On Growth and Morphology of TiO2 Nanotubes on Ti6Al4V by Anodic Oxidation in Ethylene Glycol Electrolyte: Influence of Microstructure and Anodization Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ti6Al4V Specimen Preparation
2.2. Electrolytic Anodization Process
2.3. Surface Analysis and Morphology Characterization
3. Results
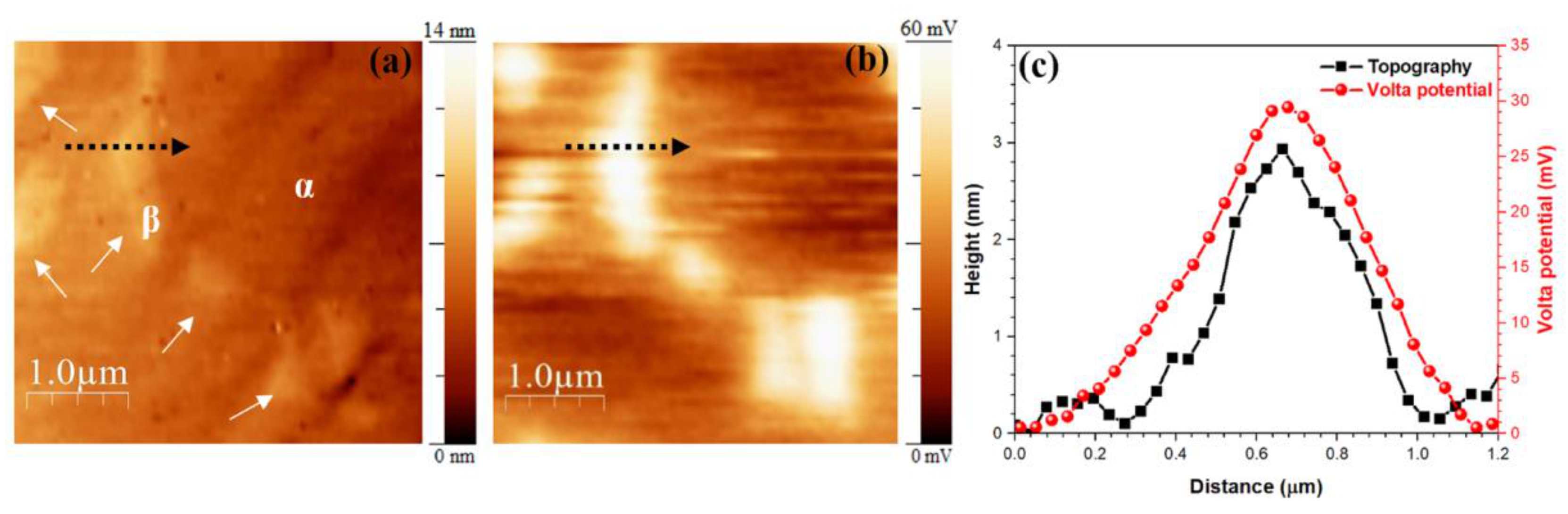
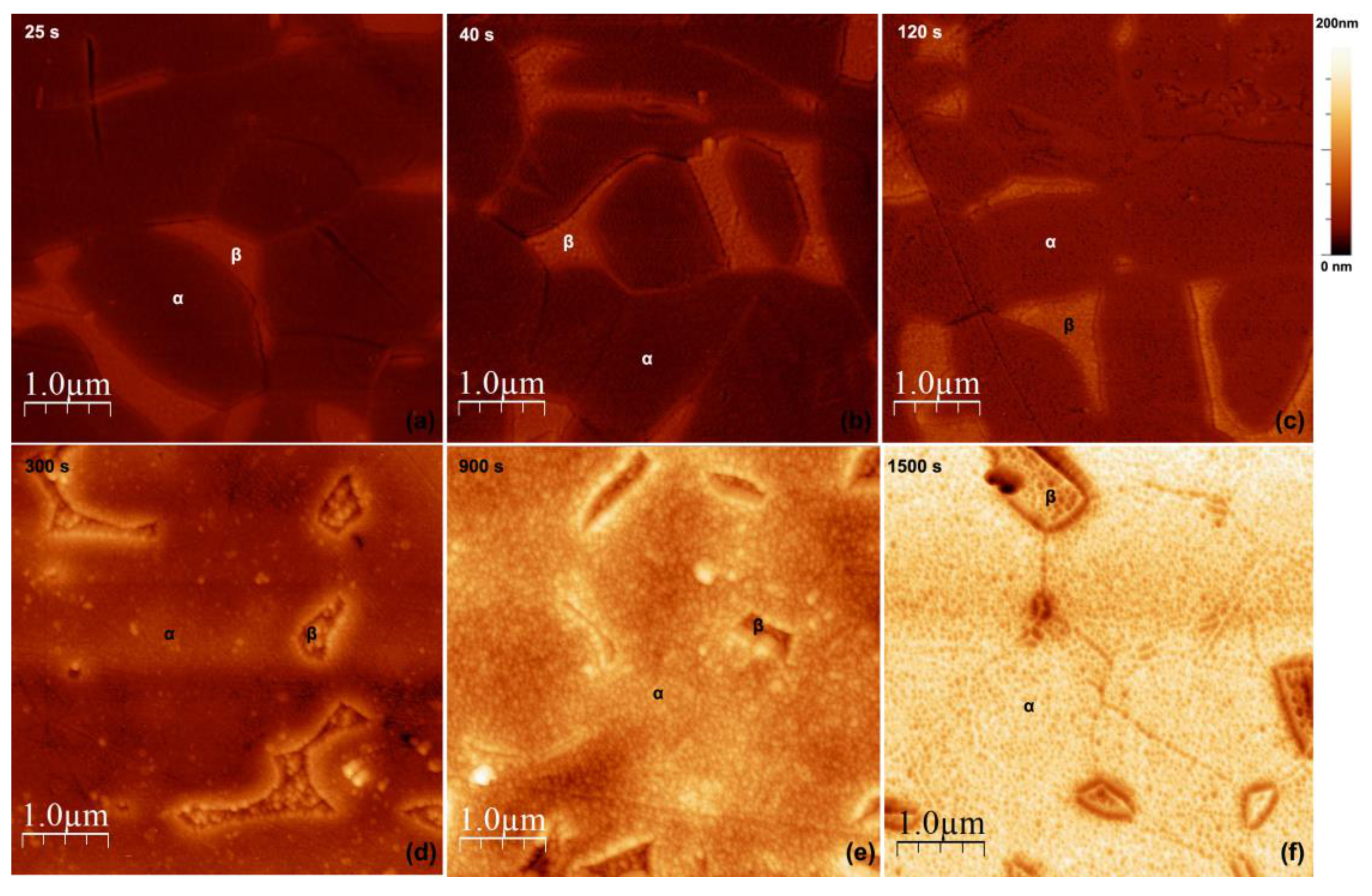
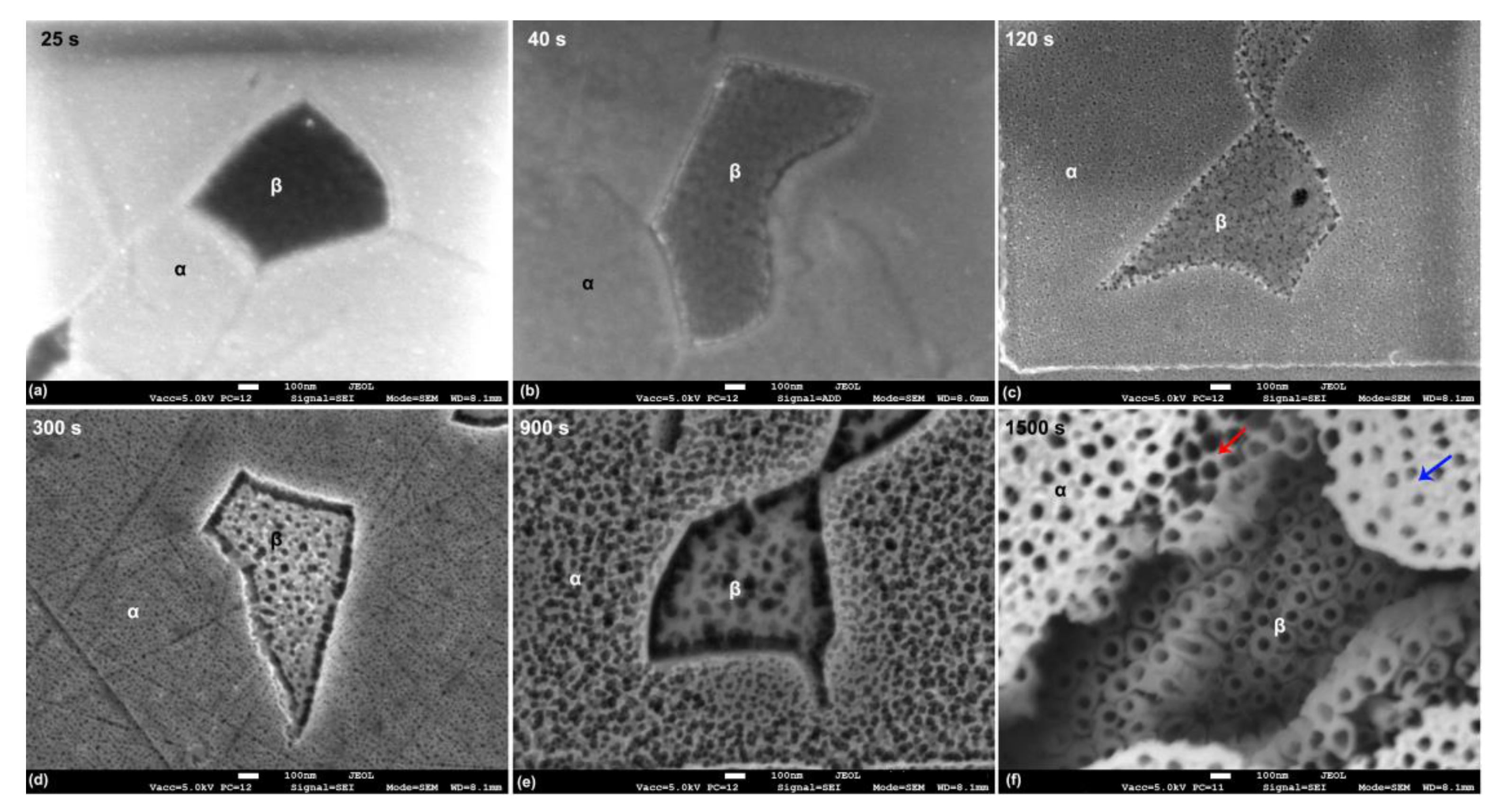
3.1. Microstructural Analysis of the Base Alloy
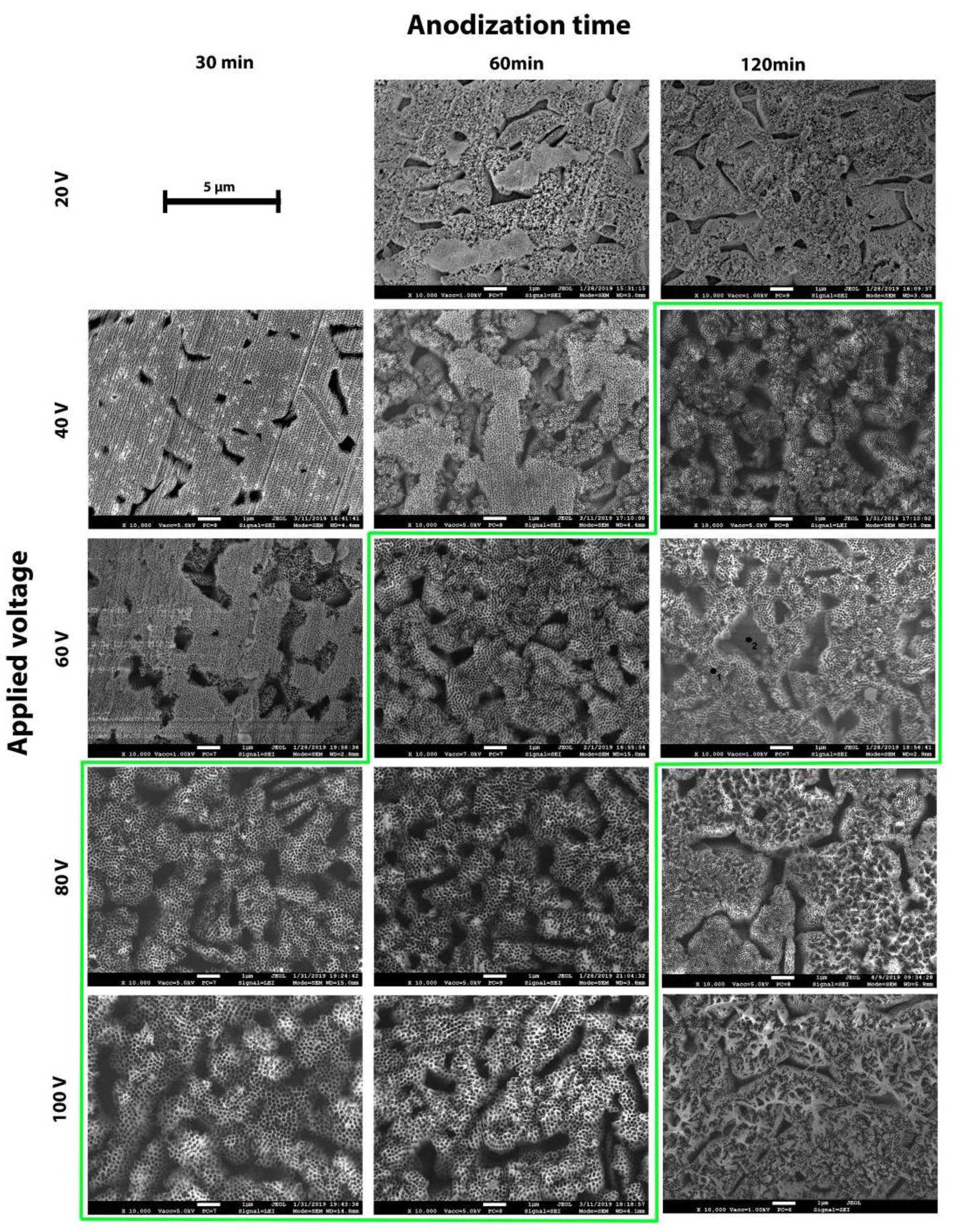
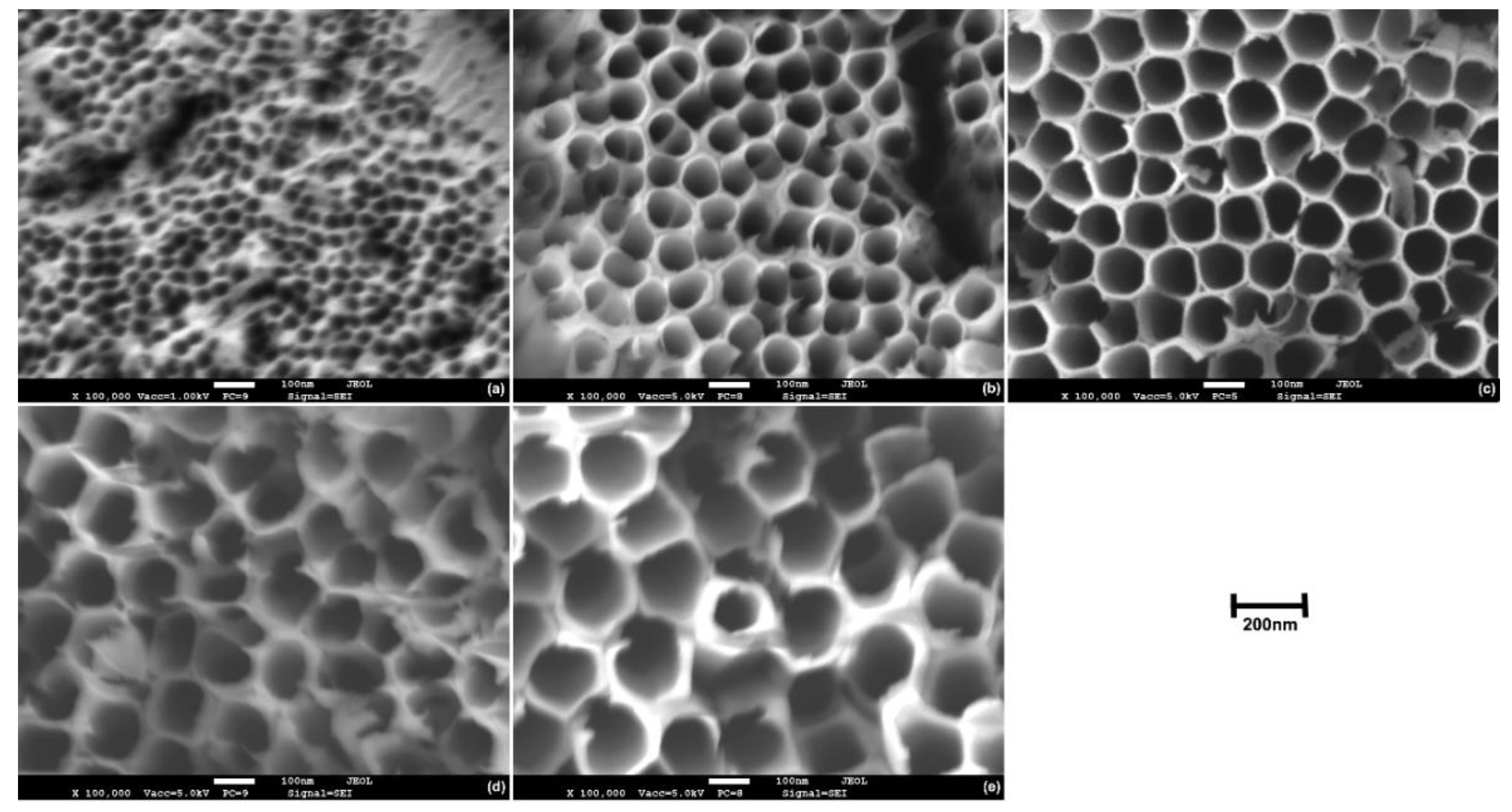
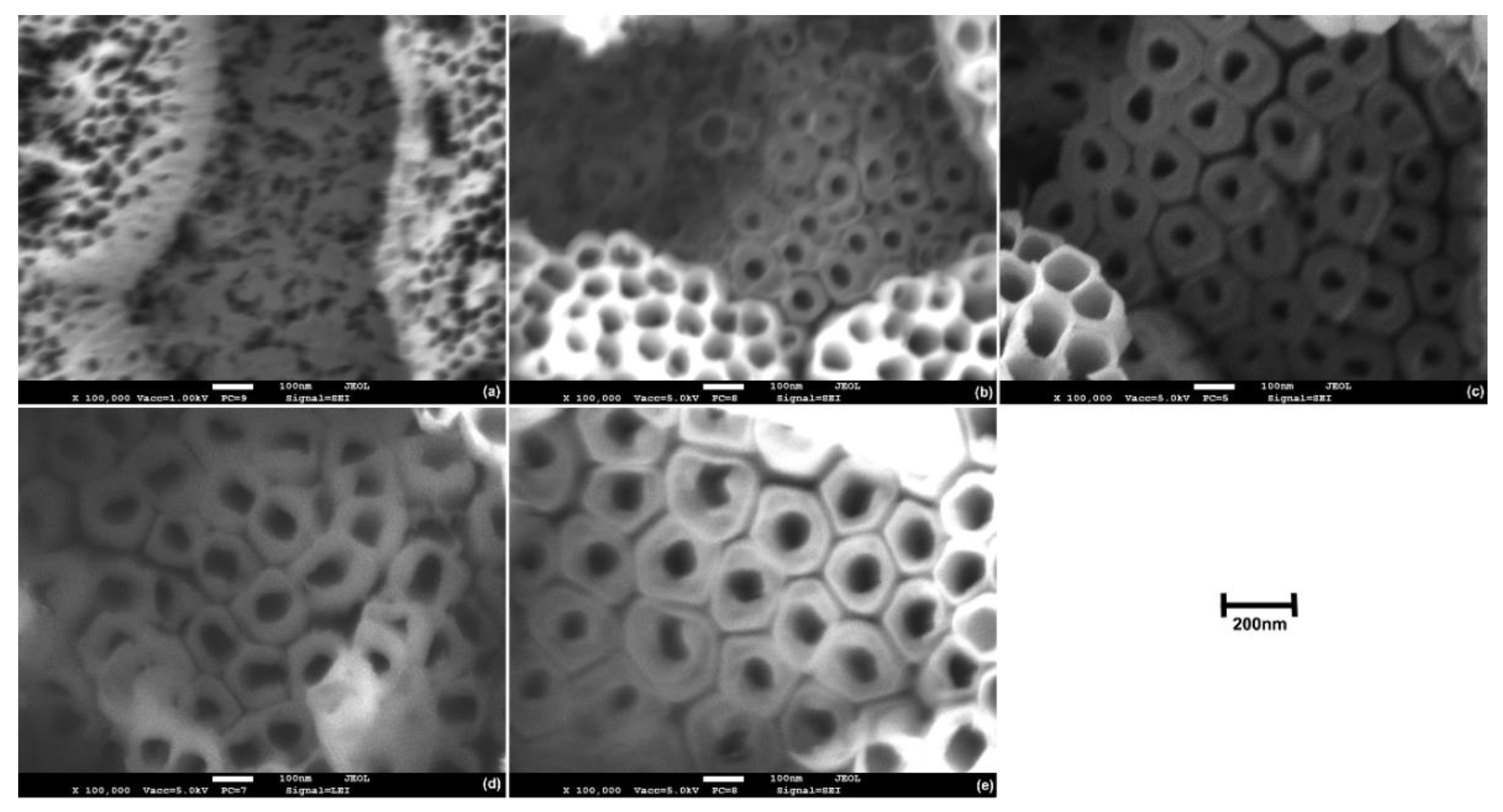
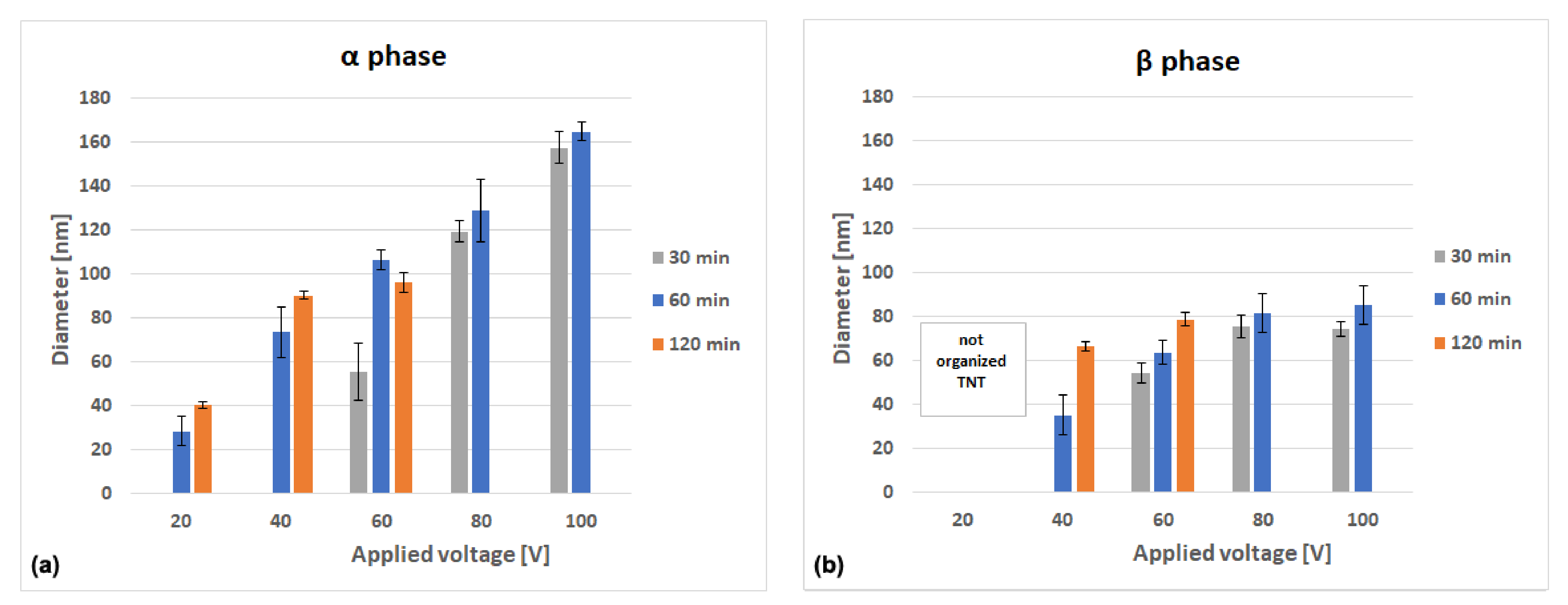
3.2. Morphology of TiO2 Nanotubes
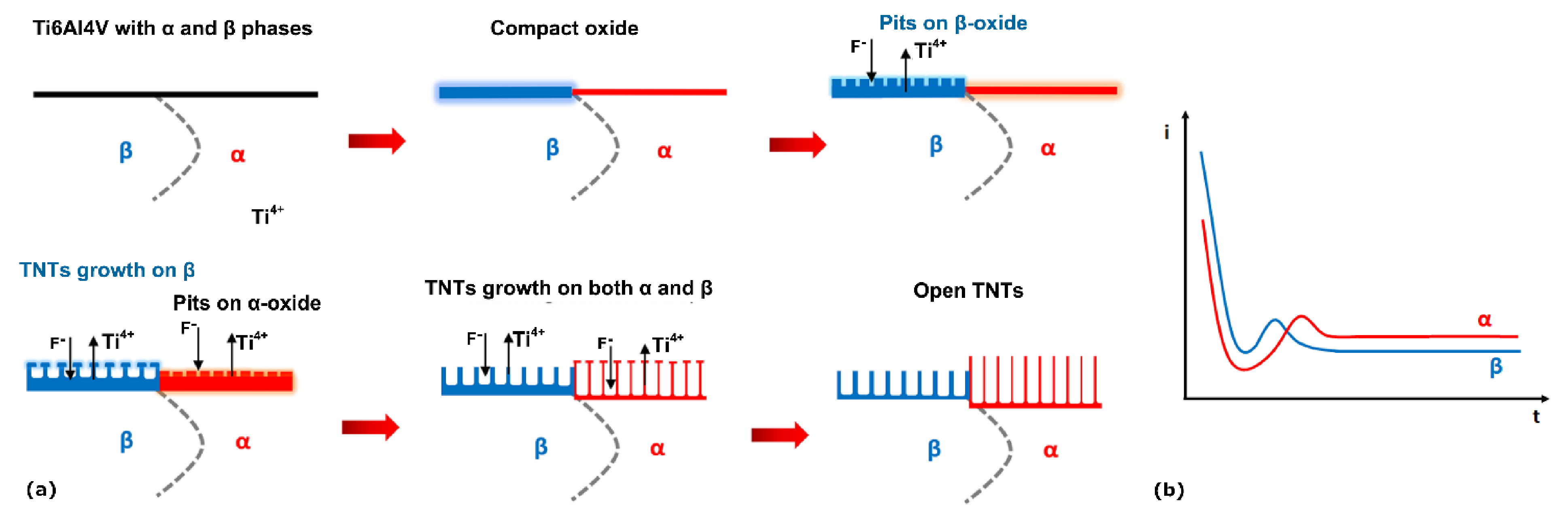
3.3. Growth Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Sreekantan, S.; Saharudin, K.A.; Wei, L.C. Formation of TiO2 nanotubes via anodization and potential applications for photocatalysts, biomedical materials, and photoelectrochemical cell. IOP Conf. Ser. Mater. Sci. Eng. 2011, 21, 012002. [Google Scholar] [CrossRef]
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S.A. Review of titania nanotubes: Fabrication and cellular response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A review on TiO2 nanotubes: Influence of anodization parameters, formation mechanism, properties, corrosion behavior, and biomedical applications. J. Bio- Tribo-Corrosion 2015, 1, 28. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Aitola, K.; Lund, P.D. TiO2 nanotubes for dye-sensitized solar cells—A review. Energy Sci. Eng. 2020, 1–17. [Google Scholar] [CrossRef]
- Cao, W.; Chen, K.; Xue, D. Highly ordered TiO2 nanotube arrays with engineered electrochemical energy storage performances. Materials 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.Y.; Aucouturier, M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anaysis 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef] [Green Version]
- Gulati, K.; Santos, A.; Findlay, D.; Losic, D. Optimizing anodization conditions for the growth of titania nanotubes on curved surfaces. J. Phys. Chem. C 2015, 119, 16033–16045. [Google Scholar] [CrossRef]
- Beranek, R.; Hildebrand, H.; Schmuki, P. Self-organized porous titanium oxide prepared in H2SO4/HF electrolytes. Electrochem. Solid State Lett. 2003, 6, 10–13. [Google Scholar] [CrossRef]
- Paulose, M.; Prakasam, H.E.; Varghese, O.K.; Peng, L.; Popat, K.C.; Mor, G.K.; Desai, T.A.; Grimes, C.A. TiO2 nanotube arrays of 1000 μm length by anodization of titanium foil: Phenol red diffusion. J. Phys. Chem. C 2007, 111, 14992–14997. [Google Scholar] [CrossRef]
- Grimes, C.A.; Mor, G.K. TiO2 Nanotube Arrays. Synthesis, Properties and Applications; Springer: New York, NJ, USA, 2009; ISBN 9781441900678. [Google Scholar]
- Sreekantan, S.; Lockman, Z.; Hazan, R.; Tasbihi, M.; Tong, L.K.; Mohamed, A.R. Influence of electrolyte pH on TiO2 nanotube formation by Ti anodization. J. Alloys Compd. 2009, 485, 478–483. [Google Scholar] [CrossRef]
- Macak, J.M.; Taveira, L.V.; Tsuchiya, H.; Sirotna, K.; Macak, J.; Schmuki, P. Influence of different fluoride containing electrolytes on the formation of self-organized titania nanotubes by Ti anodization. J. Electroceramics 2006, 16, 29–34. [Google Scholar] [CrossRef]
- Aguirre, O.R.; Echeverría, E.F. Effects of fluoride source on the characteristics of titanium dioxide nanotubes. Appl. Surf. Sci. 2018, 445, 308–319. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Aldabergerova, S.; Schmuki, P. Smooth anodic TiO2 nanotubes. Angew. Chem. Int. Ed. 2005, 44, 7463–7465. [Google Scholar] [CrossRef]
- Macak, J.M.; Hildebrand, H.; Marten-Jahns, U.; Schmuki, P. Mechanistic aspects and growth of large diameter self-organized TiO2 nanotubes. J. Electroanal. Chem. 2008, 621, 254–266. [Google Scholar] [CrossRef]
- Shankar, K.; Mor, G.K.; Fitzgerald, A.; Grimes, C.A. Cation effect on the electrochemical formation of very high aspect ratio TiO2 nanotube arrays in formamide-water mixtures. J. Phys. Chem. C 2007, 111, 21–26. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Schmuki, P. 250 μm long anodic TiO2 nanotubes with hexagonal self-ordering. Phys. Status Solidi Rapid Res. Lett. 2007, 1, 65–67. [Google Scholar] [CrossRef]
- Seçkin, E.; Ürgen, M. A kinetic model for determining morphology transitions and growth kinetics of titania nanotubes during anodization of titanium in ethylene glycol based electrolytes. Surf. Coatings Technol. 2021, 409, 126840. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Ghlcov, A.; Schmuki, P. Self-organization of anodic nanotubes on two size scales. Small 2006, 2, 888–891. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Ghicov, A.; Schmuki, P. Self-organized nanotubular oxide layers on Ti-6Al-7Nb and Ti-6Al-4V formed by anodization in NH4F solutions. J. Biomed. Mater. Res.Part A 2005, 75, 928–933. [Google Scholar] [CrossRef]
- Filova, E.; Fojt, J.; Kryslova, M.; Moravec, H.; Joska, L.; Bacakova, L. The diameter of nanotubes formed on Ti-6Al-4V alloy controls the adhesion and differentiation of Saos-2 cells. Int. J. Nanomed. 2015, 10, 7145–7163. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ding, D.; Ning, C.; Bai, S.; Huang, L.; Li, M.; Mao, D. Thermal stability and in vitro bioactivity of Ti-Al-V-O nanostructures fabricated on Ti6Al4V alloy. Nanotechnology 2009, 20, 065708. [Google Scholar] [CrossRef]
- Matykina, E.; Conde, A.; De Damborenea, J.; Marero, D.M.Y.; Arenas, M.A. Growth of TiO2-based nanotubes on Ti-6Al-4V alloy. Electrochim. Acta 2011, 56, 9209–9218. [Google Scholar] [CrossRef]
- Saharudin, K.A.; Sreekantan, S.; Aziz, S.N.Q.A.A.; Hazan, R.; Lai, C.W.; Mydin, R.B.S.M.N.; Mat, I. Surface modification and bioactivity of anodic Ti6Al4V alloy. J. Nanosci. Nanotechnol. 2013, 13, 1696–1705. [Google Scholar] [CrossRef]
- Roman, I.; Trusca, R.D.; Soare, M.L.; Fratila, C.; Krasicka-Cydzik, E.; Stan, M.S.; Dinischiotu, A. Titanium dioxide nanotube films: Preparation, characterization and electrochemical biosensitivity towards alkaline phosphatase. Mater. Sci. Eng. C 2014, 37, 374–382. [Google Scholar] [CrossRef]
- Hamlekhan, A.; Butt, A.; Patel, S.; Royhman, D.; Takoudis, C.; Sukotjo, C.; Yuan, J.; Jursich, G.; Mathew, M.T.; Hendrickson, W.; et al. Fabrication of anti-aging TiO2 nanotubes on biomedical Ti alloys. PLoS One 2014, 9, e96213. [Google Scholar] [CrossRef]
- Grotberg, J.; Hamlekhan, A.; Butt, A.; Patel, S.; Royhman, D.; Shokuhfar, T.; Sukotjo, C.; Takoudis, C.; Mathew, M.T. Thermally oxidized titania nanotubes enhance the corrosion resistance of Ti6Al4V. Mater. Sci. Eng. C 2016, 59, 677–689. [Google Scholar] [CrossRef]
- Jordanovová, V.; Losertová, M.; Štencek, M.; Lukášová, T.; Martynková, G.S.; Peikertová, P. Microstructure and properties of nanostructured coating on Ti6Al4V. Materials 2020, 13, 708. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, E.; Offoiach, R.; Baert, K.; Terryn, H.; Lekka, M.; Fedrizzi, L. Role of phosphate, calcium species and hydrogen peroxide on albumin protein adsorption on surface oxide of Ti6Al4V alloy. Materialia 2021, 15, 100988. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, K.; Macak, J.M.; Berger, S.; Ghicov, A.; Schmuki, P. Mechanistic aspects of the self-organization process for oxide nanotube formation on valve metals. J. Electrochem. Soc. 2007, 154, C472. [Google Scholar] [CrossRef]
- Sarraf, M.; Zalnezhad, E.; Bushroa, A.R.; Hamouda, A.M.S.; Rafieerad, A.R.; Nasiri-Tabrizi, B. Effect of microstructural evolution on wettability and tribological behavior of TiO2 nanotubular arrays coated on Ti-6Al-4V. Ceram. Int. 2015, 41, 7952–7962. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Moreno-Ulloa, A.; Valdez-Salas, B.; Velasquillo, C.; Carrillo, M.; Escamilla, A.; Valdez, E.; Villarreal, F. Improved osteoblast and chondrocyte adhesion and viability by surface-modified Ti6Al4V alloy with anodized TiO2 nanotubes using a super-oxidative solution. Materials 2015, 8, 867–883. [Google Scholar] [CrossRef] [Green Version]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochim. Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Zhang, H.; Man, C.; Wang, L.; Dong, C.; Wang, L.; Kong, D.; Wang, X. Different corrosion behaviors between α and β phases of Ti6Al4V in fluoride-containing solutions: Influence of alloying element Al. Corros. Sci. 2020, 169, 108605. [Google Scholar] [CrossRef]
- Marino, C.E.B.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Voltammetric stability of anodic films on the Ti6Al4V alloy in chloride medium. Electrochim. Acta 2006, 51, 6580–6583. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Berger, S.; Macak, J.M.; Ghicov, A.; Schmuki, P. Self-organized porous and tubular oxide layers on TiAl alloys. Electrochem. Commun. 2007, 9, 2397–2402. [Google Scholar] [CrossRef]
- Berger, S.; Tsuchiya, H.; Schmuki, P.; Erlangen-nuremberg, U.V.; Erlangen, D.; February, R.V.; Re, V.; Recei, M.; March, V. Transition from nanopores to nanotubes: Self-ordered anodic oxide structures on titanium-aluminides. Chem. Mater. 2008, 20, 3245–3247. [Google Scholar] [CrossRef]
- Butail, G.; Ganesan, P.G.; Teki, R.; Mahima, R.; Ravishankar, N.; Duquette, D.J.; Ramanath, G. Branched titania nanotubes through anodization voltage control. Thin Solid Films 2011, 520, 235–238. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van der Heyde, H.; Jin, S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef]
- Nevárez-Martínez, M.C.; Mazierski, P.; Kobylański, M.P.; Szczepańska, G.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.J.; Zaleska-Medynska, A. Growth, structure, and photocatalytic properties of hierarchical V2O5-TiO2 nanotube arrays obtained from the one-step anodic oxidation of Ti-V alloys. Molecules 2017, 22, 580. [Google Scholar] [CrossRef] [Green Version]


| Element | Alloy | α | β |
|---|---|---|---|
| Ti | 89.71 | 90.72 | 81.83 |
| Al | 6.42 | 6.47 | 4.79 |
| V | 3.87 | 2.56 | 13.38 |
| Element | Upper Surface | Cavity |
|---|---|---|
| O | 44.6 ± 3.2 | 6.8 ± 5.1 |
| Ti | 49.3 ± 2.9 | 85.14 ± 3.8 |
| Al | 3.7 ± 0.2 | 2.1 ± 0.9 |
| V | 2.5 ± 0.2 | 6.0 ± 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, B.; Offoiach, R.; Rahimi, E.; Salatin, E.; Lekka, M.; Fedrizzi, L. On Growth and Morphology of TiO2 Nanotubes on Ti6Al4V by Anodic Oxidation in Ethylene Glycol Electrolyte: Influence of Microstructure and Anodization Parameters. Materials 2021, 14, 2540. https://doi.org/10.3390/ma14102540
Ribeiro B, Offoiach R, Rahimi E, Salatin E, Lekka M, Fedrizzi L. On Growth and Morphology of TiO2 Nanotubes on Ti6Al4V by Anodic Oxidation in Ethylene Glycol Electrolyte: Influence of Microstructure and Anodization Parameters. Materials. 2021; 14(10):2540. https://doi.org/10.3390/ma14102540
Chicago/Turabian StyleRibeiro, Bruno, Ruben Offoiach, Ehsan Rahimi, Elisa Salatin, Maria Lekka, and Lorenzo Fedrizzi. 2021. "On Growth and Morphology of TiO2 Nanotubes on Ti6Al4V by Anodic Oxidation in Ethylene Glycol Electrolyte: Influence of Microstructure and Anodization Parameters" Materials 14, no. 10: 2540. https://doi.org/10.3390/ma14102540






