 | 243.8 | 374.6 | 7.5 | 5.65 |
| 510 | 261.6 | 444.4 | 14.9 | 13.4 | |
| 70 | 490 | 251.65 | 398.35 | 8.85 | 7.3 |
| 510 | 268.7 | 450.5 | 14.65 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grażyna, M.-N.; Gancarczyk, K.; Nowotnik, A.; Dychtoń, K.; Boczkal, G. Microstructure and Properties of As-Cast and Heat-Treated 2017A Aluminium Alloy Obtained from Scrap Recycling. Materials 2021, 14, 89. https://doi.org/10.3390/ma14010089
Grażyna M-N, Gancarczyk K, Nowotnik A, Dychtoń K, Boczkal G. Microstructure and Properties of As-Cast and Heat-Treated 2017A Aluminium Alloy Obtained from Scrap Recycling. Materials. 2021; 14(1):89. https://doi.org/10.3390/ma14010089
Chicago/Turabian StyleGrażyna, Mrówka-Nowotnik, Kamil Gancarczyk, Andrzej Nowotnik, Kamil Dychtoń, and Grzegorz Boczkal. 2021. "Microstructure and Properties of As-Cast and Heat-Treated 2017A Aluminium Alloy Obtained from Scrap Recycling" Materials 14, no. 1: 89. https://doi.org/10.3390/ma14010089
APA StyleGrażyna, M.-N., Gancarczyk, K., Nowotnik, A., Dychtoń, K., & Boczkal, G. (2021). Microstructure and Properties of As-Cast and Heat-Treated 2017A Aluminium Alloy Obtained from Scrap Recycling. Materials, 14(1), 89. https://doi.org/10.3390/ma14010089





