Cardiovascular Stents: A Review of Past, Current, and Emerging Devices
Abstract
:1. Introduction
2. Evolution of Cardiovascular Stents
2.1. Bare-Metal Stents
2.2. Drug-Eluting Stents
2.3. Bioresorbable Stents
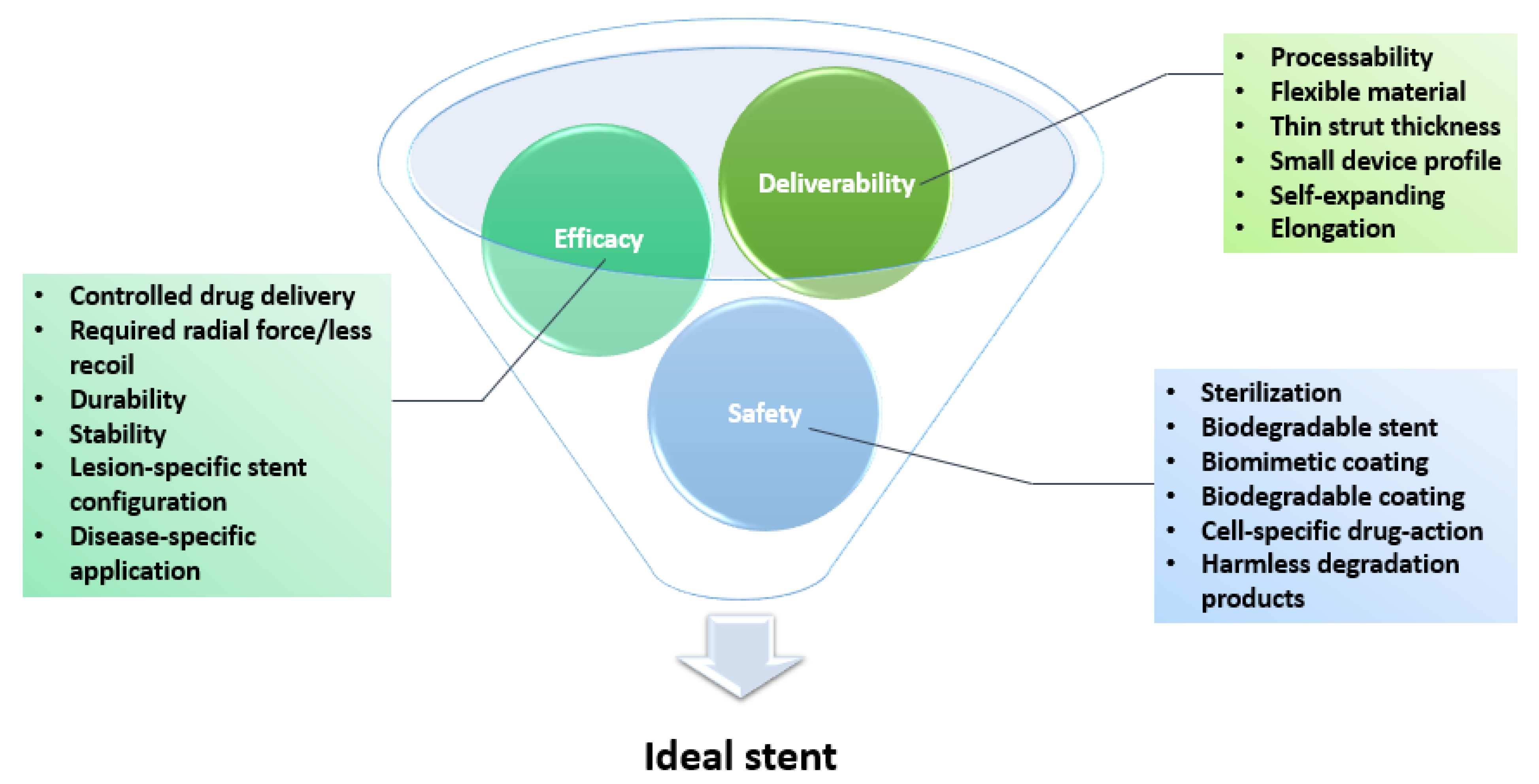
3. Stent Optimization
3.1. Features of an Ideal Stent
3.2. Novel Platforms
3.3. Surface Modifications
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulkarni, P.; Rawtani, D.; Kumar, M.; Lahoti, S.R. Cardiovascular drug delivery: A review on the recent advancements in nanocarrier based drug delivery with a brief emphasis on the novel use of magnetoliposomes and extracellular vesicles and ongoing clinical trial research. J. Drug Deliv. Sci. Technol. 2020, 60, 102029. [Google Scholar] [CrossRef]
- Bukala, J.; Buszman, P.P.; Małachowski, J.; Mazurkiewicz, L.; Sybilski, K. Experimental Tests, FEM Constitutive Modeling and Validation of PLGA Bioresorbable Polymer for Stent Applications. Materials 2020, 13, 2003. [Google Scholar] [CrossRef]
- Morciano, G.; Patergnani, S.; Bonora, M.; Pedriali, G.; Tarocco, A.; Bouhamida, E.; Marchi, S.; Ancora, G.; Anania, G.; Wieckowski, M.R.; et al. Mitophagy in Cardiovascular Diseases. J. Clin. Med. 2020, 9, 892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Synthesis of new azaindeno-acetonitrile derivative with inotropic activity against heart failure model. Biointerface Res. Appl. Chem. 2019, 9, 4598–4604. [CrossRef]
- Microscopic and submicroscopic structure of the heart atria and auricles in condition of the experimental thermal trauma. Biointerface Res. Appl. Chem. 2020, 10, 5237–5242. [CrossRef]
- Ho, M.-Y.; Chen, C.-C.; Wang, C.-Y.; Chang, S.-H.; Hsieh, M.-J.; Lee, C.-H.; Wu, V.C.-C.; Hsieh, I.-C. The Development of Coronary Artery Stents: From Bare-Metal to Bio-Resorbable Types. Metals 2016, 6, 168. [Google Scholar] [CrossRef] [Green Version]
- Canfield, J.; Totary-Jain, H. 40 Years of Percutaneous Coronary Intervention: History and Future Directions. J. Pers. Med. 2018, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease; NCBI: Bethesda, MD, USA, 2020. [Google Scholar]
- Emery, C.; Torreton, E.; Briere, J.-B.; Evers, T.; Fagnani, F. Economic burden of coronary artery disease or peripheral artery disease in patients at high risk of ischemic events in the French setting: A claims database analysis. J. Med. Econ. 2020, 23, 513–520. [Google Scholar] [CrossRef]
- Darba, S.; Safaei, N.; Mahboub–Ahari, A.; Nosratnejad, S.; Alizadeh, G.; Ameri, H.; Yousefi, M. Direct and Indirect Costs Associated with Coronary Artery (Heart) Disease in Tabriz, Iran. Risk Manag. Healthc. Policy 2020, 13, 969–978. [Google Scholar] [CrossRef]
- Duhan, N.; Barak, S.; Mudgil, D. Bioactive Lipids: Chemistry & Health Benefits. Biointerface Res. Appl. Chem. 2020, 10, 6676–6687. [Google Scholar] [CrossRef]
- Khan, W.; Farah, S.; Domb, A.J. Drug eluting stents: Developments and current status. J. Control. Release 2012, 161, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Blake, Y. Biocompatible materials for cardiovascular stents. Zenodo 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Beshchasna, N.; Ho, A.Y.K.; Saqib, M.; Kraśkiewicz, H.; Wasyluk, Ł.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Trusca, R.D.; Ficai, A.; et al. Surface evaluation of titanium oxynitride coatings used for developing layered cardiovascular stents. Mater. Sci. Eng. C 2019, 99, 405–416. [Google Scholar] [CrossRef]
- Choubey, R.K.; Pradhan, S.K. Prediction of strength and radial recoil of various stents using FE analysis. Mater. Today Proc. 2020, 27, 2254–2259. [Google Scholar] [CrossRef]
- Chaparro-Rico, B.D.M.; Sebastiano, F.; Cafolla, D. A Smart Stent for Monitoring Eventual Restenosis: Computational Fluid Dynamic and Finite Element Analysis in Descending Thoracic Aorta. Machines 2020, 8, 81. [Google Scholar] [CrossRef]
- Yelamanchili, V.S.; Hajouli, S. Coronary Artery Stents; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Cockerill, I.; See, C.W.; Young, M.L.; Wang, Y.; Zhu, D. Designing Better Cardiovascular Stent Materials: A Learning Curve. Adv. Funct. Mater. 2021, 31, 2005361. [Google Scholar] [CrossRef]
- Lee, Y.; Veerubhotla, K.; Jeong, M.H.; Lee, C.H. Deep Learning in Personalization of Cardiovascular Stents. J. Cardiovasc. Pharmacol. Ther. 2019, 25, 110–120. [Google Scholar] [CrossRef]
- Arafat, M.; Fouladian, P.; Blencowe, A.; Albrecht, H.; Song, Y.; Garg, S. Drug-Eluting non-Vascular stents for localised drug targeting in obstructive gastrointestinal cancers. J. Control. Release 2019, 308, 209–231. [Google Scholar] [CrossRef]
- Ranade, S.V.; Miller, K.M.; Richard, R.E.; Chan, A.K.; Allen, M.J.; Helmus, M.N. Physical characterization of controlled release of paclitaxel from the TAXUS? Express2? drug-Eluting stent. J. Biomed. Mater. Res. 2004, 71, 625–634. [Google Scholar] [CrossRef]
- Schmidt, T.; Abbott, J.D. Coronary Stents: History, Design, and Construction. J. Clin. Med. 2018, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Ako, J.; Bonneau, H.N.; Honda, Y.; Fitzgerald, P.J. Design Criteria for the Ideal Drug-Eluting Stent. Am. J. Cardiol. 2007, 100, S3–S9. [Google Scholar] [CrossRef]
- Fischell, T.A.; Balmuri, A.; Agarwal, S.; Verhye, S. Integrated Stent Delivery System: A Next Generation of Stent Delivery and Drug-Eluting Stent. Cardiovasc. Revasc. Med. 2020, 21, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, T.; Muller, O.; Heg, D.; Roffi, M.; Kurz, D.J.; Moarof, I.; Weilenmann, D.; Kaiser, C.; Tapponnier, M.; Losdat, S.; et al. Biodegradable-Versus Durable-Polymer Drug-Eluting Stents for STEMI. JACC Cardiovasc. Interv. 2021, 14, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Holmes, D.R. Drug-Eluting Coronary-Artery Stents. N. Engl. J. Med. 2013, 368, 254–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Feng, X.; Shi, Z.; Song, C.; Cui, X.; Zhang, J.; Li, T.; Toft, E.S.; Ge, J.; Wang, L.; et al. Research on elastic recoil and restoration of vessel pulsatility of Zn-Cu biodegradable coronary stents. Biomed. Tech. Eng. 2020, 65, 219–227. [Google Scholar] [CrossRef]
- Iqbal, J.; Gunn, J.; Serruys, P.W. Coronary stents: Historical development, current status and future directions. Br. Med. Bull. 2013, 106, 193–211. [Google Scholar] [CrossRef] [Green Version]
- Montero-Baker, M.; Braun, J.D.; Weinkauf, C.; Leon, L.R. Technological Advances in Endovascular Surgery. In Technological Advances in Surgery, Trauma and Critical Care; Latifi, R., Rhee, P., Gruessner, R.W.G., Eds.; Springer: New York, NY, USA, 2015; pp. 323–354. [Google Scholar]
- Hermawan, H.; Mantovani, D. Process of prototyping coronary stents from biodegradable Fe–Mn alloys. Acta Biomater. 2013, 9, 8585–8592. [Google Scholar] [CrossRef]
- Beshchasna, N.; Saqib, M.; Kraskiewicz, H.; Wasyluk, Ł.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Ghizdavet, Z.; Marin, A.; Ficai, A.; et al. Recent Advances in Manufacturing Innovative Stents. Pharmaceutics 2020, 12, 349. [Google Scholar] [CrossRef] [Green Version]
- Yue, R.; Niu, J.; Li, Y.; Ke, G.; Huang, H.; Pei, J.; Ding, W.; Yuan, G. In Vitro cytocompatibility, hemocompatibility and antibacterial properties of biodegradable Zn-Cu-Fe alloys for cardiovascular stents applications. Mater. Sci. Eng. C 2020, 113, 111007. [Google Scholar] [CrossRef] [PubMed]
- Saraf, A.R.; Yadav, S.P. 2-Fundamentals of bare-metal stents. In Functionalised Cardiovascular Stents; Wall, J.G., Podbielska, H., Wawrzyńska, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–44. [Google Scholar] [CrossRef]
- Azaouzi, M.; Lebaal, N.; Makradi, A.; Belouettar, S. Optimization based simulation of self-expanding Nitinol stent. Mater. Des. 2013, 50, 917–928. [Google Scholar] [CrossRef]
- Mwangi, J.W.; Nguyen, L.T.; Bui, V.D.; Berger, T.; Zeidler, H.; Schubert, A. Nitinol manufacturing and micromachining: A review of processes and their suitability in processing medical-grade nitinol. J. Manuf. Process. 2019, 38, 355–369. [Google Scholar] [CrossRef]
- Schuessler, A.; Bayer, U.; Siekmeyer, G.; Steegmueller, R.; Strobel, M.; Schuessler, A. Manufacturing of stents: Optimize the stent with new manufacturing technologies. In Proceedings of the 5th European Symposium of Vascular Biomaterials ESVB, Strasbourg, France, 17–19 October 2007. [Google Scholar]
- Obayi, C.S.; Tolouei, R.; Mostavan, A.; Paternoster, C.; Turgeon, S.; Okorie, B.A.; Obikwelu, D.O.; Mantovani, D. Effect of grain sizes on mechanical properties and biodegradation behavior of pure iron for cardiovascular stent application. Biomatter 2016, 6, e959874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grogan, J.; Leen, S.; McHugh, P. Comparing coronary stent material performance on a common geometric platform through simulated bench testing. J. Mech. Behav. Biomed. Mater. 2012, 12, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grogan, J.A.; Leen, S.B.; McHugh, P.E. Optimizing the design of a bioabsorbable metal stent using computer simulation methods. Biomaterials 2013, 34, 8049–8060. [Google Scholar] [CrossRef]
- Alicea, L.A.; Aviles, J.I.; López, I.A.; Mulero, L.E.; Sánchez, L.A. Mechanics biomaterials: Stents, 2004. Course Materials in the Department of General Engineering, University of Puerto Rico, Mayaguez. Available online: https://blogs.epfl.ch/stents/documents/biomechanics%20of%20stents.pdf (accessed on 25 March 2021).
- Brailovski, V.; Prokoshkin, S.; Gauthier, M.; Inaekyan, K.; Dubinskiy, S.; Petrzhik, M.; Filonov, M. Bulk and porous metastable beta Ti–Nb–Zr(Ta) alloys for biomedical applications. Mater. Sci. Eng. C 2011, 31, 643–657. [Google Scholar] [CrossRef] [Green Version]
- Poncin, P.; Millet, C.; Chevy, J.; Proft, J.L. Comparing and optimizing Co-Cr tubing for stent applications. In Proceedings of the Material and Processes for Medical Devices Conference, Palm Desert, CA, USA, 25–27 October 2004. [Google Scholar]
- Commandeur, S.; Van Beusekom, H.M.; Van Der Giessen, W.J. Polymers, drug release, and drug-eluting stents. J. Interv. Cardiol. 2006, 19, 500–506. [Google Scholar] [CrossRef]
- Qian, M.; Liu, Q.; Wei, Y.; Guo, Z.; Zhao, Q. In-Situ biotransformation of nitric oxide by functionalized surfaces of cardiovascular stents. Bioact. Mater. 2021, 6, 1464–1467. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Hsieh, M.-J.; Chen, C.-C.; Chang, S.-H.; Wang, C.-Y.; Chen, D.-Y.; Yang, C.-H.; Yeh, J.-K.; Ho, M.-Y.; Hsieh, I.-C. Comparison of 9-Month Angiographic Follow-Up and Long-Term Clinical Outcomes of Biodegradable Polymer Drug-Eluting Stents and Second-Generation Durable Polymer Drug-Eluting Stents in Patients Undergoing Single Coronary Artery Stenting. Acta Cardiol. Sin. 2020, 36, 97–104. [Google Scholar]
- Pendyala, L.K.; Yin, X.; Li, J.; Chen, J.P.; Chronos, N.; Hou, D. The First-Generation Drug-Eluting Stents and Coronary Endothelial Dysfunction. JACC Cardiovasc. Interv. 2009, 2, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Ali, R.M.; Kader, M.A.S.A.; Ahmad, W.A.W.; Ong, T.K.; Liew, H.B.; Omar, A.-F.; Zuhdi, A.S.M.; Nuruddin, A.A.; Schnorr, B.; Scheller, B. Treatment of Coronary Drug-Eluting Stent Restenosis by a Sirolimus- or Paclitaxel-Coated Balloon. JACC Cardiovasc. Interv. 2019, 12, 558–566. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, Y.; Zhu, X.; Miao, L.; Liang, X.; Duan, J.; Li, H.; Tian, X.; Pang, L.; Wei, Y.; et al. Significant difference between sirolimus and paclitaxel nanoparticles in anti-proliferation effect in normoxia and hypoxia: The basis of better selection of atherosclerosis treatment. Bioact. Mater. 2021, 6, 880–889. [Google Scholar] [CrossRef]
- Kobo, O.; Saada, M.; Meisel, S.R.; Hellou, E.; Frimerman, A.; Abu Fanne, R.; Mohsen, J.; Danon, A.; Roguin, A. Modern Stents: Where Are We Going? Rambam Maimonides Med. J. 2020, 11, e0017. [Google Scholar] [CrossRef]
- Kalachev, L.V. Modelling Simple Experimental Platform for In Vitro Study of Drug Elution from Drug Eluting Stents (DES). J. Phys. Conf. Ser. 2016, 727, 12005. [Google Scholar] [CrossRef] [Green Version]
- Byrne, R.A.; Joner, M.; Kastrati, A. Stent thrombosis and restenosis: What have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur. Heart J. 2015, 36, 3320–3331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidlitz, A. Drug-Eluting Stents. In In Vitro Drug Release Testing of Special Dosage Forms; Fotaki, N., Klein, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; Volume 4, pp. 87–117. [Google Scholar] [CrossRef]
- Martin, D.M.; Boyle, F.J. Drug-Eluting stents for coronary artery disease: A review. Med Eng. Phys. 2011, 33, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Vale, N.; Madeira, S.; Almeida, M.; Raposo, L.; Freitas, P.; Castro, M.; Rodrigues, G.; Oliveira, A.; Brito, J.; Leal, S.; et al. Ten-year survival of patients undergoing coronary angioplasty with first-generation sirolimus-eluting stents and bare-metal stents. Rev. Port. Cardiol. 2020, 39, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Sambola, A.; Rello, P.; Soriano, T.; Bhatt, D.L.; Pasupuleti, V.; Cannon, C.P.; Gibson, C.M.; Dewilde, W.J.; Lip, G.Y.; Peterson, E.D.; et al. Safety and efficacy of drug eluting stents vs bare metal stents in patients with atrial fibrillation: A systematic review and meta-analysis. Thromb. Res. 2020, 195, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Lee, S.-H.; Kang, M.; Choi, J.-H. Impact of medication adherence to dual antiplatelet therapy on the long-term outcome of drug-Eluting or bare-Metal stents. PLoS ONE 2020, 15, e0244062. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; Brieger, D.; Dalby, A.J.; Park, S.-J.; Mehran, R. Duration of Dual Antiplatelet Therapy After Coronary Stenting. J. Am. Coll. Cardiol. 2015, 66, 832–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. Circulation 2011, 124, e574–e651. [Google Scholar] [CrossRef] [PubMed]
- Members, T.F.; Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.-P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.W.; Head, S.J.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Yeh, R.W.; Massaro, J.M.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Gershlick, A.H.; Darius, H.; Meredith, I.T.; Ormiston, J.; et al. Antiplatelet Therapy Duration Following Bare Metal or Drug-Eluting Coronary Stents. JAMA 2015, 313, 1113–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, F.; Nakano, M.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathologic Etiologies of Late and Very Late Stent Thrombosis following First-Generation Drug-Eluting Stent Placement. Thrombosis 2012, 2012, 608593. [Google Scholar] [CrossRef] [Green Version]
- De Luca, G.; Smits, P.; Hofma, S.H.; Di Lorenzo, E.; Vlachojannis, G.J.; Hof, A.W.V.; van Boven, A.J.; Kedhi, E.; Stone, G.W.; Suryapranata, H. Everolimus eluting stent vs. first generation drug-Eluting stent in primary angioplasty: A pooled patient-Level meta-Analysis of randomized trials. Int. J. Cardiol. 2017, 244, 121–127. [Google Scholar] [CrossRef]
- Partida, R.A.; Yeh, R.W. Contemporary drug-Eluting stent platforms: Design, safety, and clinical efficacy. Cardiol. Clin. 2017, 35, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Xue, Y.-F.; Chen, T.-T.; Huang, D.-N.; Wang, Y.-X.; Ren, K.-F.; Wang, Y.-B.; Fu, G.-S.; Ji, J. Biodegradable phosphorylcholine copolymer for cardiovascular stent coating. J. Mater. Chem. B 2020, 8, 5361–5368. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Nishi, S.; Ishibashi-Ueda, H. Fabrication of drug-Eluting covered stents with micropores and differential coating of heparin and FK506. Cardiovasc. Radiat. Med. 2003, 4, 77–82. [Google Scholar] [CrossRef]
- Livingston, M.; Tan, A. Coating Techniques and Release Kinetics of Drug-Eluting Stents. J. Med. Devices 2015, 10, 010801. [Google Scholar] [CrossRef] [PubMed]
- Grabow, N.; Schmitt, L.; Pfensig, S.; Reske, T.; Rehme, H.; Senz, V.; Sternberg, K.; Schmitz, K.-P. Spray-Coating process development, manufacture, quality assessment and drug release behavior of peripheral drug-eluting stents. Biomed. Tech. Eng. 2012, 57, 867–868. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Yanamala, C.M.; Huang, W.-Q. Comparing Stent Thrombosis associated with Zotarolimus Eluting Stents versus Everolimus Eluting Stents at 1 year follow up: A systematic review and meta-analysis of 6 randomized controlled trials. BMC Cardiovasc. Disord. 2017, 17, 84. [Google Scholar] [CrossRef] [Green Version]
- Roleder, T.; Kedhi, E.; Berta, B.; Gasior, P.; Wanha, W.; Roleder, M.; Fluder, J.; Smolka, G.; Ochala, A.; Wojakowski, W. Short-term stent coverage of second-Generation zotarolimus-Eluting durable polymer stents: Onyx one-Month optical coherence tomography study. Adv. Interv. Cardiol. 2019, 15, 143–150. [Google Scholar] [CrossRef]
- Kalra, A.; Rehman, H.; Khera, S.; Thyagarajan, B.; Bhatt, D.L.; Kleiman, N.S.; Yeh, R.W. New-Generation Coronary Stents: Current Data and Future Directions. Curr. Atheroscler. Rep. 2017, 19, 14. [Google Scholar] [CrossRef]
- Kawashima, H.; Zocca, P.; Buiten, R.A.; Smits, P.C.; Onuma, Y.; Wykrzykowska, J.J.; De Winter, R.J.; Von Birgelen, C.; Serruys, P.W. The 2010s in clinical drug-Eluting stent and bioresorbable scaffold research: A Dutch perspective. Neth. Heart J. 2020, 28, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.; Iqbal, J.; Trust, S.S.T.H.N.F. Comparison of Contemporary Drug-Eluting Coronary Stents–Is Any Stent Better than the Others? Heart Int. 2020, 14. [Google Scholar] [CrossRef]
- Hwang, C.-W.; Wu, D.; Edelman, E.R. Impact of transport and drug properties on the local pharmacology of drug-Eluting stents. Int. J. Cardiovasc. Interv. 2003, 5, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Escuer, J.; Cebollero, M.; Peña, E.; McGinty, S.; Martínez, M.A. How does stent expansion alter drug transport properties of the arterial wall? J. Mech. Behav. Biomed. Mater. 2020, 104, 103610. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Jinnouchi, H.; Torii, S.; Virmani, R.; Finn, A.V. Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View. Bioengineering 2018, 5, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; Cui, Y.; Zeng, Z.; Wei, L.; Li, L.; Wang, H.; Hu, H.; Liu, T.; Huang, N.; Chen, J.; et al. Heparin/poly-l-lysine nanoplatform with growth factor delivery for surface modification of cardiovascular stents: The influence of vascular endothelial growth factor loading. J. Biomed. Mater. Res. Part A 2020, 108, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Nenna, A.; Larobina, D.; Martuscelli, G.; Singh, S.S.A.; Chello, M.; Ambrosio, L. The Use of Bioactive Polymers for Intervention and Tissue Engineering: The New Frontier for Cardiovascular Therapy. Polymers 2021, 13, 446. [Google Scholar] [CrossRef]
- Aljihmani, L.; Alic, L.; Boudjemline, Y.; Hijazi, Z.M.; Mansoor, B.; Serpedin, E.; Qaraqe, K. Magnesium-Based Bioresorbable Stent Materials: Review of Reviews. J. Bio Tribo Corros. 2019, 5, 26. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Fang, Y.; Sun, W. Design, Characterization, and 3D Printing of Cardiovascular Stents with Zero Poisson’s Ratio in Longitudinal Deformation. Engineering 2020. [Google Scholar] [CrossRef]
- Schieber, R.; Raymond, Y.; Caparrós, C.; Bou, J.; Acero, E.H.; Guebitz, G.; Canal, C.; Pegueroles, M. Functionalization Strategies and Fabrication of Solvent-Cast PLLA for Bioresorbable Stents. Appl. Sci. 2021, 11, 1478. [Google Scholar] [CrossRef]
- Waksman, R. Biodegradable stents: They do their job and disappear. J. Invasive Cardiol. 2006, 18, 70–74. [Google Scholar]
- Toong, D.W.Y.; Ng, J.C.K.; Huang, Y.; Wong, P.E.H.; Leo, H.L.; Venkatraman, S.S.; Ang, H.Y. Bioresorbable metals in cardiovascular stents: Material insights and progress. Materialia 2020, 12, 100727. [Google Scholar] [CrossRef]
- Omar, W.A.; Kumbhani, D.J. The Current Literature on Bioabsorbable Stents: A Review. Curr. Atheroscler. Rep. 2019, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Su, Y.; Qin, Y.-X.; Zheng, Y.; Wang, Y.; Zhu, D. Evolution of metallic cardiovascular stent materials: A comparative study among stainless steel, magnesium and zinc. Biomaterials 2020, 230, 119641. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Tiasha, T.; Shanov, V.N.; Yun, Y. Expandable Mg-Based Helical Stent Assessment using Static, Dynamic, and Porcine Ex Vivo Models. Sci. Rep. 2017, 7, 1173. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Giridharan, V.; Shanov, V.; Xu, Z.; Collins, B.; White, L.; Jang, Y.; Sankar, J.; Huang, N.; Yun, Y. Flow-Induced corrosion behavior of absorbable magnesium-Based stents. Acta Biomater. 2014, 10, 5213–5223. [Google Scholar] [CrossRef]
- Paryab, N.; Cronin, D.; Lee-Sullivan, P.; Ying, X.; Boey, F.Y.C.; Venkatraman, S.; Venkatraman, S. Uniform Expansion of a Polymeric Helical Stent. J. Med. Devices 2012, 6, 021012. [Google Scholar] [CrossRef]
- Su, S.-H.; Chao, R.Y.N.; Landau, C.L.; Nelson, K.D.; Timmons, R.B.; Meidell, R.S.; Eberhart, R.C. Expandable bioresorbable endovascular stent. I. Fabrication and properties. Ann. Biomed. Eng. 2003, 31, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Sun, J.; Xue, W.; Wang, F.; King, M.W.; Yu, C.; Jiao, Y.; Sun, K.; Wang, L. Development of a polycaprolactone/poly(p-dioxanone) bioresorbable stent with mechanically self-reinforced structure for congenital heart disease treatment. Bioact. Mater. 2021, 6, 2969–2982. [Google Scholar] [CrossRef]
- Sharma, U.; Concagh, D.; Core, L.; Kuang, Y.; You, C.; Pham, Q.; Zugates, G.; Busold, R.; Webber, S.; Merlo, J.; et al. The development of bioresorbable composite polymeric implants with high mechanical strength. Nat. Mater. 2017, 17, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Xue, W.; Wang, F.; Sun, J.; Lin, J.; Liu, L.; Sun, K.; Wang, L. Braided bioresorbable cardiovascular stents mechanically reinforced by axial runners. J. Mech. Behav. Biomed. Mater. 2019, 89, 19–32. [Google Scholar] [CrossRef]
- Han, X.; Wu, X.; Kelly, M.; Chen, X. Fabrication and Optimal Design of Biodegradable Polymeric Stents for Aneurysms Treatments. J. Funct. Biomater. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Park, S.A.; Lee, S.J.; Lim, K.S.; Bae, I.H.; Lee, J.H.; Kim, W.D.; Jeong, M.H.; Park, J.-K. In Vivo evaluation and characterization of a bio-Absorbable drug-Coated stent fabricated using a 3D-Printing system. Mater. Lett. 2015, 141, 355–358. [Google Scholar] [CrossRef]
- Van Lith, R.; Baker, E.; Ware, H.; Yang, J.; Farsheed, A.C.; Sun, C.; Ameer, G. 3D-Printing Strong High-Resolution Antioxidant Bioresorbable Vascular Stents. Adv. Mater. Technol. 2016, 1, 1600138. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, K.; Li, Y.; Chen, H.; Hu, Q. Combining 3D Printing and Electrospinning for the Fabrication of a Bioabsorbable Poly-p-dioxanone Stent. Adv. Transdiscipl. Eng. 2014, 1, 343–350. [Google Scholar]
- Lee, C.-H.; Hsieh, M.-J.; Liu, S.-C.; Chen, J.-K.; Liu, S.-J.; Hsieh, I.-C.; Wen, M.-S.; Hung, K.-C. Novel bifurcation stents coated with bioabsorbable nanofibers with extended and controlled release of rosuvastatin and paclitaxel. Mater. Sci. Eng. C 2018, 88, 61–69. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Loffredo, S.; Demir, A.; Previtali, B.; Mantovani, D.; Beanland, R.; Vedani, M. Novel Zn-Based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef]
- Flege, C.; Vogt, F.; Höges, S.; Jauer, L.; Borinski, M.; Schulte, V.A.; Hoffmann, R.; Poprawe, R.; Meiners, W.; Jobmann, M.; et al. Development and characterization of a coronary polylactic acid stent prototype generated by selective laser melting. J. Mater. Sci. Mater. Electron. 2013, 24, 241–255. [Google Scholar] [CrossRef]
- Ang, H.Y.; Huang, Y.Y.; Lim, S.T.; Wong, P.; Joner, M.; Foin, N. Mechanical behavior of polymer-based vs. metallic-based bioresorbable stents. J. Thorac. Dis. 2017, 9, S923–S934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montava-Jorda, S.; Chacon, V.; Lascano, D.; Sanchez-Nacher, L.; Montanes, N. Manufacturing and Characterization of Functionalized Aliphatic Polyester from Poly(lactic acid) with Halloysite Nanotubes. Polymers 2019, 11, 1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Fang, G.; Zhao, Y.-H.; Zhou, J. Improvement of Mechanical Performance of Bioresorbable Magnesium Alloy Coronary Artery Stents through Stent Pattern Redesign. Appl. Sci. 2018, 8, 2461. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.-Y.; Chen, Y.-C.; Liang, T.-H.; Cai, Z.-Y. Fabrication of Edge Rounded Polylactic Acid Biomedical Stents by the Multi-Axis Micro-Milling Process. Appl. Sci. 2020, 10, 2809. [Google Scholar] [CrossRef] [Green Version]
- Borhani, S.; Hassanajili, S.; Tafti, S.H.A.; Rabbani, S. Cardiovascular stents: Overview, evolution, and next generation. Prog. Biomater. 2018, 7, 175–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onuma, Y.; Serruys, P. Bioresorbable Scaffold. Circulation 2011, 123, 779–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfamariam, B. Bioresorbable vascular scaffolds: Biodegradation, drug delivery and vascular remodeling. Pharmacol. Res. 2016, 107, 163–171. [Google Scholar] [CrossRef]
- Das, D.; Zhang, Z.; Winkler, T.; Mour, M.; Günter, C.I.; Morlock, M.M.; Machens, H.-G.; Schilling, A.F. Bioresorption and Degradation of Biomaterials. Adv. Biochem. Eng. Biotechnol. 2011, 126, 317–333. [Google Scholar] [CrossRef]
- Moravej, M.; Mantovani, D. Biodegradable Metals for Cardiovascular Stent Application: Interests and New Opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, J.; Wu, W.; Shi, Y.; Jin, L.; Petrini, L.; Shen, L.; Yuan, G.; Ding, W.; Ge, J.; et al. In Vivo and in Vitro evaluation of a biodegradable magnesium vascular stent designed by shape optimization strategy. Biomaterials 2019, 221, 119414. [Google Scholar] [CrossRef]
- Kang, M.-H.; Cheon, K.-H.; Jo, K.-I.; Ahn, J.-H.; Kim, H.-E.; Jung, H.-D.; Jang, T.-S. An asymmetric surface coating strategy for improved corrosion resistance and vascular compatibility of magnesium alloy stents. Mater. Des. 2020, 196, 109182. [Google Scholar] [CrossRef]
- Kandala, B.S.P.K.; Zhang, G.; Hopkins, T.M.; An, X.; Pixley, S.K.; Shanov, V. In Vitro and In Vivo Testing of Zinc as a Biodegradable Material for Stents Fabricated by Photo-Chemical Etching. Appl. Sci. 2019, 9, 4503. [Google Scholar] [CrossRef] [Green Version]
- Maeng, M.; Jensen, L.O.; Falk, E.; Andersen, H.R.; Thuesen, L. Negative vascular remodelling after implantation of bioabsorbable magnesium alloy stents in porcine coronary arteries: A randomised comparison with bare-metal and sirolimus-eluting stents. Heart 2008, 95, 241–246. [Google Scholar] [CrossRef]
- Waksman, R.; Barbash, I.M.; Dvir, D.; Torguson, R.; Ben-Dor, I.; Maluenda, G.; Xue, Z.; Satler, L.F.; Suddath, W.O.; Kent, K.M.; et al. Safety and Efficacy of the XIENCE V Everolimus-Eluting Stent Compared to First-Generation Drug-Eluting Stents in Contemporary Clinical Practice. Am. J. Cardiol. 2012, 109, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Foin, N.; Lee, R.D.; Torii, R.; Guitierrez-Chico, J.L.; Mattesini, A.; Nijjer, S.; Sen, S.; Petraco, R.; Davies, J.E.; Di Mario, C.; et al. Impact of stent strut design in metallic stents and biodegradable scaffolds. Int. J. Cardiol. 2014, 177, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Baquet, M.; Jochheim, D.; Mehilli, J. Polymer-Free drug-Eluting stents for coronary artery disease. J. Interv. Cardiol. 2018, 31, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Abizaid, A.; Mehran, R.; Schofer, J.; Schuler, G.C.; Hauptmann, K.E.; Magalhães, M.A.; Parise, H.; Grube, E. Polymer-Free Biolimus A9-Coated Stents in the Treatment of De Novo Coronary Lesions. JACC Cardiovasc. Interv. 2016, 9, 51–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamburino, C.; Di Salvo, M.E.; Capodanno, D.; Capranzano, P.; Parisi, R.; Mirabella, F.; Scardaci, F.; Ussia, G.; Galassi, A.R.; Fiscella, D.; et al. Real world safety and efficacy of the Janus tacrolimus-eluting stent: Long-term clinical outcome and angiographic findings from the tacrolimus-eluting stent (TEST) registry. Catheter. Cardiovasc. Interv. 2008, 73, 243–248. [Google Scholar] [CrossRef]
- El-Hayek, G.; Bangalore, S.; Dominguez, A.C.; Devireddy, C.; Jaber, W.; Kumar, G.; Mavromatis, K.; Tamis-Holland, J.; Samady, H. Meta-Analysis of Randomized Clinical Trials Comparing Biodegradable Polymer Drug-Eluting Stent to Second-Generation Durable Polymer Drug-Eluting Stents. JACC Cardiovasc. Interv. 2017, 10, 462–473. [Google Scholar] [CrossRef]
- Medtronic. Endeavor (R) Drug-Eluting Coronary Stent; Medtronic: Dublin, Ireland, 2007. [Google Scholar]
- Kim, S.; Kang, S.; Lee, J.M.; Chung, W.; Park, J.J.; Yoon, C.; Suh, J.; Cho, Y.; Doh, J.; Cho, J.M.; et al. Three-Year clinical outcome of biodegradable hybrid polymer Orsiro sirolimus-eluting stent and the durable biocompatible polymer Resolute Integrity zotarolimus-eluting stent: A randomized controlled trial. Catheter. Cardiovasc. Interv. 2020, 96, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Kuramitsu, S.; Hiromasa, T.; Enomoto, S.; Shinozaki, T.; Iwabuchi, M.; Mazaki, T.; Domei, T.; Yamaji, K.; Soga, Y.; Hyodo, M.; et al. Incidence and Clinical Impact of Stent Fracture After PROMUS Element Platinum Chromium Everolimus-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2015, 8, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Rapetto, C.; Leoncini, M. Magmaris: A new generation metallic sirolimus-Eluting fully bioresorbable scaffold: Present status and future perspectives. J. Thorac. Dis. 2017, 9, S903–S913. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, B.; Abizaid, A.; Carrié, D.; Frey, N.; Lutz, M.; Weber-Albers, J.; Dudek, D.; Weng, S.-C.; Akodad, M.; Anderson, J.; et al. Clinical and Angiographic Outcomes With a Novel Radiopaque Sirolimus-Eluting Bioresorbable Vascular Scaffold. Circ. Cardiovasc. Interv. 2019, 12, e007283. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Khawaja, S.A.; Vesga, B.; Hernandez, H.; Moncada, M.; Delgado, J.A.; Esposito, G.; Ferrone, M.; Dager, A.; Arana, C.; et al. First in human evaluation of a novel Sirolimus-eluting ultra-high molecular weight bioresorbable scaffold: 9-, 24-and 36-months imaging and clinical results from the multi-center renascent study. Int. J. Cardiol. 2020, 321, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Hernandez, J.M.D.L.T. The Newest Generation of Drug-eluting Stents and Beyond. Eur. Cardiol. Rev. 2018, 13, 54–59. [Google Scholar] [CrossRef]
- Regazzoli, D.; Leone, P.P.; Colombo, A.; Latib, A. New generation bioresorbable scaffold technologies: An update on novel devices and clinical results. J. Thorac. Dis. 2017, 9, S979–S985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kereiakes, D.J.; Ellis, S.G.; Metzger, C.; Caputo, R.P.; Rizik, D.G.; Teirstein, P.S.; Litt, M.R.; Kini, A.; Kabour, A.; Marx, S.O.; et al. 3-Year Clinical Outcomes With Everolimus-Eluting Bioresorbable Coronary Scaffolds. J. Am. Coll. Cardiol. 2017, 70, 2852–2862. [Google Scholar] [CrossRef]
- Rizik, D.G.; Hermiller, J.B.; Kereiakes, D.J. The ABSORB bioresorbable vascular scaffold: A novel, fully resorbable drug-eluting stent: Current concepts and overview of clinical evidence. Catheter. Cardiovasc. Interv. 2015, 86, 664–677. [Google Scholar] [CrossRef]
- Guerra, A.J.; San, J.; Ciurana, J. Fabrication of PCL/PLA Composite Tube for Stent Manufacturing. Procedia CIRP 2017, 65, 231–235. [Google Scholar] [CrossRef]
- Chichareon, P.; Katagiri, Y.; Asano, T.; Takahashi, K.; Kogame, N.; Modolo, R.; Tenekecioglu, E.; Chang, C.-C.; Tomaniak, M.; Kukreja, N.; et al. Mechanical properties and performances of contemporary drug-eluting stent: Focus on the metallic backbone. Expert Rev. Med Devices 2019, 16, 211–228. [Google Scholar] [CrossRef]
- Bink, N.; Mohan, V.B.; Fakirov, S. Recent advances in plastic stents: A comprehensive review. Int. J. Polym. Mater. 2021, 70, 54–74. [Google Scholar] [CrossRef]
- Wholey, M.H.; Finol, E.A. Designing the ideal stent. Endovasc. Today 2007, 6, 25–34. [Google Scholar]
- Watson, T.I.; Webster, M.W.; Ormiston, J.; Ruygrok, P.N.; Stewart, J.T. Long and short of optimal stent design. Open Heart 2017, 4, e000680. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Haverich, A.; Serruys, P.; Bonow, R.O.; Kappetein, P.; Falk, V.; Velazquez, E.; Diegeler, A.; Sigusch, H. PCI and CABG for Treating Stable Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 73, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Dola, J.; Morawiec, B.; Muzyk, P.; Nowalany-Kozielska, E.; Kawecki, D. Ideal coronary stent: Development, characteristics, and vessel size impact. Ann. Acad. Med. Silesiensis 2020, 74, 191–197. [Google Scholar] [CrossRef]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Ii, R.J.G.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [Green Version]
- Nazneen, F.; Herzog, G.; Arrigan, D.W.; Caplice, N.; Benvenuto, P.; Galvin, P.; Thompson, M. Surface chemical and physical modification in stent technology for the treatment of coronary artery disease. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1989–2014. [Google Scholar] [CrossRef]
- Polanec, B.; Kramberger, J.; Glodez, S. A review of production technologies and materials for manufacturing of cardiovascular stents. Adv. Prod. Eng. Manag. 2020, 15, 390–402. [Google Scholar] [CrossRef]
- Rab, T.; Abbott, J.D.; Basir, M.B.; Latib, A.; Kumar, G.; Meraj, P.; Croce, K.; Davé, R. Summary of Practice Considerations for Percutaneous Coronary Intervention of Left Main Bifurcation Disease. Heart Int. 2020, 14, 69–72. [Google Scholar] [CrossRef]
- Gil, R.J.; Kern, A.; Pawłowski, T.; Bil, J. Twelve-Month clinical results from the new cobalt-chromium sirolimus-eluting dedicated bifurcation stent BiOSS LIM C Registry. Arch. Med. Sci. 2020, 16. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S. Dedicated bifurcation stents–Mechanistic, hardware, and technical aspects. Indian Heart J. 2016, 68, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Ramzy, J.; Burgess, S.; Zaman, S. Percutaneous Coronary Intervention for Coronary Bifurcation Lesions: Latest Evidence. Curr. Treat. Options Cardiovasc. Med. 2020, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Guillory, R.J.; Shearier, E.R.; Seitz, J.-M.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng. C 2015, 56, 467–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W.; et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef]
- Huang, L.; Pu, C.; Fisher, R.K.; Mountain, D.J.; Gao, Y.; Liaw, P.K.; Zhang, W.; He, W. A Zr-Based bulk metallic glass for future stent applications: Materials properties, finite element modeling, and In Vitro human vascular cell response. Acta Biomater. 2015, 25, 356–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miskovic, D.M.; Laws, K.J.; Ferry, M. 9-Metallic glasses. In Structural Biomaterials; Wen, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 275–300. [Google Scholar] [CrossRef]
- Hasannaeimi, V.; Sadeghilaridjani, M.; Mukherjee, S. Electrochemical and Corrosion Behavior of Metallic Glasses; MDPI Books: Basel, Switzerland, 2021. [Google Scholar] [CrossRef]
- Kiani, F.; Wen, C.; Li, Y. Prospects and strategies for magnesium alloys as biodegradable implants from crystalline to bulk metallic glasses and composites—A review. Acta Biomater. 2020, 103, 1–23. [Google Scholar] [CrossRef]
- Motru, S.; Sachidananda, M.; Avyaktha, K.; Kumar, G.P.; Patil, N. Structural analysis of bulk metallic glass cardio-vascular stent under dynamic and failure criteria. Mater. Today Proc. 2020, 28, 767–775. [Google Scholar] [CrossRef]
- Kumar, G.P.; Tavakoli, R.; Cui, F.; Jafary-Zadeh, M. Feasibility of using bulk metallic glass for self-expandable stent applications. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2016, 105, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Jafary-Zadeh, M.; Kumar, G.P.; Branicio, P.S.; Seifi, M.; Lewandowski, J.J.; Cui, F. A Critical Review on Metallic Glasses as Structural Materials for Cardiovascular Stent Applications. J. Funct. Biomater. 2018, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, G.; Wang, X. Metallic Glasses for Biomedical Applications. In Novel Structured Metallic and Inorganic Materials; Setsuhara, Y., Kamiya, T., Yamaura, S.-I., Eds.; Springer: Singapore, 2019; pp. 421–433. [Google Scholar] [CrossRef]
- Meagher, P.; O’Cearbhaill, E.D.; Byrne, J.H.; Browne, D.J. Bulk Metallic Glasses for Implantable Medical Devices and Surgical Tools. Adv. Mater. 2016, 28, 5755–5762. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, H.-J.; Pang, S.-J.; Zhang, T. Ti–Zr–Cu–Fe–Sn–Si–Ag–Ta bulk metallic glasses with good corrosion resistance as potential biomaterials. Rare Met. 2020, 39, 688–694. [Google Scholar] [CrossRef]
- Yeazel, T.R.; Becker, M.L. Advancing Toward 3D Printing of Bioresorbable Shape Memory Polymer Stents. Biomacromolecules 2020, 21, 3957–3965. [Google Scholar] [CrossRef]
- Kapoor, D. Nitinol for Medical Applications: A Brief Introduction to the Properties and Processing of Nickel Titanium Shape Memory Alloys and their Use in Stents. Johns. Matthey Technol. Rev. 2017, 61, 66–76. [Google Scholar] [CrossRef]
- Jia, H.; Gu, S.-Y.; Chang, K. 3D printed self-expandable vascular stents from biodegradable shape memory polymer. Adv. Polym. Technol. 2018, 37, 3222–3228. [Google Scholar] [CrossRef]
- Omid, S.O.; Goudarzi, Z.; Kangarshahi, L.M.; Mokhtarzade, A.; Bahrami, F. Self-expanding stents based on shape memory alloys and shape memory polymers. J. Compos. Compd. 2020, 2, 92–98. [Google Scholar] [CrossRef]
- Holman, H.; Kavarana, M.N.; Rajab, T.K. Smart materials in cardiovascular implants: Shape memory alloys and shape memory polymers. Artif. Organs 2021, 45, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Fukushima, S.; Sasaki, K. Shape Memory Effect and Superelasticity of Textured NiTi Alloy Wire. In Advances in Shape Memory Materials: In Commemoration of the Retirement of Professor Hisaaki Tobushi; Sun, Q., Matsui, R., Takeda, K., Pieczyska, E.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 43–50. [Google Scholar] [CrossRef]
- Ghobadi, E.; Shutov, A.; Steeb, H. Parameter Identification and Validation of Shape-Memory Polymers within the Framework of Finite Strain Viscoelasticity. Materials 2021, 14, 2049. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Davoodi, P.; Ramakrishna, S. Electrospun Shape Memory Polymer Micro-/Nanofibers and Tailoring Their Roles for Biomedical Applications. Nanomaterials 2021, 11, 933. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.-Y.; Shen, Y.-F.; Astuti, S.D.; Lee, A.K.-X.; Lin, S.-H.; Dwijaksara, N.L.B.; Chen, Y.-W. Review of Polymeric Materials in 4D Printing Biomedical Applications. Polymers 2019, 11, 1864. [Google Scholar] [CrossRef] [Green Version]
- Basit, A.; L’Hostis, G.; Durand, B. The recovery properties under load of a shape memory polymer composite material. Mater. Werkst. 2019, 50, 1555–1559. [Google Scholar] [CrossRef]
- Mather, P.T.; Luo, X.; Rousseau, I.A. Shape Memory Polymer Research. Annu. Rev. Mater. Res. 2009, 39, 445–471. [Google Scholar] [CrossRef]
- Lee, A.Y.; An, J.; Chua, C.K. Two-Way 4D Printing: A Review on the Reversibility of 3D-Printed Shape Memory Materials. Engineering 2017, 3, 663–674. [Google Scholar] [CrossRef]
- Chu, C.; Xiang, Z.; Wang, J.; Xie, H.; Xiang, T.; Zhou, S. A near-infrared light-triggered shape-memory polymer for long-time fluorescence imaging in deep tissues. J. Mater. Chem. B 2020, 8, 8061–8070. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.M.; Zhao, Y.; Wang, C.C.; Ding, Z.; Purnawali, H.; Tang, C.; Zhang, J.L. Thermo/chemo-responsive shape memory effect in polymers: A sketch of working mechanisms, fundamentals and optimization. J. Polym. Res. 2012, 19, 9952. [Google Scholar] [CrossRef]
- Svedman, C.; Bruze, M. Coronary Stents. In Contact Dermatitis; Johansen, J., Mahler, V., Lepoittevin, J.P., Frosch, P., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 1–13. [Google Scholar] [CrossRef]
- Nezami, F.R.; Athanasiou, L.S.; Edelman, E.R. Chapter 28-Endovascular drug-delivery and drug-elution systems. In Biomechanics of Coronary Atherosclerotic Plaque; Ohayon, J., Finet, G., Pettigrew, R.I., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 4, pp. 595–631. [Google Scholar]
- Worthley, S.G.; Abizaid, A.; Kirtane, A.J.; Simon, D.I.; Windecker, S.; Brar, S.; Meredith, I.T.; Shetty, S.; Sinhal, A.; Almonacid, A.P.; et al. First-in-Human Evaluation of a Novel Polymer-Free Drug-Filled Stent. JACC Cardiovasc. Interv. 2017, 10, 147–156. [Google Scholar] [CrossRef]
- Yerasi, C.; Case, B.C.; Forrestal, B.J.; Torguson, R.; Weintraub, W.S.; Garcia-Garcia, H.M.; Waksman, R. Drug-Coated Balloon for De Novo Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 75, 1061–1073. [Google Scholar] [CrossRef]
- Giacoppo, D.; Alfonso, F.; Xu, B.; Claessen, B.E.; Adriaenssens, T.; Jensen, C.; Pérez-Vizcayno, M.J.; Kang, D.-Y.; Degenhardt, R.; Pleva, L.; et al. Drug-Coated Balloon Angioplasty Versus Drug-Eluting Stent Implantation in Patients with Coronary Stent Restenosis. J. Am. Coll. Cardiol. 2020, 75, 2664–2678. [Google Scholar] [CrossRef]
- Fahrni, G.; Scheller, B.; Coslovsky, M.; Gilgen, N.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Weilenmann, D.; Wöhrle, J.; Cuculi, F.; et al. Drug-coated balloon versus drug-eluting stent in small coronary artery lesions: Angiographic analysis from the BASKET-SMALL 2 trial. Clin. Res. Cardiol. 2020, 109, 1114–1124. [Google Scholar] [CrossRef]
- Majewska, P.; Oledzka, E.; Sobczak, M. Overview of the latest developments in the field of drug-eluting stent technology. Biomater. Sci. 2019, 8, 544–551. [Google Scholar] [CrossRef]
- Schieber, R.; Lasserre, F.; Hans, M.; Fernández-Yagüe, M.; Díaz-Ricart, M.; Escolar, G.; Ginebra, M.-P.; Mücklich, F.; Pegueroles, M. Direct Laser Interference Patterning of CoCr Alloy Surfaces to Control Endothelial Cell and Platelet Response for Cardiovascular Applications. Adv. Healthc. Mater. 2017, 6, 1700327. [Google Scholar] [CrossRef]
- Lee, S.J.; Jo, H.H.; Lim, K.S.; Lim, D.; Lee, S.; Lee, J.H.; Kim, W.D.; Jeong, M.H.; Lim, J.Y.; Kwon, I.K.; et al. Heparin coating on 3D printed poly (l-lactic acid) biodegradable cardiovascular stent via mild surface modification approach for coronary artery implantation. Chem. Eng. J. 2019, 378, 122116. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, S.; Rasser, C.; Mesnier, J.; Chevallier, P.; Gallet, R.; Choqueux, C.; Even, G.; Sayah, N.; Chaubet, F.; Nicoletti, A.; et al. Coronary stent CD31-mimetic coating favours endothelialization and reduces local inflammation and neointimal development in vivo. Eur. Heart J. 2021, 42, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, G.; Pei, Y.; Zhang, H. Layered hydroxide/polydopamine/hyaluronic acid functionalized magnesium alloys for enhanced anticorrosion, biocompatibility and antithrombogenicity in vascular stents. J. Biomater. Appl. 2020, 34, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Kang, S.N.; Kim, D.H.; Kim, H.-B.; Im, K.S.; Park, W.; Hong, Y.J.; Han, D.K.; Joung, Y.K. Late endothelial progenitor cell-capture stents with CD146 antibody and nanostructure reduce in-stent restenosis and thrombosis. Acta Biomater. 2020, 111, 91–101. [Google Scholar] [CrossRef]
- Blanco, E.; Segura-Ibarra, V.; Bawa, D.; Wu, S.; Liu, H.; Ferrari, M.; Lumsden, A.B.; Shah, D.J.; Lin, C.H. Functionalization of endovascular devices with superparamagnetic iron oxide nanoparticles for interventional cardiovascular magnetic resonance imaging. Biomed. Microdevices 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, Q.; Huo, J.; Zhang, Y.; Yang, W.; Li, X. Crystallization in additive manufacturing of metallic glasses: A review. Addit. Manuf. 2020, 36, 101568. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, C.H. Augmented reality for personalized nanomedicines. Biotechnol. Adv. 2018, 36, 335–343. [Google Scholar] [CrossRef]
- Salavitabar, A.; Armstrong, A.K. Personalized Interventions: A Reality in the Next 20 Years or Pie in the Sky. Pediatr. Cardiol. 2020, 41, 486–502. [Google Scholar] [CrossRef]
- Lee, H.; Tajmir, S.; Lee, J.; Zissen, M.; Yeshiwas, B.A.; Alkasab, T.K.; Choy, G.; Do, S. Fully Automated Deep Learning System for Bone Age Assessment. J. Digit. Imaging 2017, 30, 427–441. [Google Scholar] [CrossRef] [Green Version]
- Balu, A.; Nallagonda, S.; Xu, F.; Krishnamurthy, A.; Hsu, M.-C.; Sarkar, S. A Deep Learning Framework for Design and Analysis of Surgical Bioprosthetic Heart Valves. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]






| Stent Material | Young’s Modulus (GPa) | Ultimate Tensile Strength (MPa) | Equivalent Von-Mises Stress (MPa) | Elongation at Break (%) | References |
|---|---|---|---|---|---|
| Iron | 211 | 270 | - | 40 | [33,37,38,39] |
| Stainless steel | 193 | 595 | 231.14 | 40 | [15,33] |
| Tantalum | 186 | 285 | 514.70 | - | [15,40] |
| Nitinol | 45–50 | 1200 | 436.12 | ~20 | [15,41] |
| Cobalt-chromium L-605 | 243 | 1020 | 536.20 | 50 | [15,42] |
| Cobalt-chromium MP35 N | 233 | 930 | 529.82 | 45 | [15,42] |
| Stent Material | Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation at Break (%) | Degradation (Months) | References |
|---|---|---|---|---|---|
| PLA | 2–4 | 65 | 2–6 | 18–30 | [99,100] |
| PDLLA | 1–3.5 | 40 | 1–2 | 3–4 | [99] |
| PLLA | 2–4 | 60–70 | 2–6 | >24 | [99] |
| PGA | 6–7 | 90–110 | 1–2 | 4–6 | [99] |
| PDLGA (50/50) | 1–4.3 | 45 | 1–4 | 1–2 | [99] |
| PLGA (82/12) | 3.3–3.5 | 65 | 2–6 | 12–18 | [99] |
| PCL | 0.34–0.36 | 23 | >4000 | 24–36 | [99] |
| PLA/PCL (70/30) | 0.02–0.04 | 2–4.5 | >100 | 12–24 | [99] |
| PC | 2–2.4 | 55–75 | 80–150 | >14 | [99] |
| AE21 | 45 | - | - | 2–3 | [78,101] |
| AE42 | 45 | 237 | 8–10 | - | [78] |
| WE43 | 40–50 | 220–330 | 2–20 | 3–12 | [99] |
| AZ31 | 45 | 235 | 7–21 | <4 | [78] |
| Device | Stent Specifications | Observations | References | |||
|---|---|---|---|---|---|---|
| Platform Material | Strut Thickness (μm) | Coating Material | Drug | |||
| Cypher | Stainless steel | 140 | Parylene C | Sirolimus | Drug-eluting time: 80% elutes in the first 30 days, while the remainder is released by the end of 90 days Outcomes at 1 year (percent from the total number of patients in the trial):
| [31,112,113] |
| Taxus | Stainless steel | 132 | Polystyrene-b-isobutylene-b-styrene (translute) polymer | Paclitaxel | Drug-eluting time: elutes over 90 days Outcomes at 1 year (percent from the total number of patients in the trial):
| [31,112,113] |
| Axxion | Stainless steel | 117 | - | Paclitaxel | Drug-eluting time: 40–50% in the first week, while the remainder is released by the end of 4 weeks | [31,114] |
| Achieve | Stainless steel | - | Paclitaxel | Drug-eluting time: 28% within 4 days; 69% within 2 weeks | [31] | |
| Amazonia PAX | Cobalt-chromium L-605 | 73 | - | Paclitaxel | Drug-eluting time: 60% within 2 days, while the remainder is released by the end of 7 weeks | [31,114] |
| Cre8 | Cobalt-chromium L-605 | 70–80 | - | Amphilimus | Drug-eluting time: 50% on the first day, while the remainder is released by the end of 3 weeks | [31,114] |
| BioFreedom | Stainless steel | 119 | - | Biolimus A9 | Drug-eluting time: 98% within 4 weeks Outcomes at 1 year (percent from the total number of patients in the trial):
| [31,114,115] |
| JANUS | Stainless steel | Carbofilm | Tacrolimus | Drug-eluting time: 50% within the first 4 weeks Outcomes at 22 months (percent from the total number of patients in the trial):
| [31,116] | |
| NANO + | Stainless steel | 90 | - | Sirolimus | Drug-eluting time: 85% during the first 4 weeks | [31,114] |
| BioMatri × Flex | Stainless steel | 120 | PLLA | Biolimus A9 | Polymer coating degradation: 6 to 9 months | [113] |
| Endeavor | Cobalt-chromium MP35 N | 91 | Phosphorylcholine | Zotarolimus | Drug-eluting time: 80% during the first 10 days | [31,117,118] |
| Orsiro | Cobalt-chromium alloy | 60 | PLLA with silicon carbide layer | Sirolimus | Polymer coating degradation: 12 months Outcomes at 1 year (percent from the total number of patients in the trial):
| [113,119] |
| Synergy | Platinum-chromium | 74 | PLGA | Everolimus | Polymer coating degradation: 3 months | [113] |
| Promus Element | Platinum-chromium alloy | 81 | Permanent fluorinated polymer | Everolimus | Outcomes at 9 months (from total number of patients in the trial):
| [113,117,120] |
| MiStent | Cobalt-chromium alloy | 64 | PLGA | Sirolimus | Polymer coating degradation: 3 months | [113] |
| Mitsu | Cobalt-chromium alloy | 40 × 80 | Lipid nano-spheres | Merilimus | Polymer coating degradation: 1.5 months | [113] |
| Xience V | Cobalt-chromium L-605 | 81 | Poly(vinyldenefluoride-co-hexafluoropropylene) | Everolimus | Drug-eluting time: 80% during first 30 days Outcomes at 1 year (percent from the total number of patients in the trial):
| [112,113,117] |
| Resolute Integrity | Cobalt-chromium alloy | 91 | BioLinx polymer | Zotarolimus | Outcomes at 1 year (percent from the total number of patients in the trial):
| [113,117,119] |
| Magmaris | Magnesium alloy | 120–150 | PLLA | Sirolimus | Resorption time: 12 months Drug-eluting time: 90 days | [83,121] |
| AMS 1.0 | Magnesium alloy | 165 | - | - | Resorption time: <4 months | [113] |
| AMS 2.0 | Magnesium alloy | 120 | - | - | Resorption time: >4 months | [113] |
| DREAMS 1 | Magnesium alloy | 125 | PLGA | Paclitaxel | Resorption time: 9 months | [113] |
| DREAMS 2 | Magnesium alloy | 150 | PLLA | Sirolimus | Resorption time: 9 months | [113] |
| Fantom | Tyrosine polycarbonate | 125 | - | Sirolimus | Resorption time: 36 months Outcomes at 1 year (percent from the total number of patients in the trial):
| [83,122] |
| ReZolve | Poly-tyrosine-derived polycarbonate | 114–228 | - | Sirolimus | Resorption time: 48 months | [113] |
| REVA Gen I | Poly-tyrosine-derived polycarbonate | 200 | - | Paclitaxel | Resorption time: 48 months | [113] |
| IDEAL BioStent Gen I | Polylactide anhydride mixed with a polymer of salycilic acid with a sebaic acid linker | 200 | Salicylate linked with adipic acid | Sirolimus | Resorption time: 6 to 9 months | [113] |
| Fortitude | PLLA | 150 | Sirolimus:polymer matrix (1:1) | Sirolimus | Drug-eluting time: 90% during first 90 days Resorption time: 10 months Higher mechanical strength, expansion capabilities, and resistance to fracture than other BVSs | [83,123] |
| MeRes 100 | PLLA | 100 | PDLLA | Everolimus/Sirolimus | Resorption time: 24–36 months Drug-eluting time: 90 days | [83,124] |
| DESolve | PLLA | 150 | PLLA | Myolimus/Novolimus | Resorption time: 12 to 24 months | [83,113] |
| Magnitude | PLLA | <100 | - | Sirolimus | Resorption time: 24–36 months | [83,125] |
| ABSORB BVS | PLLA | 150 | PDLLA | Everolimus | Resorption time: 36 months Drug-eluting time: 90 days Early and sustained safety in both simple lesions of stable patients and more complex anatomic and clinical settings | [83,124,126,127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scafa Udriște, A.; Niculescu, A.-G.; Grumezescu, A.M.; Bădilă, E. Cardiovascular Stents: A Review of Past, Current, and Emerging Devices. Materials 2021, 14, 2498. https://doi.org/10.3390/ma14102498
Scafa Udriște A, Niculescu A-G, Grumezescu AM, Bădilă E. Cardiovascular Stents: A Review of Past, Current, and Emerging Devices. Materials. 2021; 14(10):2498. https://doi.org/10.3390/ma14102498
Chicago/Turabian StyleScafa Udriște, Alexandru, Adelina-Gabriela Niculescu, Alexandru Mihai Grumezescu, and Elisabeta Bădilă. 2021. "Cardiovascular Stents: A Review of Past, Current, and Emerging Devices" Materials 14, no. 10: 2498. https://doi.org/10.3390/ma14102498
APA StyleScafa Udriște, A., Niculescu, A.-G., Grumezescu, A. M., & Bădilă, E. (2021). Cardiovascular Stents: A Review of Past, Current, and Emerging Devices. Materials, 14(10), 2498. https://doi.org/10.3390/ma14102498








