Hydrogels Based on Poly(Ether-Ester)s as Highly Controlled 5-Fluorouracil Delivery Systems—Synthesis and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
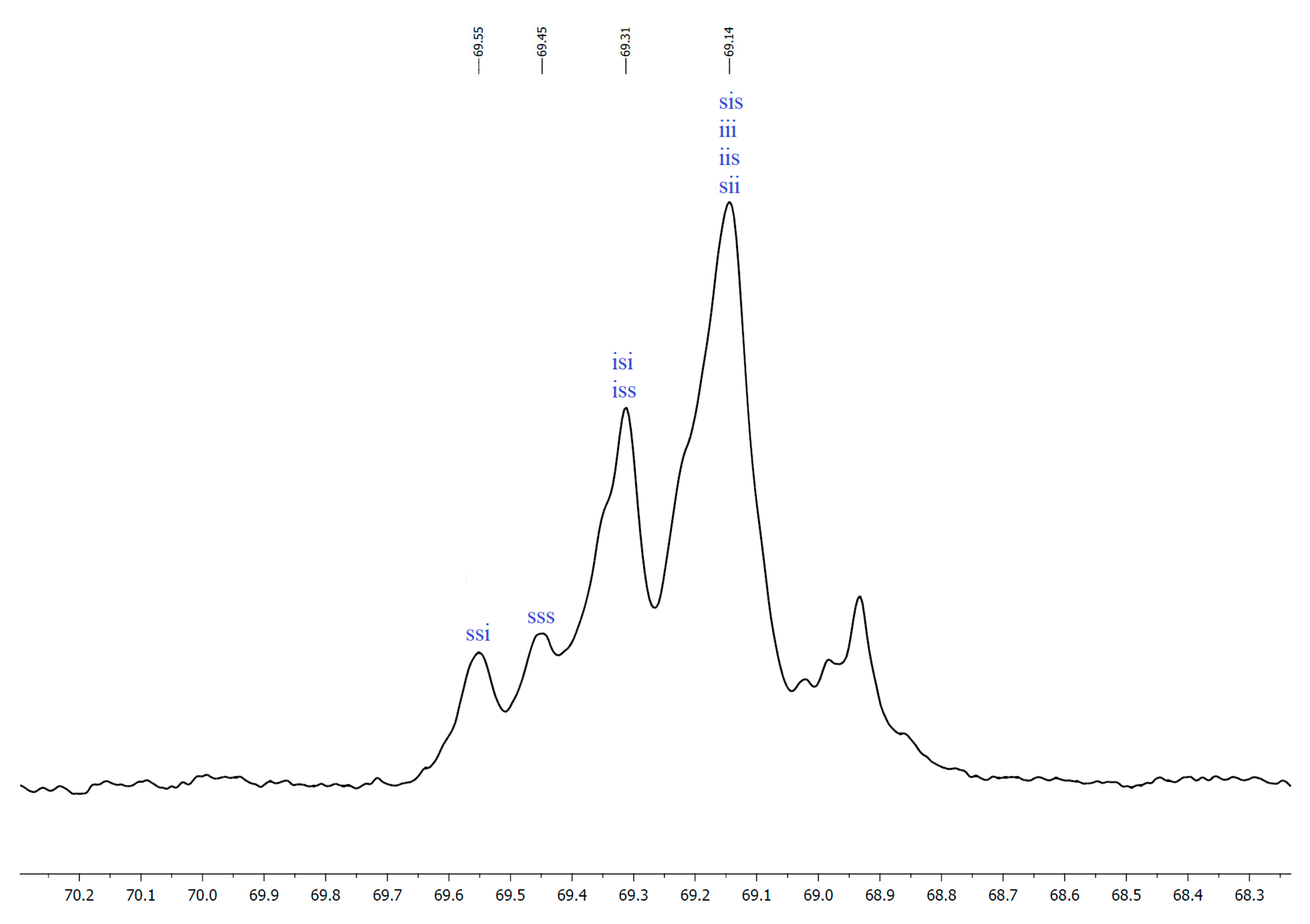
2.2. NMR Data
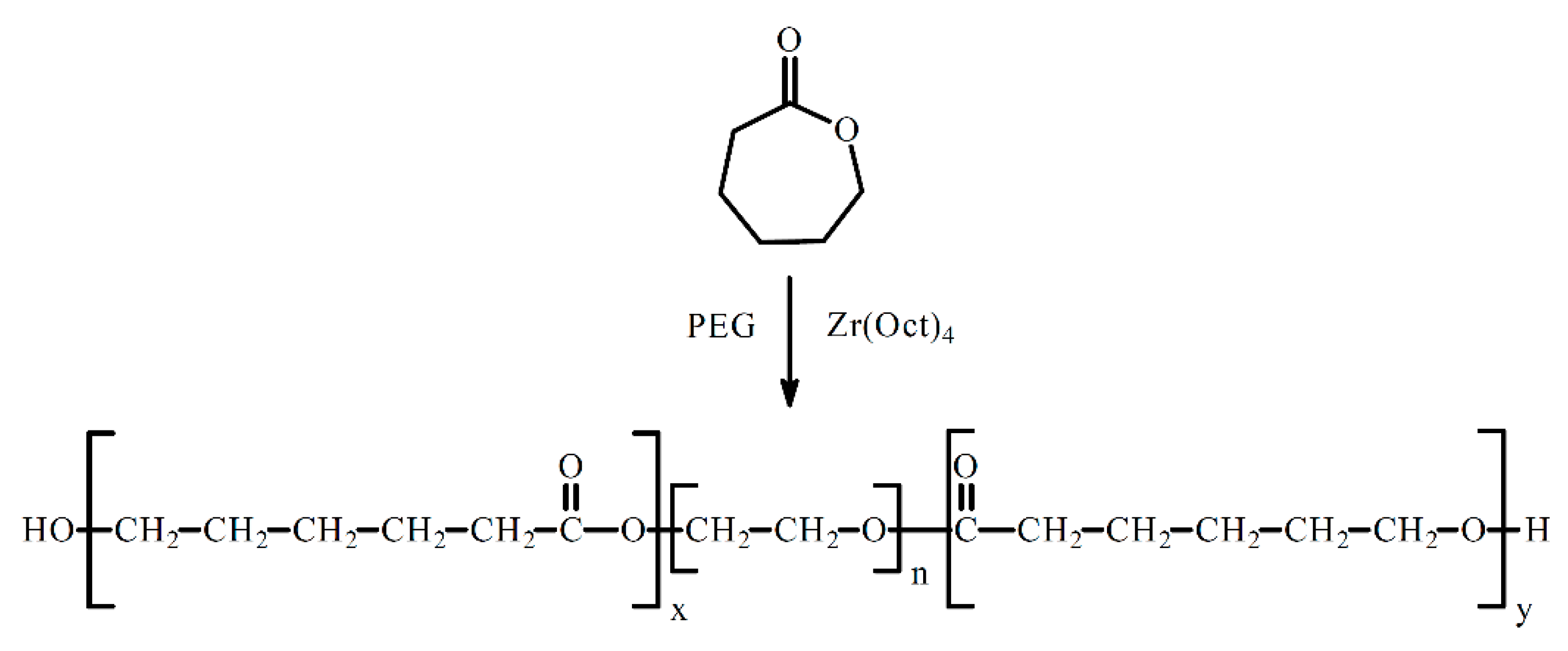
2.3. Synthesis of Copolymers CL, LA and PEG
2.4. Preparation of Hydrogels
2.5. In Vitro Studies of 5-FU Release from Hydrogels
2.5.1. 5‑FU Loading
2.5.2. 5-FU Release Studies
2.6. Measurements
2.6.1. Structural Analysis
2.6.2. HPLC Analysis
2.7. Mathematical Models for 5‑FU Release Studies
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carugo, A.; Draetta, G.F. Academic Discovery of Anticancer Drugs: Historic and Future Perspectives. Annu. Rev. Cancer Biol. 2019, 3, 385–408. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Yu, Y.-T. Incorporation of 5-fluorouracil into U2 snRNA blocks pseudouridylation and pre-mRNA splicing in vivo. Nucleic Acids Res. 2006, 35, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Mojardín, L.; Botet, J.; Quintales, L.; Moreno, S.; Salas, M. New Insights into the RNA-Based Mechanism of Action of the Anticancer Drug 5′-Fluorouracil in Eukaryotic Cells. PLoS ONE 2013, 8, e78172. [Google Scholar] [CrossRef] [Green Version]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Apetoh, L.; Ghiringhelli, F. Enhancing the anticancer effects of 5-fluorouracil: Current challenges and future perspectives. Biomed. J. 2015, 38, 111. [Google Scholar] [CrossRef]
- Guerra, G.D.; Cerrai, P.; Tricoli, M.; Maltinti, S. Release of 5-fluorouracil by biodegradable poly(ester-ether-ester)s. Part I: Release by fused thin sheets. J. Mater. Sci. Mater. Electron. 2001, 12, 313–317. [Google Scholar] [CrossRef]
- Hou, J.; Li, C.; Cheng, L.; Guo, S.; Zhang, Y.; Tang, T. Study on hydrophilic 5-fluorouracil release from hydrophobic poly(ϵ-caprolactone) cylindrical implants. Drug Dev. Ind. Pharm. 2011, 37, 1068–1075. [Google Scholar] [CrossRef]
- Rahman, Z.; Kohli, K.; Khar, R.K.; Ali, M.; Charoo, N.A.; Shamsher, A.A.A. Characterization of 5-fluorouracil microspheres for colonic delivery. AAPS PharmSciTech 2006, 7, E113–E121. [Google Scholar] [CrossRef] [Green Version]
- Martini, L.G.; Collett, J.H.; Attwood, D. The Release of 5-Fluorouracil from Microspheres of Poly(ε-Caprolactone- co -ethylene Oxide). Drug Dev. Ind. Pharm. 2000, 26, 7–12. [Google Scholar] [CrossRef]
- He-Ping, L.; Li, H.; Wang, Z.-D.; Zhang, J.-J.; Deng, M.-F.; Chen, S.-L. Preparation and In Vitro Release of Ramose Chitosan-Based-5-Fluorouracil Microspheres. J. Korean Chem. Soc. 2013, 57, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Lai, L.; Guo, H. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int. J. Pharm. 2011, 404, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.-T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil—An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C 2021, 119, 111591. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Affram, K.; Nottingham, E.L.; Han, B.; Amissah, F.; Krishnan, S.; Trevino, J.; Agyare, E. Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Mitschang, F.; Agarwal, S. Biocompatible Drug Delivery System for Photo-Triggered Controlled Release of 5-Fluorouracil. Biomacromolecules 2011, 12, 3684–3691. [Google Scholar] [CrossRef]
- Doi, N.; Sasai, Y.; Yamauchi, Y.; Adachi, T.; Kuzuya, M.; Kondo, S.-I. Development of Novel Polymeric Prodrugs Synthesized by Mechanochemical Solid-State Copolymerization of Hydroxyethylcellulose and Vinyl Monomers. Chem. Pharm. Bull. 2015, 63, 992–997. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Chung, N.; JeongHo, A. Poly(d,l-lactide-ran-ε-caprolactone)-poly(ethylene glycol)-poly(d,l-lactide-ran-ε-caprolactone) as parenteral drug-delivery systems. Biomaterials 2004, 25, 3733–3742. [Google Scholar] [CrossRef]
- Dalwadi, C.; Gc, P. Thermosensitive nanohydrogel of 5-fluorouracil for head and neck cancer: Preparation, characterization and cytotoxicity assay. Int. J. Nanomed. 2018, 13, 31–33. [Google Scholar] [CrossRef] [Green Version]
- Mishra, G.P.; Kinser, R.; Wierzbicki, I.H.; Alany, R.G.; Alani, A. In situ gelling polyvalerolactone-based thermosensitive hydrogel for sustained drug delivery. Eur. J. Pharm. Biopharm. 2014, 88, 397–405. [Google Scholar] [CrossRef]
- Seo, H.W.; Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Jin, L.M.; Lee, B.; Kim, J.-H.; Min, B.-H.; Kim, M.S. Injectable intratumoral hydrogel as 5-fluorouracil drug depot. Biomaterials 2013, 34, 2748–2757. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart Hydrogels—Synthetic Stimuli-Responsive Antitumor Drug Release Systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Ferreira, N.; Ferreira, L.; Cardoso, V.; Boni, F.; Souza, A.; Gremião, M. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qu, Y.; Shi, K.; Chu, B.; Jia, Y.; Xiao, X.; He, Q.; Qian, Z. Camptothecin@HMSNs/thermosensitive hydrogel composite for applications in preventing local breast cancer recurrence. Chin. Chem. Lett. 2018, 29, 1819–1823. [Google Scholar] [CrossRef]
- Abdullah, O.; Minhas, M.U.; Ahmad, M.; Ahmad, S.; Ahmad, A. Synthesis of hydrogels for combinatorial delivery of 5-fluorouracil and leucovorin calcium in colon cancer: Optimization, in vitro characterization and its toxicological evaluation. Polym. Bull. 2019, 76, 3017–3037. [Google Scholar] [CrossRef]
- Yun, Q.; Wang, S.S.; Xu, S.; Yang, J.P.; Fan, J.; Yang, L.L.; Chen, Y.; Fu, S.Z.; Wu, J.B. Use of 5-Fluorouracil Loaded Micelles and Cisplatin in Thermosensitive Chitosan Hydrogel as an Efficient Therapy against Colorectal Peritoneal Carcinomatosis. Macromol. Biosci. 2017, 17, 1600262. [Google Scholar] [CrossRef]
- Blanco, M.D.; Fernándezace, L.G.-; Gómez, C.; Sastre, R.L.; Teijón, J. In-vivo drug delivery of 5-fluorouracil using poly(2-hydroxyethyl methacrylate-co-acrylamide) hydrogels. J. Pharm. Pharmacol. 2000, 52, 1319–1325. [Google Scholar] [CrossRef]
- Yi, H.; Cho, H.-J.; Cho, S.-M.; Lee, D.-G.; El-Aty, A.A.; Yoon, S.-J.; Bae, G.-W.; Nho, K.; Kim, B.; Lee, C.-H.; et al. Pharmacokinetic properties and antitumor efficacy of the 5-fluorouracil loaded PEG-hydrogel. BMC Cancer 2010, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Chun, C.; Heo, J.Y.; Song, S.-C. Injectable and thermosensitive poly(organophosphazene) hydrogels for a 5-fluorouracil delivery. J. Appl. Polym. Sci. 2009, 113, 3831–3839. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Nicoletta, F.P.; Iemma, F. Injectable Hydrogels for Cancer Therapy over the Last Decade. Pharmaceutics 2019, 11, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent Advances in Designing 5-Fluorouracil Delivery Systems: A Stepping Stone in the Safe Treatment of Colorectal Cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Munim, S.A.; Raza, Z.A. Poly(lactic acid) based hydrogels: Formation, characteristics and biomedical applications. J. Porous Mater. 2018, 26, 881–901. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, C.; Yang, L.; Wu, Q.J.; Shi, S.; Shi, H.; Qian, Z.; Wei, Y.-Q. 5-FU-hydrogel inhibits colorectal peritoneal carcinomatosis and tumor growth in mice. BMC Cancer 2010, 10, 402. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Wang, C.; Wang, Y.; Wu, Q.; Zhang, D.; Luo, F.; Qian, Z. Efficient inhibition of colorectal peritoneal carcinomatosis by drug loaded micelles in thermosensitive hydrogel composites. Nanoscale 2012, 4, 3095–3104. [Google Scholar] [CrossRef]
- Dobrzyński, P. Mechanism of ε-caprolactone polymerization and ε-caprolactone/trimethylene carbonate copolymerization carried out with Zr(Acac)4. Polymer 2007, 48, 2263–2279. [Google Scholar] [CrossRef]
- Maring, J.G.; Schouten, L.; Greijdanus, B.; De Vries, E.G.E.; Uges, D.R.A. A Simple and Sensitive Fully Validated HPLC-UV Method for the Determination of 5-Fluorouracil and Its Metabolite 5,6-Dihydrofluorouracil in Plasma. Ther. Drug Monit. 2005, 27, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Singhvi, G.; Singh, M. Review: In-vitro drug release characterization models. Int. J. Pharm. Sci. Res. 2011, 2, 77–84. [Google Scholar]
- Dobrzyński, P.; Pastusiak, M.; Jaworska, J.; Kaczmarczyk, B.; Kwiecień, M.; Kawalec, M. Zirconium (IV) Acetylacetonate: Ring-Opening Initiator Mediating One-Step Synthesis of Biodegradable Polyacids. Adv. Polym. Technol. 2019, 2019, 3761430. [Google Scholar] [CrossRef] [Green Version]
- Kasperczyk, J.; Bero, M. Stereoselective polymerization of racemic dl-lactide in the presence of butyllithium and butylmagnesium. Structural investigations of the polymers. Polymer 2000, 41, 391–395. [Google Scholar] [CrossRef]
| Sample | Method | Temp. (°C) | Time (h) | % mol a CL | % mol a PEG | Mnb (g/mol) | Đc |
|---|---|---|---|---|---|---|---|
| A-MS-1 | toluene | 80 | 44 | 78 | 22 | 7100 | 1.68 |
| A-MS-2 | toluene | 80 | 44 | 81 | 19 | 6900 | 1.72 |
| A-MS-3 | toluene | 80 | 24 | 71 | 29 | 6600 | 1.64 |
| A-MS-4 | toluene | 60 | 44 | 68 | 32 | 3700 | 1.61 |
| A-MS-5 | toluene | 60 | 24 | 75 | 25 | 2800 | 1.49 |
| A-MS-11 | bulk | 100 | 45 | 93 | 7 | 9600 | 2.03 |
| A-MS-12 | bulk | 100 | 24 | 90 | 10 | 8400 | 1.89 |
| A-MS-13 | bulk | 100 | 27 | 92 | 8 | 8600 | 1.86 |
| A-MS-14 | bulk | 140 | 24 | 94 | 6 | 9500 | 1.94 |
| A-MS-15 | bulk | 140 | 48 | 95 | 5 | 10,600 | 2.11 |
| A-MS-21 | bulk | 160 | 24 | 78 | 22 | 10,400 | 2.06 |
| A-MS-22 | bulk | 160 | 42 | 81 | 19 | 12,300 | 2.26 |
| A-MS-23 | bulk | 160 | 48 | 81 | 19 | 13,600 | 2.42 |
| A-MS-24 | bulk | 120 | 24 | 84 | 16 | 8300 | 1.71 |
| A-MS-25 | bulk | 120 | 48 | 81 | 19 | 8600 | 1.76 |
| A-MS-31 | bulk | 140 | 1 | 92 | 8 | 1800 | 1.34 |
| A-MS-32 | bulk | 140 | 2 | 96 | 4 | 2100 | 1.38 |
| A-MS-33 | bulk | 140 | 3 | 97 | 3 | 2600 | 1.45 |
| A-MS-34 | bulk | 140 | 4 | 94 | 6 | 4200 | 1.54 |
| Sample | Method | Temp. (°C) | Time (h) | % mol a CL | % mol a PEG | Mnb (g/mol) | Đc | Td (%) |
|---|---|---|---|---|---|---|---|---|
| A-MS-6 | toluene | 80 | 44 | 64 | 36 | 3700 | 1.76 | 72 |
| A-MS-7 | toluene | 80 | 44 | 64 | 36 | 3300 | 1.69 | 72 |
| A-MS-8 | toluene | 80 | 24 | 55 | 45 | 2300 | 1.89 | ≈100 |
| A-MS-9 | toluene | 60 | 44 | 60 | 40 | ≈100 | ||
| A-MS-10 | toluene | 60 | 24 | 62 | 38 | ≈100 | ||
| A-MS-16 | bulk | 140 | 48 | 95 | 5 | 8900 | 2.26 | 32 |
| A-MS-17 | bulk | 140 | 24 | 95 | 5 | 7200 | 1.96 | 40 |
| A-MS-18 | bulk | 140 | 27 | 95 | 5 | 7500 | 2.41 | 40 |
| A-MS-19 | bulk | 140 | 45 | 95 | 5 | 8200 | 2.29 | 48 |
| A-MS-20 | bulk | 140 | 48 | 84 | 16 | 9700 | 2.31 | 48 |
| A-MS-26 | bulk | 160 | 24 | 85 | 15 | 9400 | 2.16 | 64 |
| A-MS-27 | bulk | 160 | 42 | 84 | 16 | 10,300 | 2.56 | 72 |
| A-MS-28 | bulk | 160 | 48 | 84 | 16 | 11,700 | 2.64 | 72 |
| A-MS-29 | bulk | 120 | 24 | 85 | 15 | 5400 | 1.62 | 48 |
| A-MS-35 | bulk | 160 | 1 | 84 | 16 | 1100 | 1.29 | 0 |
| A-MS-36 | bulk | 160 | 2 | 98 | 2 | 1600 | 1.48 | 48 |
| A-MS-37 | bulk | 160 | 3 | 96 | 4 | 2400 | 1.54 | 48 |
| A-MS-38 | bulk | 160 | 4 | 97 | 3 | 2800 | 1.59 | 56 |
| Sample | Copolymer | Temp. (°C) | Time (h) | % mol a CL | % mol a PEG | Mnb (g/mol) | Đc | Td (%) |
|---|---|---|---|---|---|---|---|---|
| A‑MS‑39 | CL‑PEG | 140 | 24 | 93 | 7 | 6 800 | 1.73 | |
| A‑MS‑40 | rac‑LA‑PEG | 140 | 24 | 96 | 4 | 10 800 | 1.98 | 32 |
| Sample | Copolymer | Microtox® | Spirotox® | |||
|---|---|---|---|---|---|---|
| PE a (%) | Test Reactions | |||||
| 15 min | 30 min | 1 h | 24 h | 48 h | ||
| A‑MS‑39 | CL‑PEG | 13 ± 1 | 18 ± 1 | none | none | none |
| A‑MS‑40 | rac‑LA‑PEG | 8 ± 1 | 6 ± 1 | none | none | none |
| Copolymer | Sample | PEG 600/Copolymer (mol/mol) | HDI/Copolymer (mol/mol) | Copolymer Weight (g) | Water Absorption Capacity (%) |
|---|---|---|---|---|---|
| CL‑PEG | A | 1 | 4.1 | 0.5325 | 57 |
| B | 1 | 4.1 | 0.5214 | 21 | |
| C | 2 | 4.1 | 0.5729 | 16 | |
| G | 3 | 4.1 | 0.685 | 15 | |
| H | 7 | 8.3 | 0.898 | 14 | |
| I | 5 | 8.3 | 0.958 | 9 | |
| rac‑LA‑PEG | D | 1 | 6.6 | 0.2649 | 114 |
| E | 0.5 | 6.6 | 0.2829 | 84 | |
| F | 2 | 6.6 | 0.3597 | 107 | |
| J | 3 | 6.6 | 0.2275 | 291 | |
| K | 7 | 13.2 | 0.2143 | 397 | |
| L | 9 | 13.2 | 0.2022 | 470 |
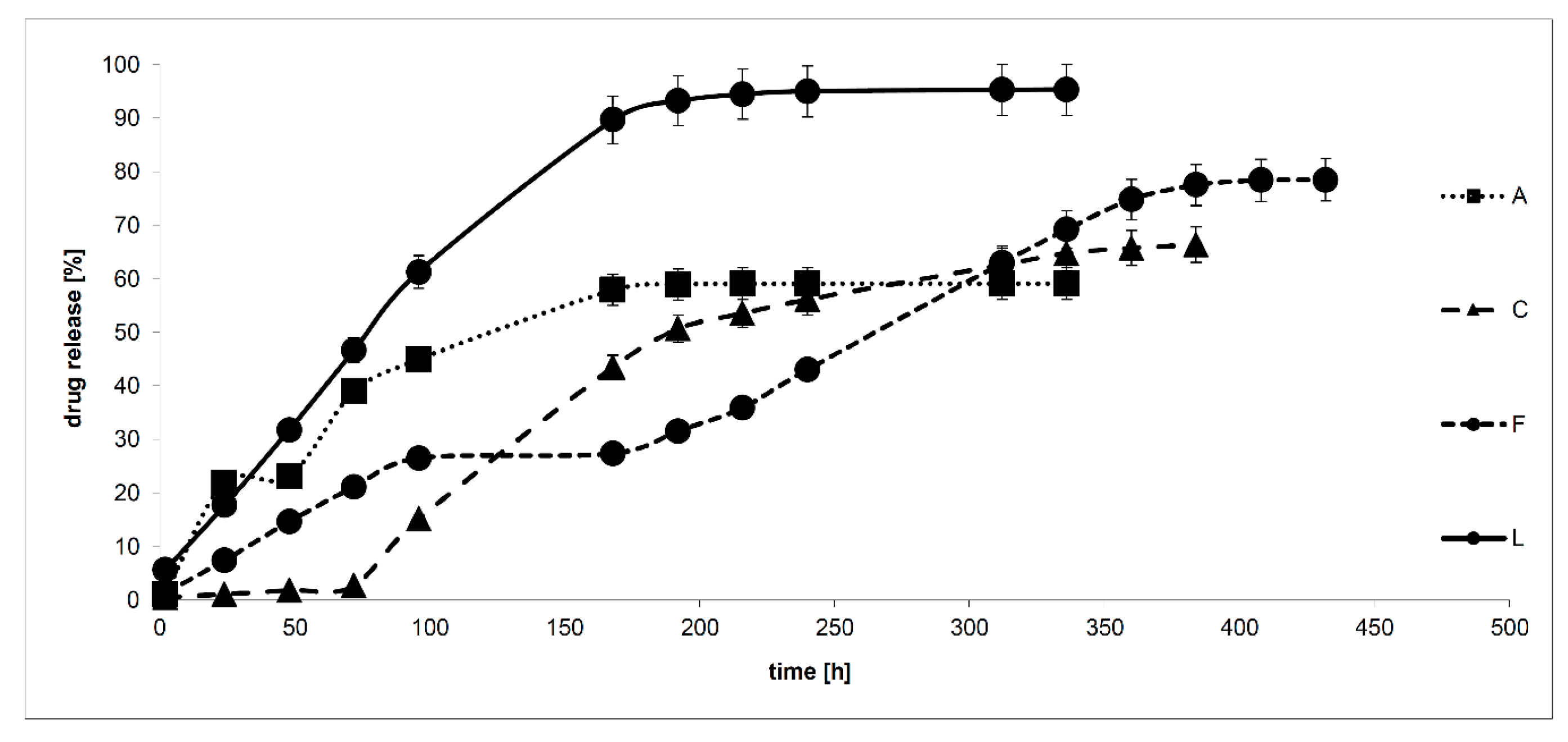
| No. | Zero-Order Model | First-Order Model | Korsmeyer–Peppas Model | Drug Transport Mechanism | |
|---|---|---|---|---|---|
| R2 | R2 | R2 | n | ||
| A | 0.7407 | 0.7943 | 0.9178 | 0.752 | non-Fickian transport |
| C | 0.9157 | 0.9575 | 0.7786 | 1.037 | unknown |
| F | 0.9712 | 0.9325 | 0.9825 | 0.741 | non-Fickian transport |
| L | 0.8259 | 0.9101 | 0.9702 | 0.601 | non-Fickian transport |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Nałęcz-Jawecki, G.; Drobniewska, A.; Sobczak, M. Hydrogels Based on Poly(Ether-Ester)s as Highly Controlled 5-Fluorouracil Delivery Systems—Synthesis and Characterization. Materials 2021, 14, 98. https://doi.org/10.3390/ma14010098
Kasiński A, Zielińska-Pisklak M, Oledzka E, Nałęcz-Jawecki G, Drobniewska A, Sobczak M. Hydrogels Based on Poly(Ether-Ester)s as Highly Controlled 5-Fluorouracil Delivery Systems—Synthesis and Characterization. Materials. 2021; 14(1):98. https://doi.org/10.3390/ma14010098
Chicago/Turabian StyleKasiński, Adam, Monika Zielińska-Pisklak, Ewa Oledzka, Grzegorz Nałęcz-Jawecki, Agata Drobniewska, and Marcin Sobczak. 2021. "Hydrogels Based on Poly(Ether-Ester)s as Highly Controlled 5-Fluorouracil Delivery Systems—Synthesis and Characterization" Materials 14, no. 1: 98. https://doi.org/10.3390/ma14010098
APA StyleKasiński, A., Zielińska-Pisklak, M., Oledzka, E., Nałęcz-Jawecki, G., Drobniewska, A., & Sobczak, M. (2021). Hydrogels Based on Poly(Ether-Ester)s as Highly Controlled 5-Fluorouracil Delivery Systems—Synthesis and Characterization. Materials, 14(1), 98. https://doi.org/10.3390/ma14010098






