Crystallographic Characteristic Effect of Cu Substrate on Serrated Cathode Dissolution in Cu/Sn–3.0Ag–0.5Cu/Cu Solder Joints during Electromigration
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
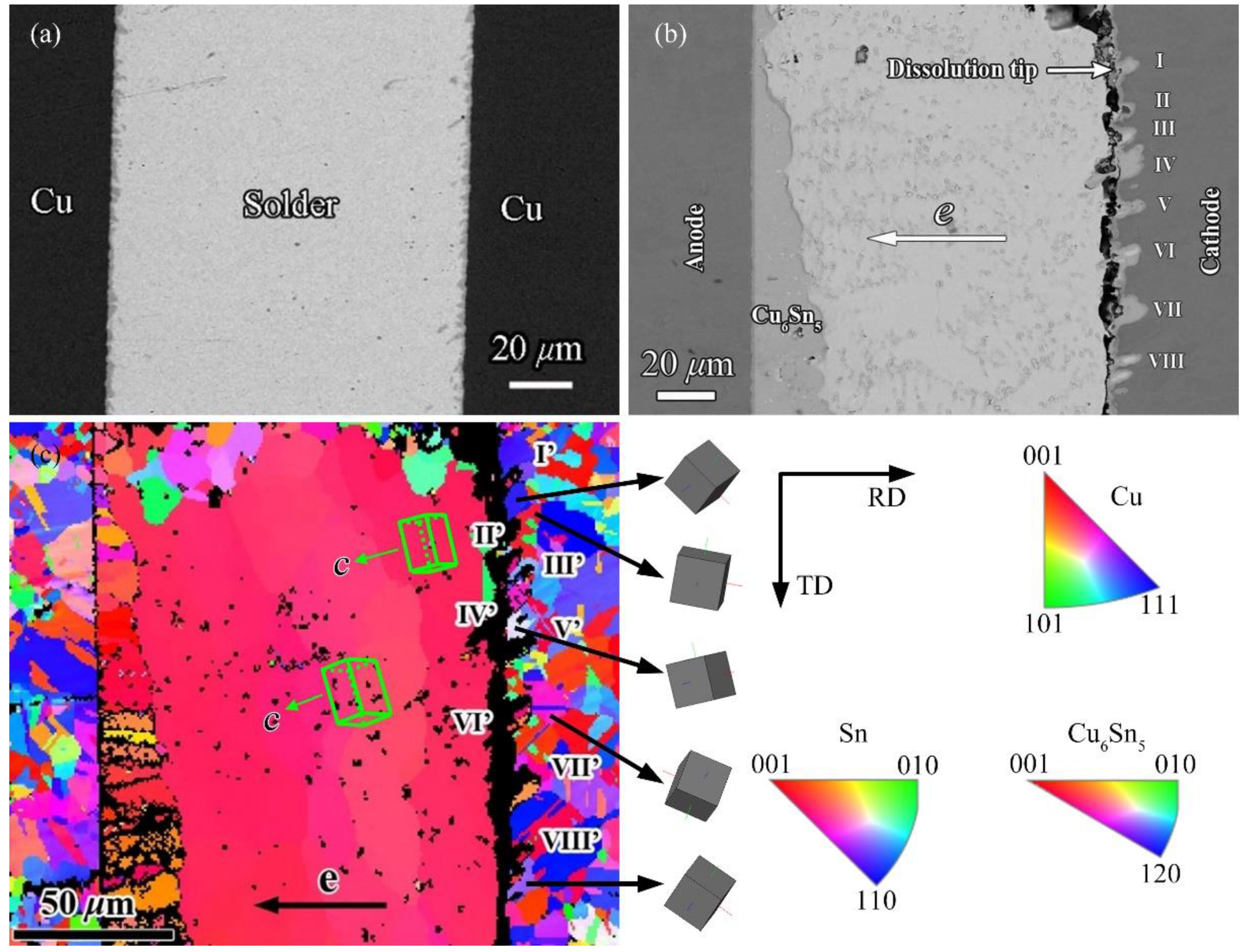
3.1. Realization of Crystallographic Characteristic of Cu Substrate and Cathode Dissolution
3.2. Mechanism of Crystallographic Characteristic Effect of Cu Substrate on Serrated Cathode Dissolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brandeburg, S. Electromigration studies of flip chip bump solder joints. In Proceedings of the Surface Mount International Conference and Exposition, Edina, MN, USA, 23–27 August 1998; pp. 337–343. [Google Scholar]
- Chan, Y.C.; Yang, D. Failure mechanisms of solder interconnects under current stressing in advanced electronic packages. Prog. Mater. Sci. 2010, 55, 428–475. [Google Scholar] [CrossRef]
- Tu, K.N. Recent advances on electromigration in very-large-scale-integration of interconnects. J. Appl. Phys. 2003, 94, 5451–5473. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, S.B.; Wang, J.H. New challenges of miniaturization of electronic devices: Electromigration and thermomigration in lead-free solder joints. Mater. Des. 2020, 192, 108726. [Google Scholar] [CrossRef]
- An, R.; Tian, Y.H.; Zhang, R.; Wang, C.Q. Electromigration-induced intermetallic growth and voids formation in symmetrical Cu/Sn/Cu and Cu/Intermetallic compounds (IMCs)/Cu joints. J. Mater. Sci. Mater. Electron. 2015, 26, 2674–2681. [Google Scholar] [CrossRef]
- Gan, H.; Tu, K.N. Polarity effect of electromigration on kinetics of intermetallic compound formation in Pb-free solder V-groove samples. J. Appl. Phys. 2005, 97, 63514. [Google Scholar] [CrossRef]
- Ouyang, F.Y.; Chen, K.; Tu, K.N.; Lai, Y.S. Effect of current crowding on whisker growth at the anode in flip chip solder joints. Appl. Phys. Lett. 2007, 91, 231919. [Google Scholar] [CrossRef]
- Ren, F.; Nah, J.W.; Tu, K.N.; Xiong, B.S.; Xu, L.H.; Pang, J.H. Electromigration induced ductile-to-brittle transition in lead-free solder joints. Appl. Phys. Lett. 2006, 89, 141914. [Google Scholar] [CrossRef]
- Xie, H.X.; Friedman, D.; Mirpuri, K.; Chawla, N. Electromigration Damage Characterization in Sn-3.9Ag-0.7Cu and Sn-3.9Ag-0.7Cu-0.5Ce Solder Joints by Three-Dimensional X-ray Tomography and Scanning Electron Microscopy. J. Electron. Mater. 2014, 43, 33–42. [Google Scholar] [CrossRef]
- Zhang, X.F.; Guo, J.D.; Shang, J.K. Abnormal polarity effect of electromigration on intermetallic compound formation in Sn–9Zn solder interconnect. Scr. Mater. 2007, 57, 513–516. [Google Scholar] [CrossRef]
- Chiu, S.H.; Shao, T.L.; Chen, C.; Yao, D.J.; Hsu, C.Y. Infrared microscopy of hot spots induced by Joule heating in flip-chip SnAg solder joints under accelerated electromigration. Appl. Phys. Lett. 2006, 88, 022110. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.L.; Zhou, Q.; Zhao, N.; Zhang, Z.J. Abnormal Diffusion Behavior of Zn in Cu/Sn-9 wt.%Zn/Cu Interconnects During Liquid–Solid Electromigration. J. Electron. Mater. 2013, 42, 2975–2982. [Google Scholar] [CrossRef]
- Liang, S.B.; Ke, C.B.; Huang, J.Q.; Zhou, M.B.; Zhang, X.P. Phase field simulation of microstructural evolution and thermomigration-induced phase segregation in Cu/Sn58Bi/Cu interconnects under isothermal aging and temperature gradient. Microelectron. Reliab. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Kim, J.; Jung, K.H.; Kim, J.H.; Lee, C.J.; Jung, S.B. Electromigration behaviors of Sn58%Bi solder containing Ag-coated MWCNTs with OSP surface finished PCB. J. Alloys. Compd. 2019, 775, 581–588. [Google Scholar] [CrossRef]
- Ma, L.M.; Xu, G.C.; Sun, J.; Guo, F.; Wang, X.T. Effects of Co additions on electromigration behaviors in Sn–3.0Ag–0.5Cu-based solder joint. J. Mater. Sci. 2011, 46, 4896–4905. [Google Scholar] [CrossRef]
- Yoon, J.W.; Back, J.H.; Jung, S.B. Comparative study of ENEPIG and thin ENEPIG as surface finishes for SAC305 solder joints. J. Mater. Sci. Mater. Electron. 2018, 29, 4724–4731. [Google Scholar] [CrossRef]
- Yue, W.; Qin, H.B.; Zhou, M.B.; Ma, X.; Zhang, X.P. Electromigration induced microstructure evolution and damage in asymmetric Cu/Sn-58Bi/Cu solder interconnect under current stressing. Trans. Nonferrous Met. Soc. China 2014, 24, 1619–1628. [Google Scholar] [CrossRef]
- Huang, T.C.; Yang, T.L.; Ke, J.H.; Hsueh, C.H.; Kao, C.R. Effects of Sn grain orientation on substrate dissolution and intermetallic precipitation in solder joints under electron current stressing. Scr. Mater. 2014, 80, 37–40. [Google Scholar] [CrossRef]
- Wei, S.; Ma, H.C.; Chen, J.Q.; Guo, J.D. Extreme anisotropy of electromigration: Nickel in single-crystal tin. J. Alloys Compd. 2016, 687, 999–1003. [Google Scholar] [CrossRef]
- Ke, J.H.; Chuang, H.Y.; Shih, W.L.; Kao, C.R. Mechanism for serrated cathode dissolution in Cu/Sn/Cu interconnect under electron current stressing. Acta Mater. 2012, 60, 2082–2090. [Google Scholar] [CrossRef]
- Kinney, C.; Linares, X.; Lee, K.O.; Morris, J.W. The Effect of Sn Orientation on Intermetallic Compound Growth in Idealized Sn-Cu-Ag Interconnects. J. Electron. Mater. 2013, 42, 607–615. [Google Scholar] [CrossRef]
- Liu, C.Y.; Ke, L.; Chuang, Y.C.; Wang, S.J. Study of electromigration-induced Cu consumption in the flip-chip Sn/Cu solder bumps. J. Appl. Phys. 2006, 100, 83702. [Google Scholar] [CrossRef]
- Lu, M.H.; Shih, D.Y.; Lauro, P.; Goldsmith, C.; Henderson, D.W. Effect of Sn grain orientation on electromigration degradation mechanism in high Sn-based Pb-free solders. Appl. Phys. Lett. 2008, 92, 211909. [Google Scholar] [CrossRef]
- Wang, C.H.; Kuo, C.Y.; Chen, H.H.; Chen, S.W. Effects of current density and temperature on Sn/Ni interfacial reactions under current stressing. Intermetallics 2011, 19, 75–80. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chiu, T.C.; Lin, K.L.; Wu, A.T.; Jang, W.L.; Dong, C.L.; Lee, H.Y. Anisotropic dissolution behavior of the second phase in SnCu solder alloys under current stress. Scr. Mater. 2013, 68, 317–320. [Google Scholar] [CrossRef]
- Huang, M.L.; Zhao, J.F.; Zhang, Z.J.; Zhao, N. Dominant effect of high anisotropy in β-Sn grain on electromigration-induced failure mechanism in Sn-3.0Ag-0.5Cu interconnect. J. Alloys Compd. 2016, 678, 370–374. [Google Scholar] [CrossRef]
- Shen, Y.A.; Chen, C. Effect of Sn grain orientation on formation of Cu6Sn5 intermetallic compounds during electromigration. Scr. Mater. 2017, 128, 6–9. [Google Scholar] [CrossRef]
- Sukharev, V.; Zschech, E.; Nix, W.D. A model for electromigration-induced degradation mechanisms in dual-inlaid copper interconnects: Effect of microstructure. J. Appl. Phys. 2007, 102, 53505. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Mat. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Yue, W. A Study of Influence of Configuration and Microstructure Inhomogeneity on Electromigration Behavior of Microscale Lead-Free Solder Interconnects. Ph.D. Thesis, South China University of Technology, Guang Zhou, China, 2014. [Google Scholar]
- Giannuzzi, L.A.; Stevie, F.A. (Eds.) Introduction to Focused Ion Beams: Instrumentation, theory, Techniques and Practice; Springer Science + Business Media Inc.: New York, NY, USA, 2009; pp. 1–712. [Google Scholar] [CrossRef]
- Shang, P.J.; Liu, Z.Q.; Pang, X.Y.; Li, D.X.; Shang, J.K. Growth mechanisms of Cu3Sn on polycrystalline and single crystalline Cu substrates. Acta Mater. 2009, 57, 4697–4706. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, M.L. Anomalous growth of interfacial intermetallic compounds on (111)-oriented nanotwinned Cu substrate. J. Alloys Compd. 2020, 858, 158340. [Google Scholar] [CrossRef]


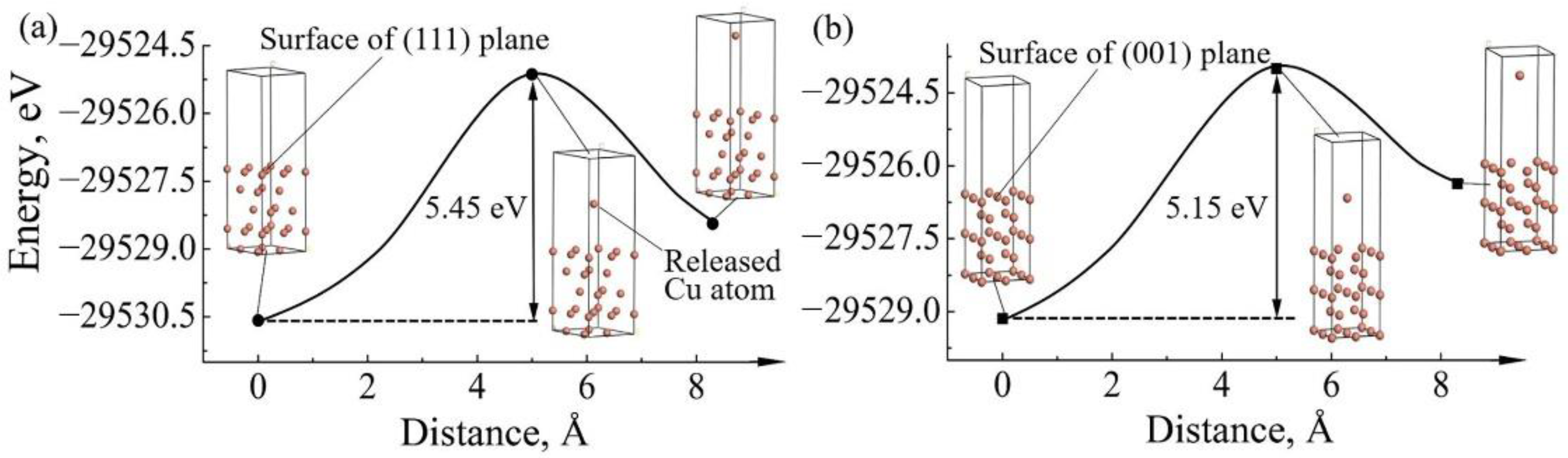
| Plane | A (10−20 m2) | Eslab (eV) | N | Ebulk (eV) | Esurf (J/m2) |
|---|---|---|---|---|---|
| (111) | 5.3543 | −10,335.3243 | 7 | −1476.6475 | 1.8077 |
| (001) | 6.1826 | −10,335.0060 | 7 | −1476.6475 | 1.9779 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, W.; Ding, C.; Qin, H.; Gong, C.; Zhang, J. Crystallographic Characteristic Effect of Cu Substrate on Serrated Cathode Dissolution in Cu/Sn–3.0Ag–0.5Cu/Cu Solder Joints during Electromigration. Materials 2021, 14, 2486. https://doi.org/10.3390/ma14102486
Yue W, Ding C, Qin H, Gong C, Zhang J. Crystallographic Characteristic Effect of Cu Substrate on Serrated Cathode Dissolution in Cu/Sn–3.0Ag–0.5Cu/Cu Solder Joints during Electromigration. Materials. 2021; 14(10):2486. https://doi.org/10.3390/ma14102486
Chicago/Turabian StyleYue, Wu, Chao Ding, Hongbo Qin, Chenggong Gong, and Junxi Zhang. 2021. "Crystallographic Characteristic Effect of Cu Substrate on Serrated Cathode Dissolution in Cu/Sn–3.0Ag–0.5Cu/Cu Solder Joints during Electromigration" Materials 14, no. 10: 2486. https://doi.org/10.3390/ma14102486






