Solution Processed PVB/Mica Flake Coatings for the Encapsulation of Organic Solar Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of Protective Layer
2.2.2. Preparation of P3HT Films for Degradation Test
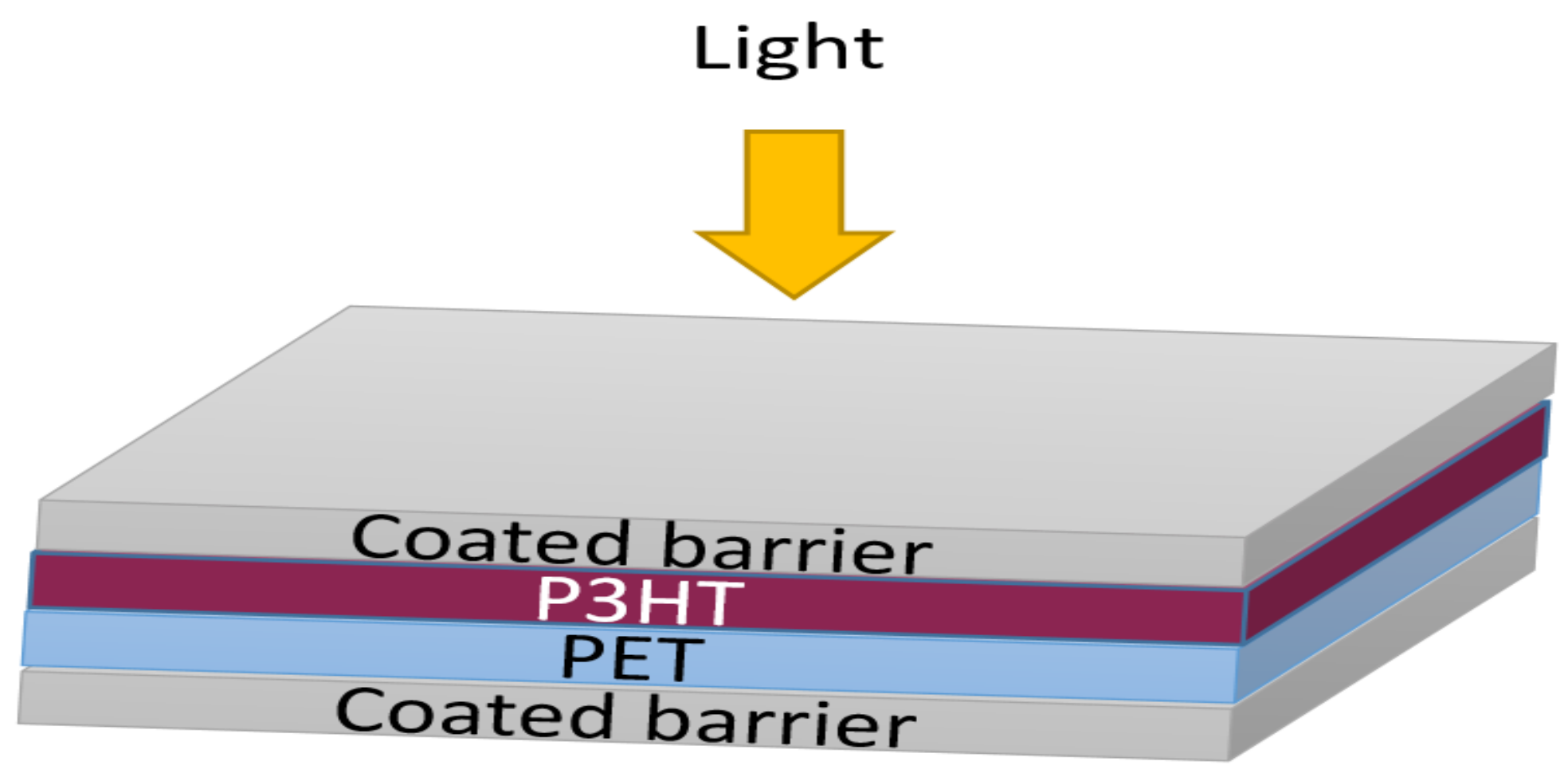
2.2.3. Encapsulation of Organic Photovoltaic Devices
2.3. Characterization
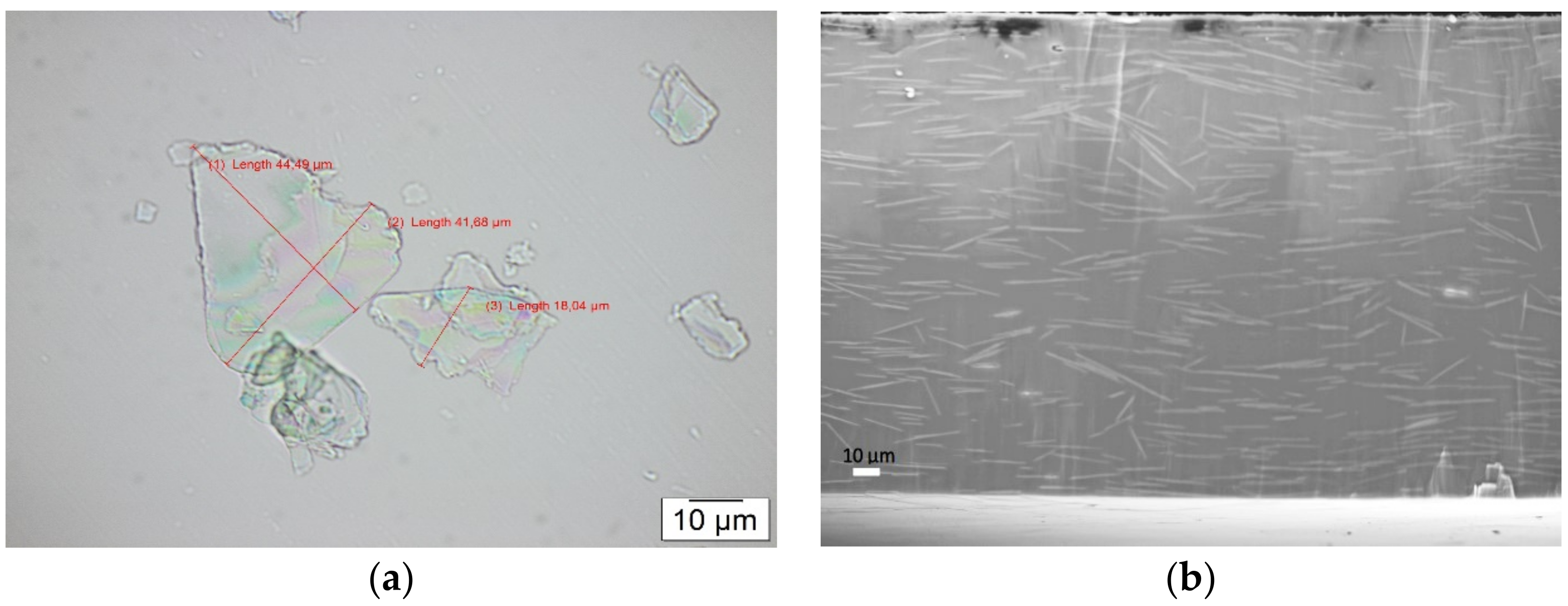
2.3.1. Microscopic Analysis
2.3.2. Spectroscopic Analysis
2.3.3. Permeability Measurements
2.3.4. Bending of the Barrier Layers
2.3.5. SEM Images
2.3.6. Accelerated Lifetime Experiments
3. Results and Discussion
3.1. Optimization of PVB/Mica Flakes Layers
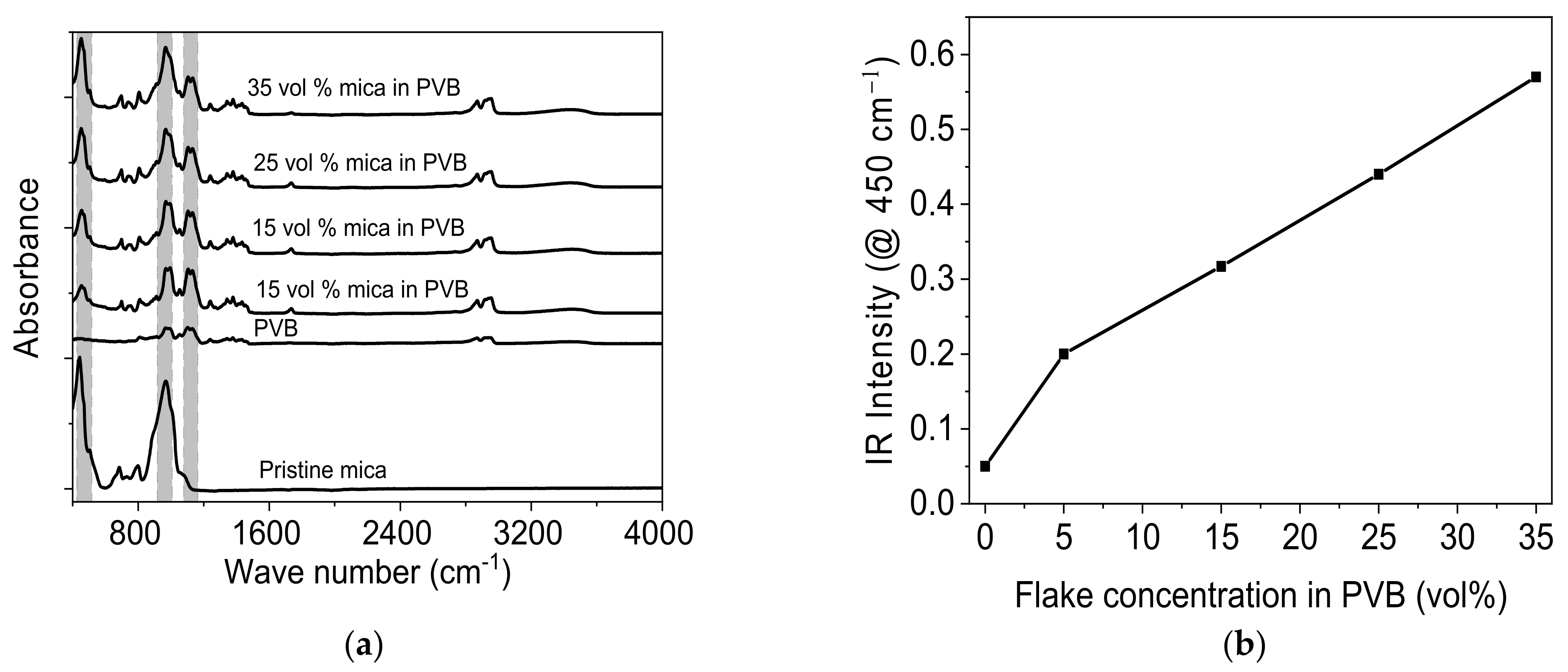
3.1.1. Analysis of PVB/Mica Coating
3.1.2. FTIR Analysis of Prepared Composite
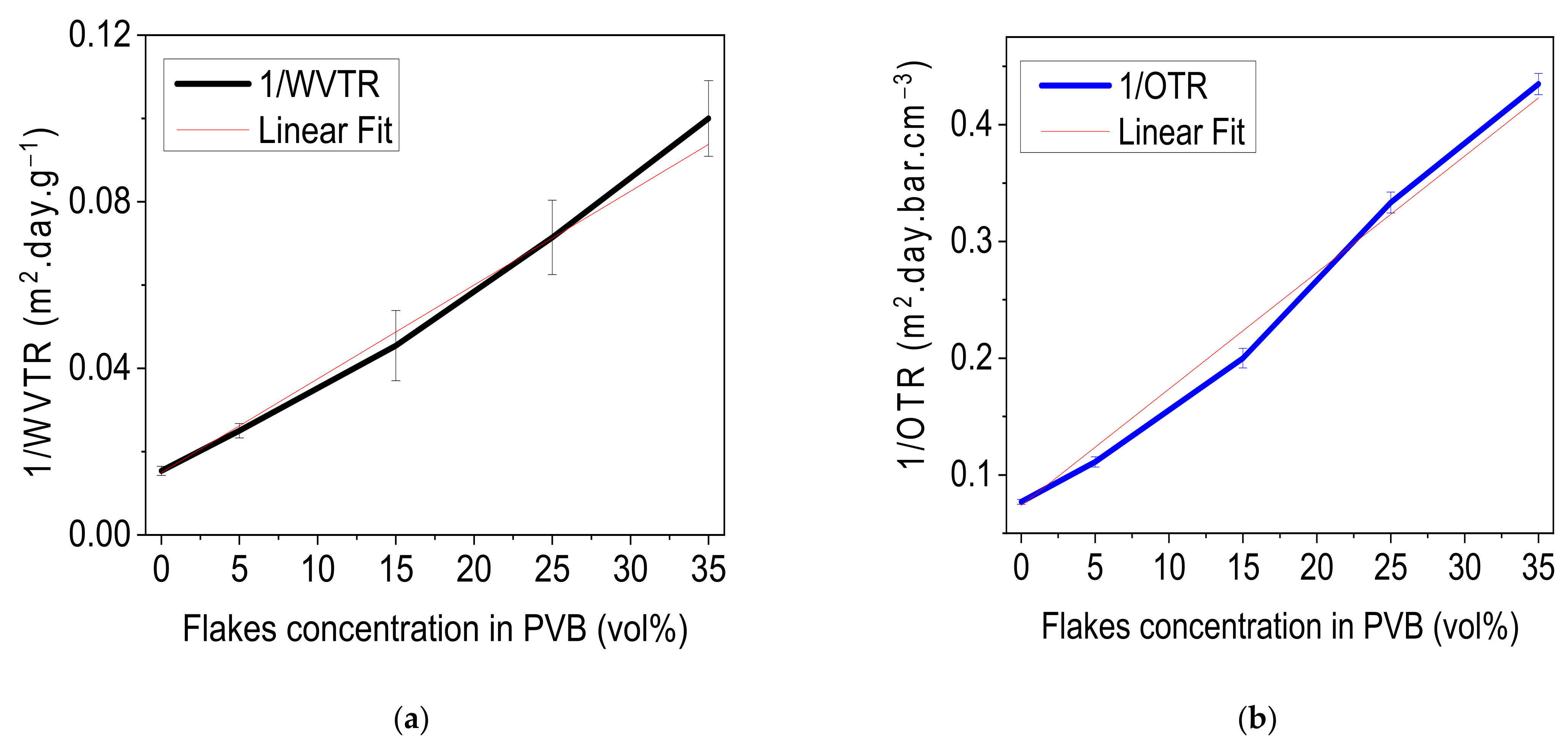
3.1.3. Barrier Characteristics of Layers
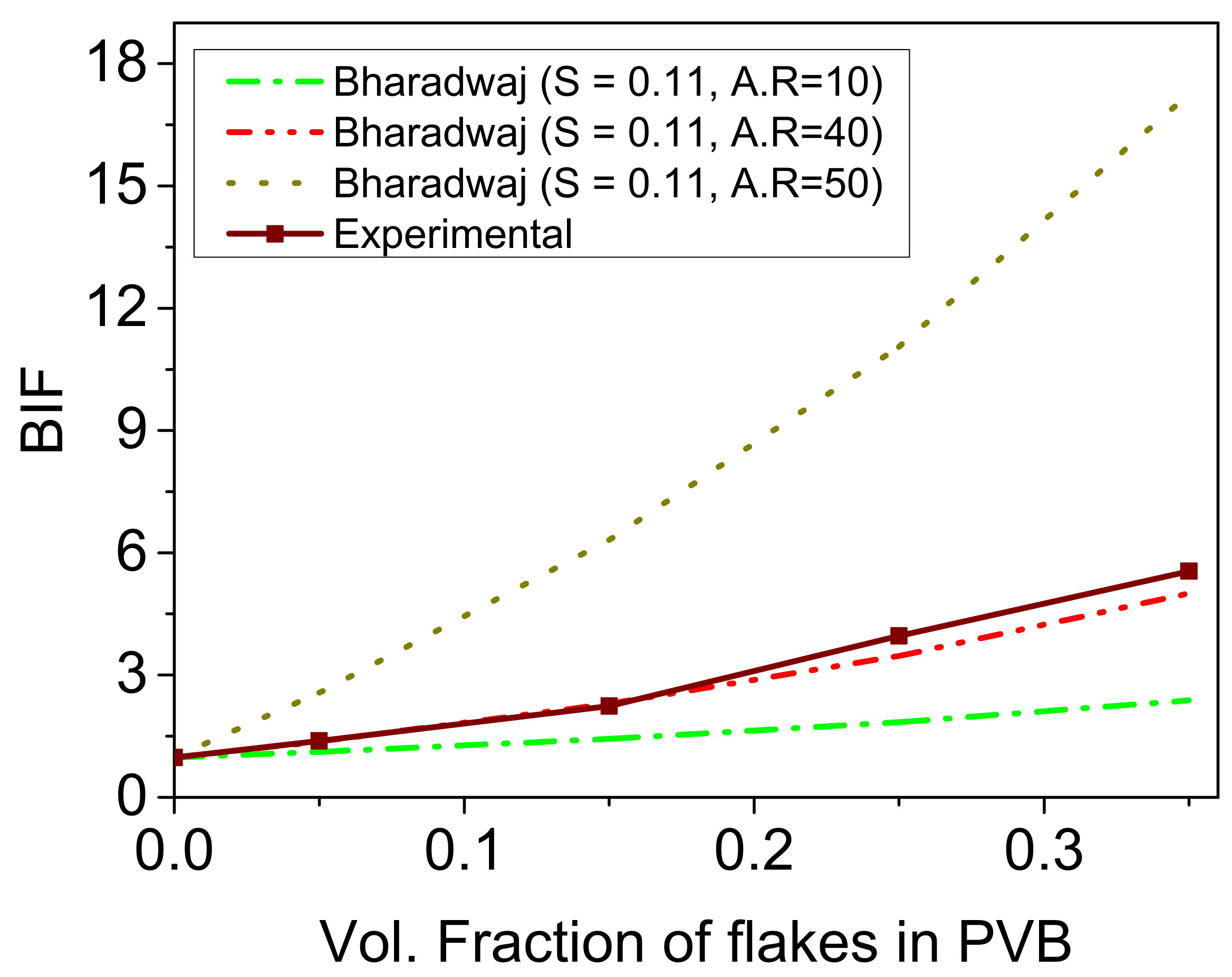
3.1.4. Validation of Barrier Characteristics
3.1.5. Transparency of the Layers
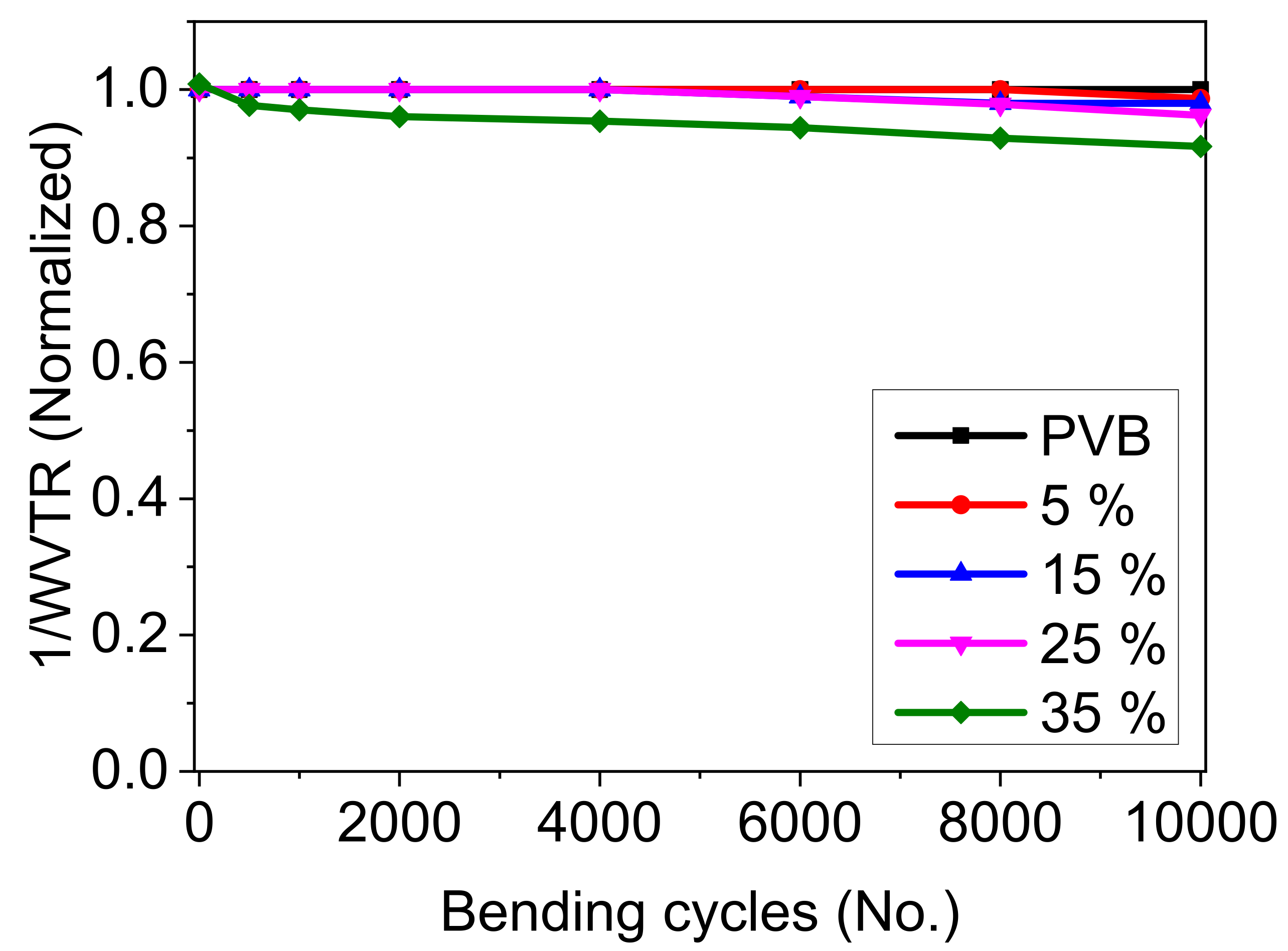
3.1.6. Bendability of the PVB/Mica Layers
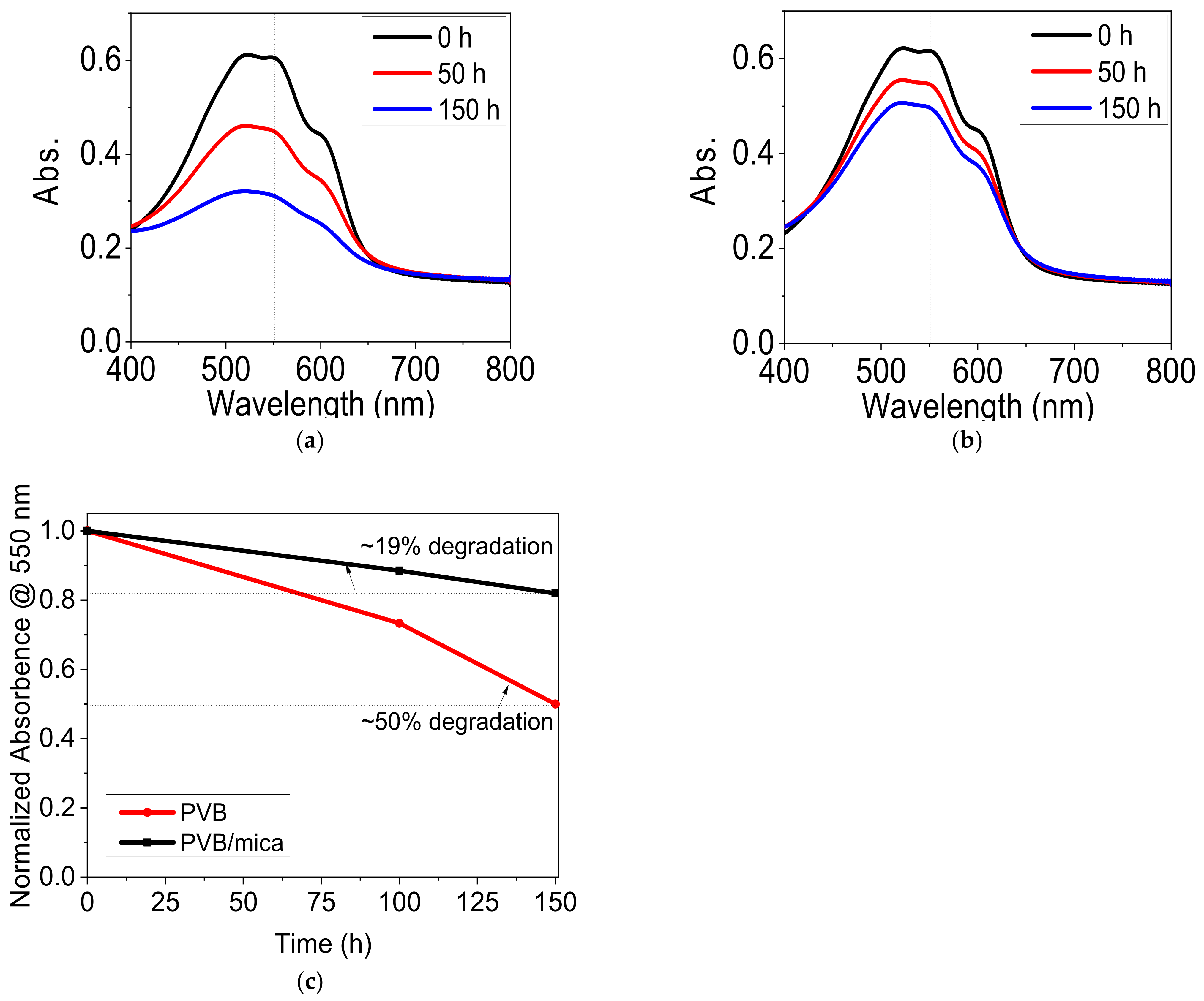
3.2. Encapsulation of P3HT Films
3.3. Encapsulation Organic Solar Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mo, C.; Yuan, W.; Lei, W.; Shijiu, Y. Effects of Temperature and Humidity on the Barrier Properties of Biaxially-oriented Polypropylene and Polyvinyl Alcohol Films. J. Appl. Packag. Res. 2014, 6, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Seemann, A.; Egelhaaf, H.-J.; Brabec, C.J.; Hauch, J.A. Influence of oxygen on semi-transparent organic solar cells with gas permeable electrodes. Org. Electron. 2009, 10, 1424–1428. [Google Scholar] [CrossRef]
- Seemann, A.; Sauermann, T.; Lungenschmied, C.; Armbruster, O.; Bauer, S.; Egelhaaf, H.-J.; Hauch, J. Reversible and irreversible degradation of organic solar cell performance by oxygen. Sol. Energy 2011, 85, 1238–1249. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Zaiser, M.; Brabec, C.J.; Egelhaaf, H. Thin Film Encapsulation of Organic Solar Cells by Direct Deposition of Polysilazanes from Solution. Adv. Energy Mater. 2019, 1900598. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, H.-P.; Kim, H.-M.; Youn, J.-H.; Nam, D.-H.; Lee, Y.-G.; Lee, J.-G.; Mohd, Y.A.R.; Jang, J. Solution processed encapsulation for organic photovoltaics. Sol. Energy Mater. Sol. Cells. 2013, 111, 97–101. [Google Scholar] [CrossRef]
- Hauch, J.A.; Schilinsky, P.; Choulis, S.A.; Rajoelson, S.; Brabec, C.J. The impact of water vapor transmission rate on the lifetime of flexible polymer solar cells. Appl. Phys. Lett. 2008, 93, 10–13. [Google Scholar] [CrossRef]
- Adams, J.; Salvador, M.; Lucera, L.; Langner, S.; Spyropoulos, G.D.; Fecher, F.W.; Voigt, M.M.; Dowland, S.A.; Osvet, A.; Egelhaaf, H.-J.; et al. Water Ingress in Encapsulated Inverted Organic Solar Cells: Correlating Infrared Imaging and Photovoltaic Performance. Adv. Energy Mater. 2015, 5, 1501065. [Google Scholar] [CrossRef]
- Hepp, J.; Machui, F.; Egelhaaf, H.-J.; Brabec, C.J.; Vetter, A. Automatized analysis of IR-images of photovoltaic modules and its use for quality control of solar cells. Energy Sci. Eng. 2016, 4, 363–371. [Google Scholar] [CrossRef]
- Channa, I.A. Development of Solution Processed Thin Film Barriers for Encapsulating Thin Film Electronics Entwicklung von lösungsprozessierten Dünnschichtbarrieren für die Verpackung von Dünnschichtelektronik. 2019. Available online: https://opus4.kobv.de/opus4-fau/frontdoor/index/index/year/2019/docId/12583 (accessed on 1 April 2021).
- Ahmad, J.; Bazaka, K.; Anderson, L.J.; White, R.D.; Jacob, M.V. Materials and methods for encapsulation of OPV: A review. Renew. Sustain. Energy Rev. 2013, 27, 104–117. [Google Scholar] [CrossRef]
- Uddin, A.; Upama, M.B.; Yi, H.; Duan, L. Encapsulation of organic and perovskite solar cells: A review. Coatings 2019, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Bazaka, K.; Ahmad, J.; Oelgemöller, M.; Uddin, A.; Jacob, M.V. Photostability of plasma polymerized γ-terpinene thin films for encapsulation of OPV. Sci. Rep. 2017, 7, 45599. [Google Scholar] [CrossRef] [Green Version]
- Majid, I.; Ahmad Nayik, G.; Mohammad Dar, S.; Nanda, V. Novel food packaging technologies: Innovations and future prospective. J. Saudi. Soc. Agric. Sci. 2016, 17, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Duan, L.; Uddin, A. Progress in Stability of Organic Solar Cells. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Sanz, Y.E.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Mokwena, K.K.; Tang, J. Ethylene Vinyl Alcohol: A Review of Barrier Properties for Packaging Shelf Stable Foods. Crit Rev. Food Sci Nutr. 2012, 52, 640–650. [Google Scholar] [CrossRef]
- Hammann, F.; Schmid, M. Determination and Quantification of Molecular Interactions in Protein Films: A Review. Materials 2014, 7, 7975–7996. [Google Scholar] [CrossRef] [Green Version]
- Figura, L.O.; Teixeira, A.A. Food Physics, Physical Proerties-Measurements and Applicartions; Springer: Berlin/Heidelberg, Germany, 2008; Volume 39. [Google Scholar]
- Siracusa, V.; Ingrao, C.; Lo Giudice, A.; Mbohwa, C.; Dalla Rosa, M. Environmental assessment of a multilayer polymer bag for food packaging and preservation: An LCA approach. Food Res. Int. 2014, 62, 151–161. [Google Scholar] [CrossRef]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of Whey-Protein-Coated Films and Laminates as Novel Recyclable Food Packaging Materials with Excellent Barrier Properties. Int. J. Polym. Sci. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Zillinger, W.; Müller, K.; Sängerlaub, S. Permeation of water vapour, nitrogen, oxygen and carbon dioxide through whey protein isolate based films and coatings—Permselectivity and activation energy. Food Packag. Shelf. Life 2015, 6 (Suppl. C), 21–29. [Google Scholar] [CrossRef]
- Reig, C.S.; Lopez, A.D.; Ramos, M.H.; Ballester, V.A.C. Nanomaterials: A Map for Their Selection in Food Packaging Applications: Nanomaterials: A map for food packaging applications. Packag. Technol. Sci. 2014, 27, 839–866. [Google Scholar] [CrossRef]
- Seethamraju, S.; Ramamurthy, P.C.; Madras, G. Ionomer based blend as water vapor barrier material for organic device encapsulation. ACS Applied Materials & Interfaces. 2013, 5, 4409–4416. [Google Scholar] [CrossRef]
- Yeo, J.H.; Lee, C.H.; Park, C.-S.; Lee, K.-J.; Nam, J.-D.; Kim, S.W. Rheological, morphological, mechanical, and barrier properties of PP/EVOH blends. Adv. Polym. Technol. 2001, 20, 191–201. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Iacovidou, E. Closing the loop on plastic packaging materials: What is quality and how does it affect their circularity? Sci. Total Environ. 2018, 630, 1394–1400. [Google Scholar] [CrossRef]
- Gaume, J.; Taviot-Gueho, C.; Cros, S.; Rivaton, A.; Thérias, S.; Gardette, J.L. Optimization of PVA clay nanocomposite for ultra-barrier multilayer encapsulation of organic solar cells. Sol. Energy Mater. Sol. Cells. 2012, 99, 240–249. [Google Scholar] [CrossRef]
- Cooksey, K. Important Factors for Selecting Food Packaging Materials Based on Permeability. In Proceedings of the Flexible Packaging Conference, Atlanta, GA, USA, 24–26 March 2004; pp. 1–12. [Google Scholar]
- Strawhecker, K.E.; Manias, E. Nanocomposites based on water soluble polymers and unmodified smectide clays. Polym. Nanocompos. 2006, 206–233. [Google Scholar] [CrossRef]
- Atai, M.; Solhi, L.; Nodehi, A.; Mirabedini, S.M.; Kasraei, S.; Akbari, K.; Babanzadeh, S. PMMA-grafted nanoclay as novel filler for dental adhesives. Dent. Mater. 2009, 25, 339–347. [Google Scholar] [CrossRef]
- Nikolaidis, A.K.; Achilias, D.S.; Karayannidis, G.P. Synthesis and characterization of PMMA/organomodified montmorillonite nanocomposites prepared by in situ bulk polymerization. Ind. Eng. Chem. Res. 2011, 50, 571–579. [Google Scholar] [CrossRef]
- Seethamraju, S.; Ramamurthy, P.C.; Madras, G. Performance of an ionomer blend-nanocomposite as an effective gas barrier material for organic devices. RSC Adv. 2014, 4, 11176–11187. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Lee, H.; Rhim, J. Effects of clay type and content on mechanical, water barrier and antimicrobial properties of agar-based nanocomposite films. Carbohydr. Polym. 2011, 86, 691–699. [Google Scholar]
- Spanring, J.; Droisner, A.; Erler, B.; Kraxner, M.; Krumlacher, W.; Plessing, A.; Ruplitsch, A.; Skringer, A.; Steiner, A. High performance barrier films for flexible photovoltaic module encapsulation. In Proceedings of the European Photovoltaic Solar Energy Conference, Hamburg, Germany, 21–25 September 2009. [Google Scholar]
- Tsai, T.-Y.; Lin, M.; Li, C.; Chang, C.-W.; Li, C.-C. Morphology and properties of poly(methyl methacrylate)/clay nanocomposites by in-situ solution polymerization. J. Phys. Chem. Solids 2010, 71, 590–594. [Google Scholar] [CrossRef]
- Carosio, F.; Colonna, S.; Fina, A.; Rydzek, G.; Hemmerlé, J.; Jierry, L.; Schaaf, P.; Boulmedais, F. Efficient gas and water vapor barrier properties of thin poly(lactic acid) packaging films: Functionalization with moisture resistant Nafion and clay multilayers. Chem. Mater. 2014, 26, 5459–5466. [Google Scholar] [CrossRef]
- Souza, D.H.S.; Dahmouche, K.; Andrade, C.T.; Dias, M.L. Applied Clay Science Structure, morphology and thermal stability of synthetic fl uorine mica and its organic derivatives. Appl. Clay Sci. 2011, 54, 226–234. [Google Scholar] [CrossRef]
- Souza, D.H.S.; Andrade, C.T.; Dias, M.L. Rheological behavior of poly (lactic acid)/synthetic mica nanocomposites. Mater. Sci. Eng. C 2013, 33, 1795–1799. [Google Scholar] [CrossRef]
- He, Y.; Fan, Y.; Chen, C.; Zhong, F.; Qing, D. Synthesis of mica-multiwalled carbon nanotube (MWCNT) hybrid material and properties of mica-MWCNT/epoxy composites coating research. High. Perform. Polym. 2015, 27, 191–199. [Google Scholar] [CrossRef]
- Kaufman, S.H.; Leidner, J.; Woodhams, R.T.; Xanthos, M. The Preparation and classification of high aspect ratio mica flakes for use in polymer reinforcement. Powder Technol. 1974, 9, 125–133. [Google Scholar] [CrossRef]
- Hajian, M.; Reisi, M.R. Preparation and characterization of Polyvinylbutyral/Graphene. Nanocomposite 2012. [Google Scholar] [CrossRef]
- Ding, R.; Luo, Z.; Ma, X.; Fan, X.; Xue, L.; Lin, X.; Chen, S. High Sensitive Sensor Fabricated by Reduced Graphene Oxide/Polyvinyl Butyral Nanofibers for Detecting Cu (II) in Water. Int. J. Anal. Chem. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- He, X.; Wu, L.L.; Wang, J.J.; Zhang, T.; Sun, H.; Shuai, N. Layer-by-layer assembly deposition of graphene oxide on poly(lactic acid) films to improve the barrier properties. High. Perform. Polym. 2015, 27, 318–325. [Google Scholar] [CrossRef]
- Bharadwaj, R.K. Modeling the barrier properties of polymer-layered silicate nanocomposites. Macromolecules 2001, 34, 9189–9192. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Egelhaaf, H.; Brabec, C.J. Solution Coated Barriers for Flexible Electronics. In Organic Flexible Electronics, Fundamentals, Devices, and Applications; Cosseddu, P., Caironi, M., Eds.; Woodhead Publishing: Cambrige, UK, 2020; Available online: https://www.elsevier.com/books/organic-flexible-electronics/cosseddu/978-0-12-818890-3 (accessed on 1 April 2021).
- Karuthedath, S.; Sauermann, T.; Egelhaaf, H. The effect of oxygen induced degradation on charge carrier dynamics in P3HT: PCBM and Si- PCPDTBT: PCBM thin fi lms and solar cells. J. Mater. Chem. A 2015, 3399–3408. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Channa, I.A.; Chandio, A.D.; Rizwan, M.; Shah, A.A.; Bhatti, J.; Shah, A.K.; Hussain, F.; Shar, M.A.; AlHazaa, A. Solution Processed PVB/Mica Flake Coatings for the Encapsulation of Organic Solar Cells. Materials 2021, 14, 2496. https://doi.org/10.3390/ma14102496
Channa IA, Chandio AD, Rizwan M, Shah AA, Bhatti J, Shah AK, Hussain F, Shar MA, AlHazaa A. Solution Processed PVB/Mica Flake Coatings for the Encapsulation of Organic Solar Cells. Materials. 2021; 14(10):2496. https://doi.org/10.3390/ma14102496
Chicago/Turabian StyleChanna, Iftikhar Ahmed, Ali Dad Chandio, Muhammad Rizwan, Aqeel Ahmed Shah, Jahanzeb Bhatti, Abdul Karim Shah, Fayaz Hussain, Muhammad Ali Shar, and Abdulaziz AlHazaa. 2021. "Solution Processed PVB/Mica Flake Coatings for the Encapsulation of Organic Solar Cells" Materials 14, no. 10: 2496. https://doi.org/10.3390/ma14102496






