An Innovative Method of Converting Ferrous Mill Scale Wastes into Superparamagnetic Nanoadsorbents for Water Decontamination
Abstract
1. Introduction
2. Materials and Methods
2.1. Steps for MS Converting into ION
2.1.1. Acidic Dissolution Step for MS-ION Preparation as Precursor
2.1.2. Coprecipitation Method for ION Preparation
2.2. Characterization and Composition of MS, MS-ION and ION
2.3. Magnetic Properties and BET Surface Analysis
2.4. Adsorption Tests
3. Results and Discussion
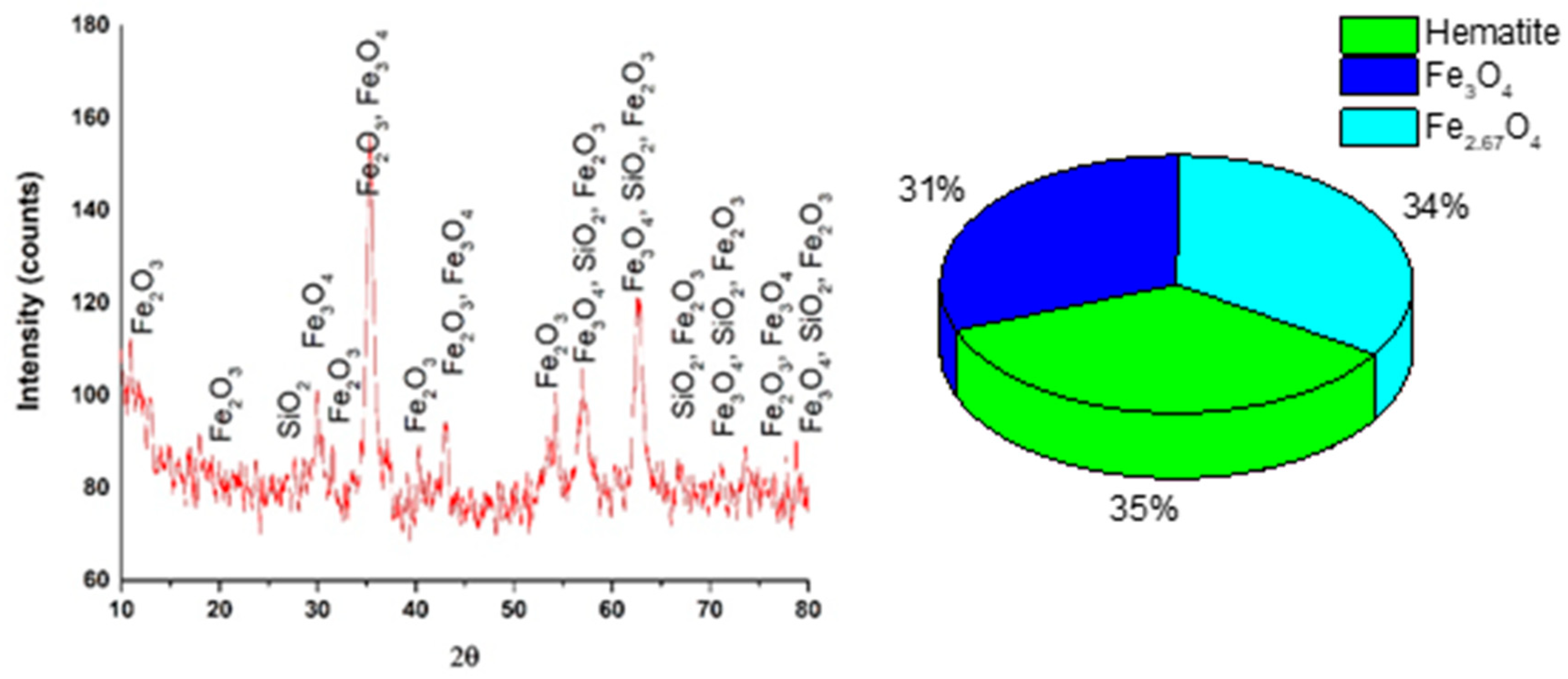
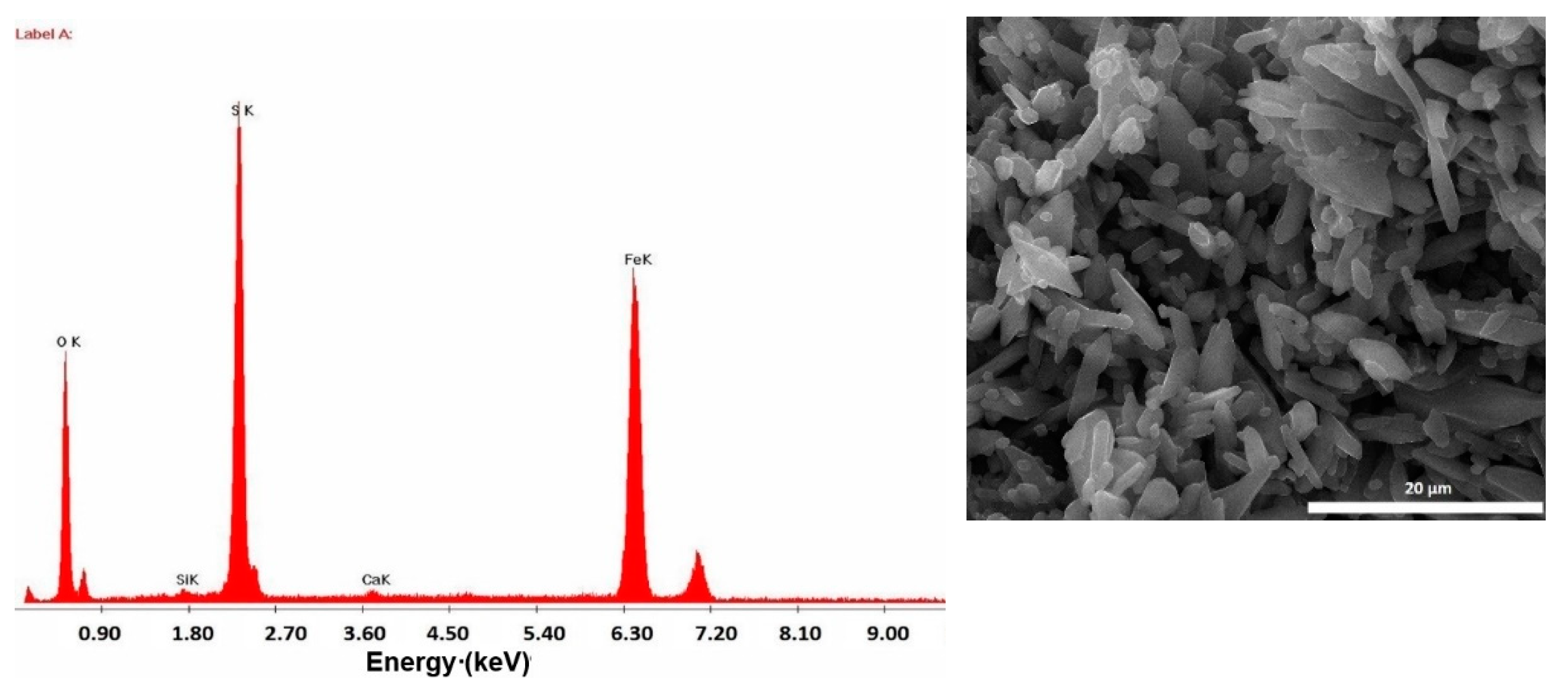
3.1. Characterization and Composition of MS
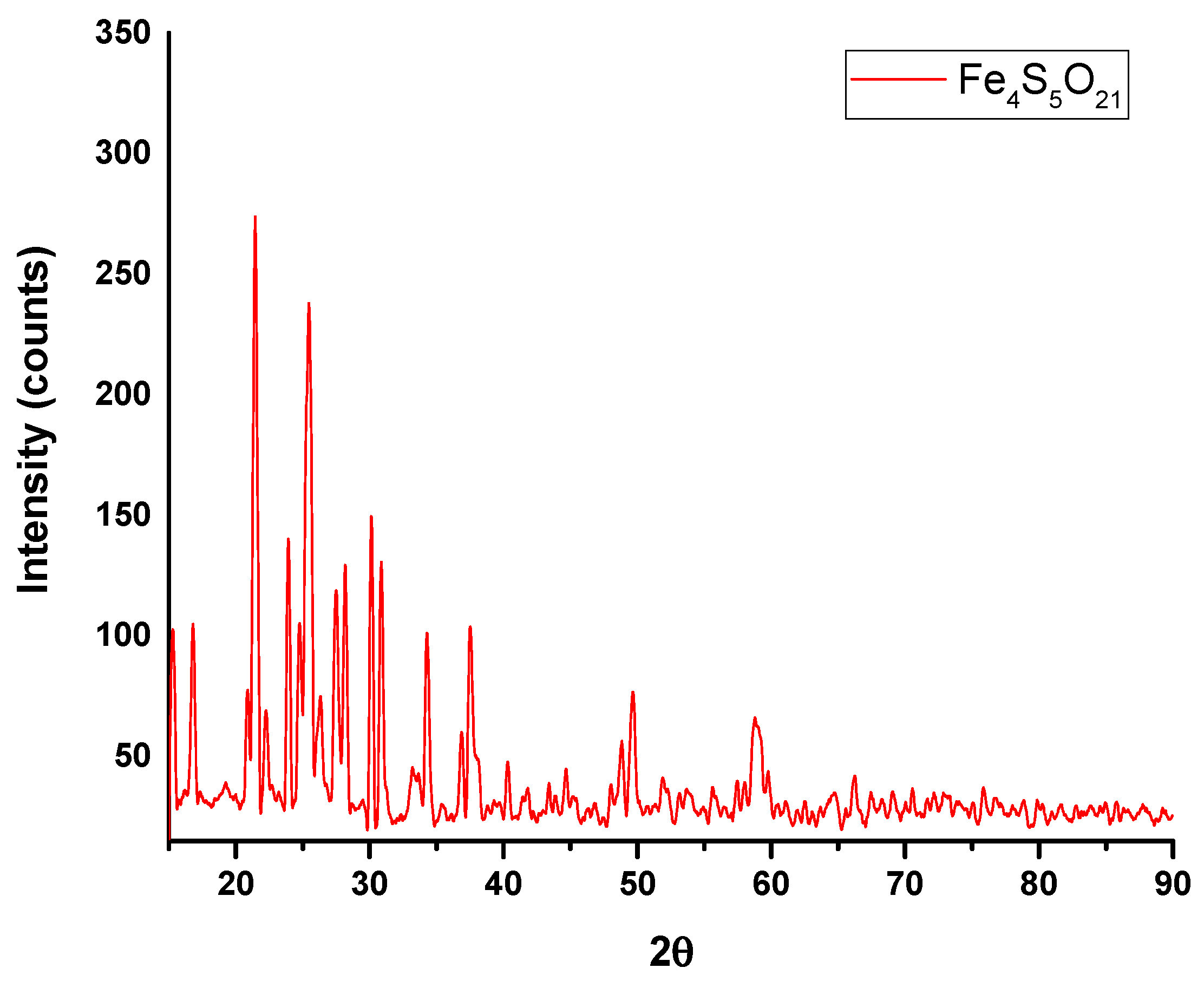
3.2. Characterization and Composition of MS-ION
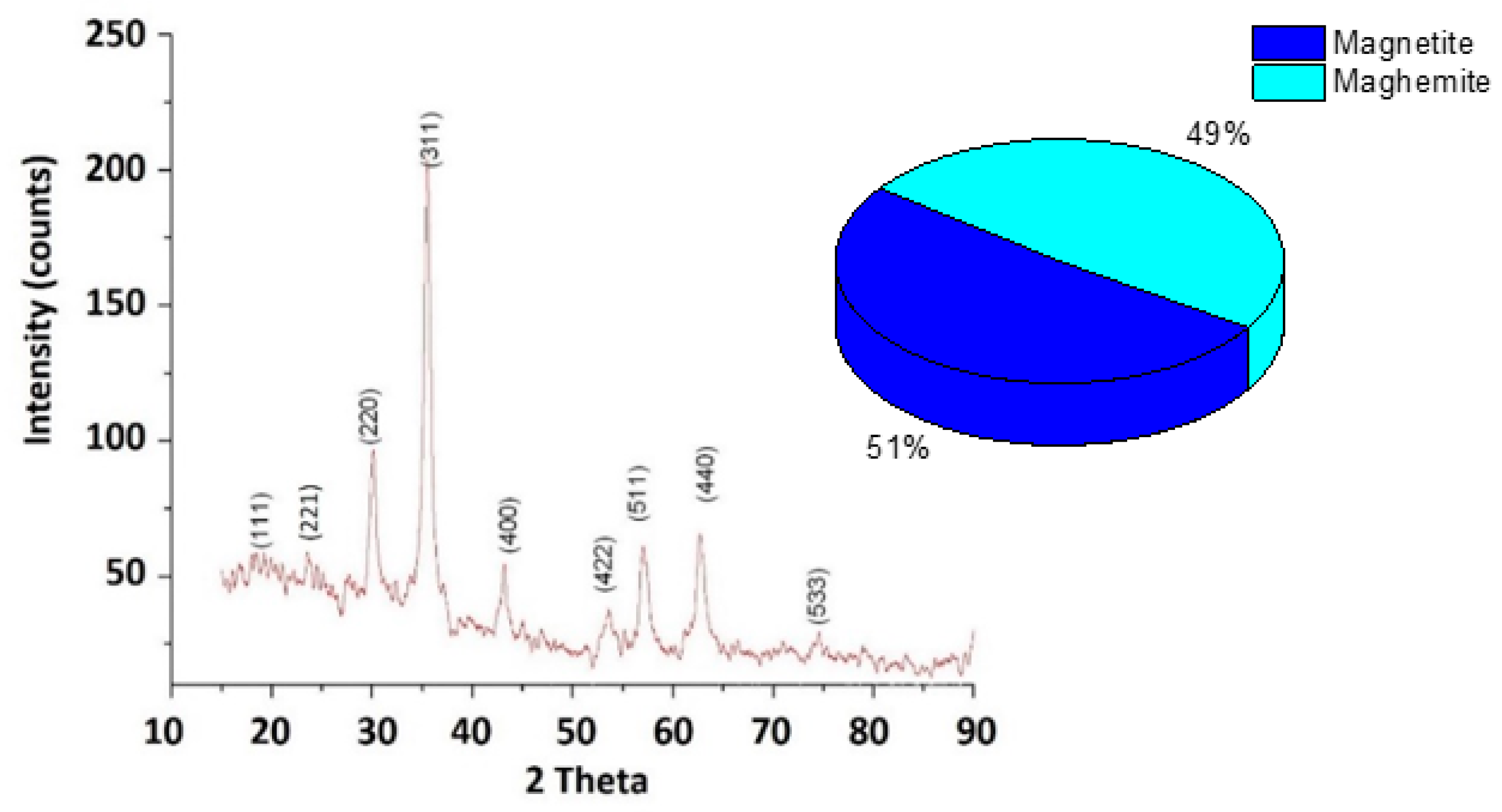
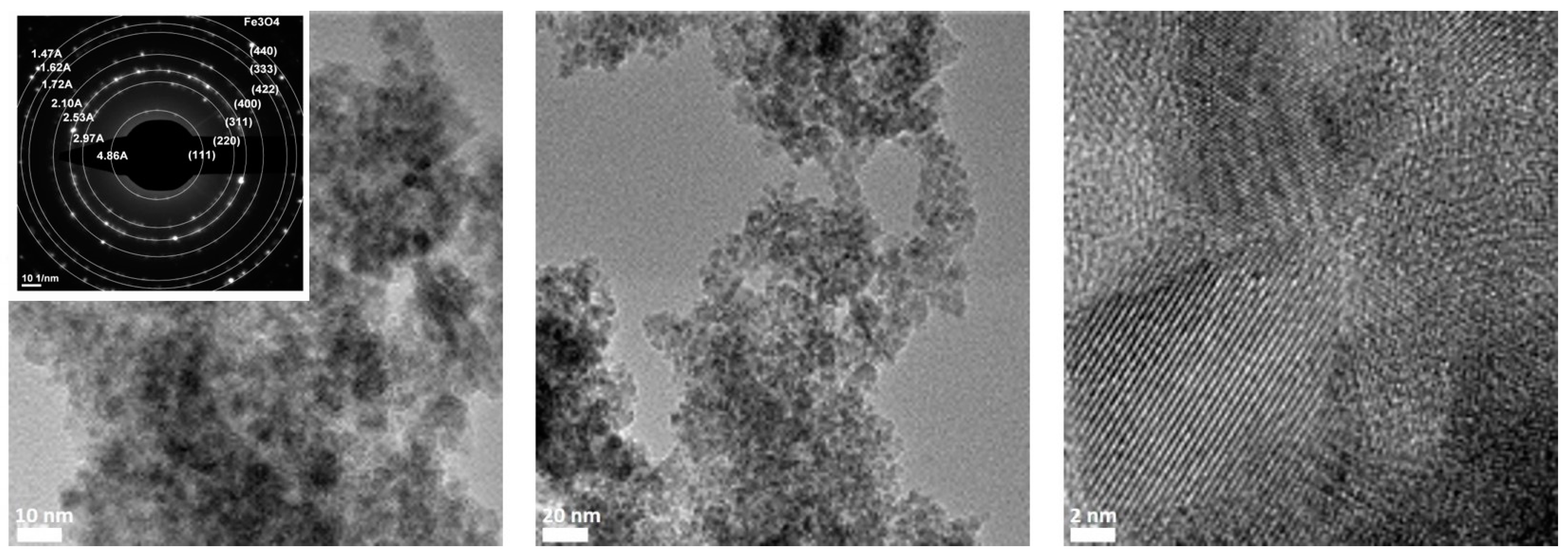
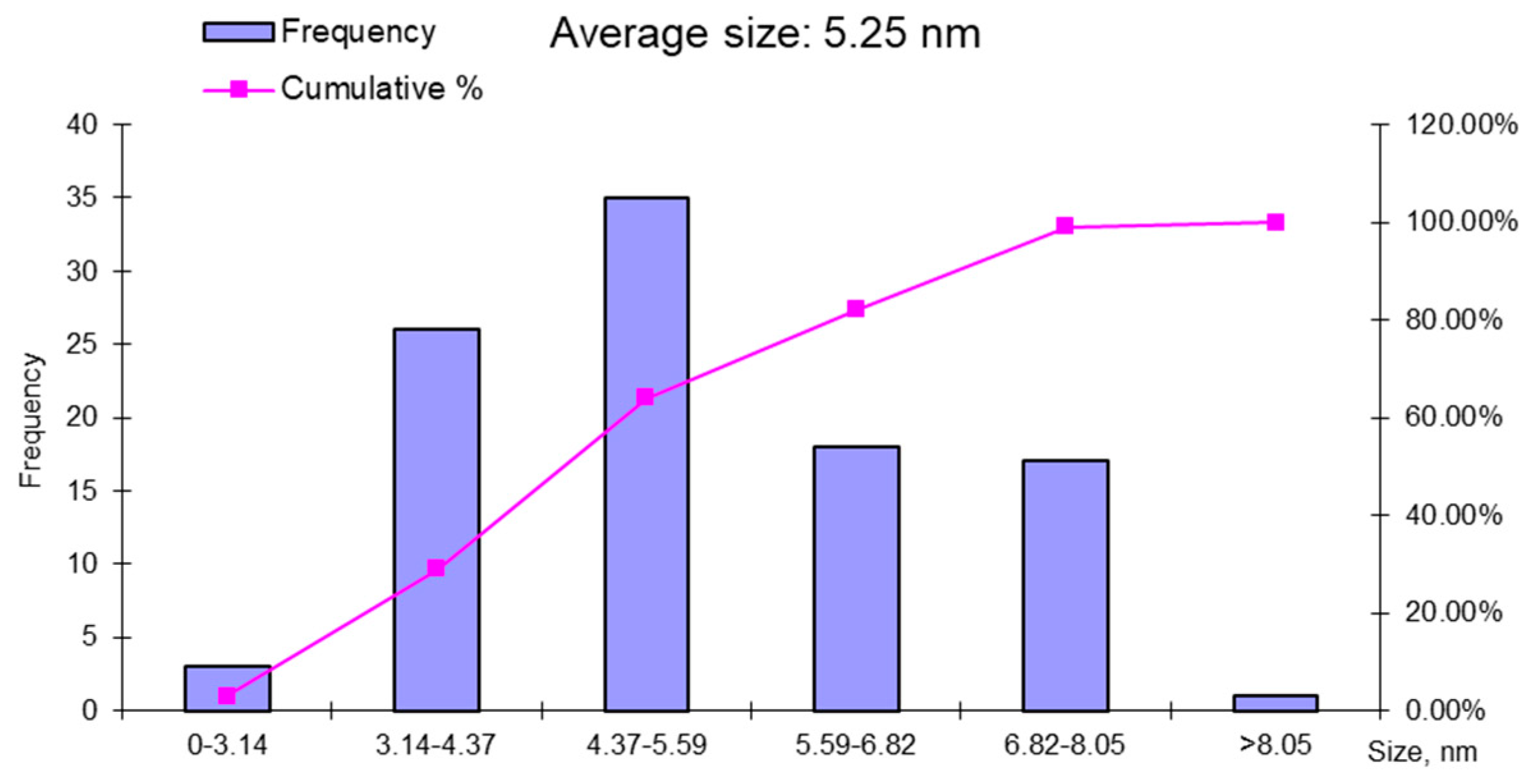
3.3. Characterization and Composition of ION
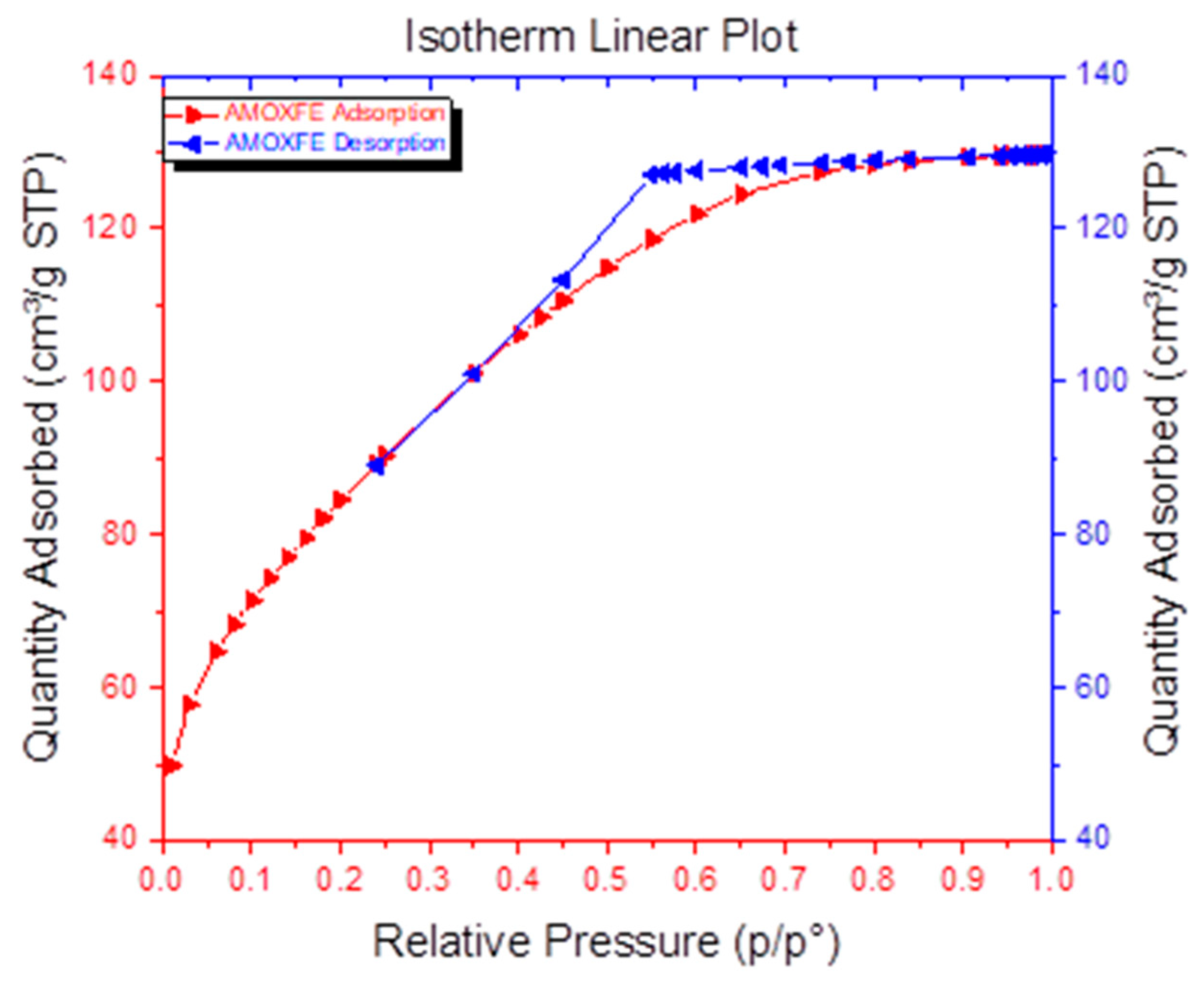
3.4. Magnetic and Surface Properties Investigations
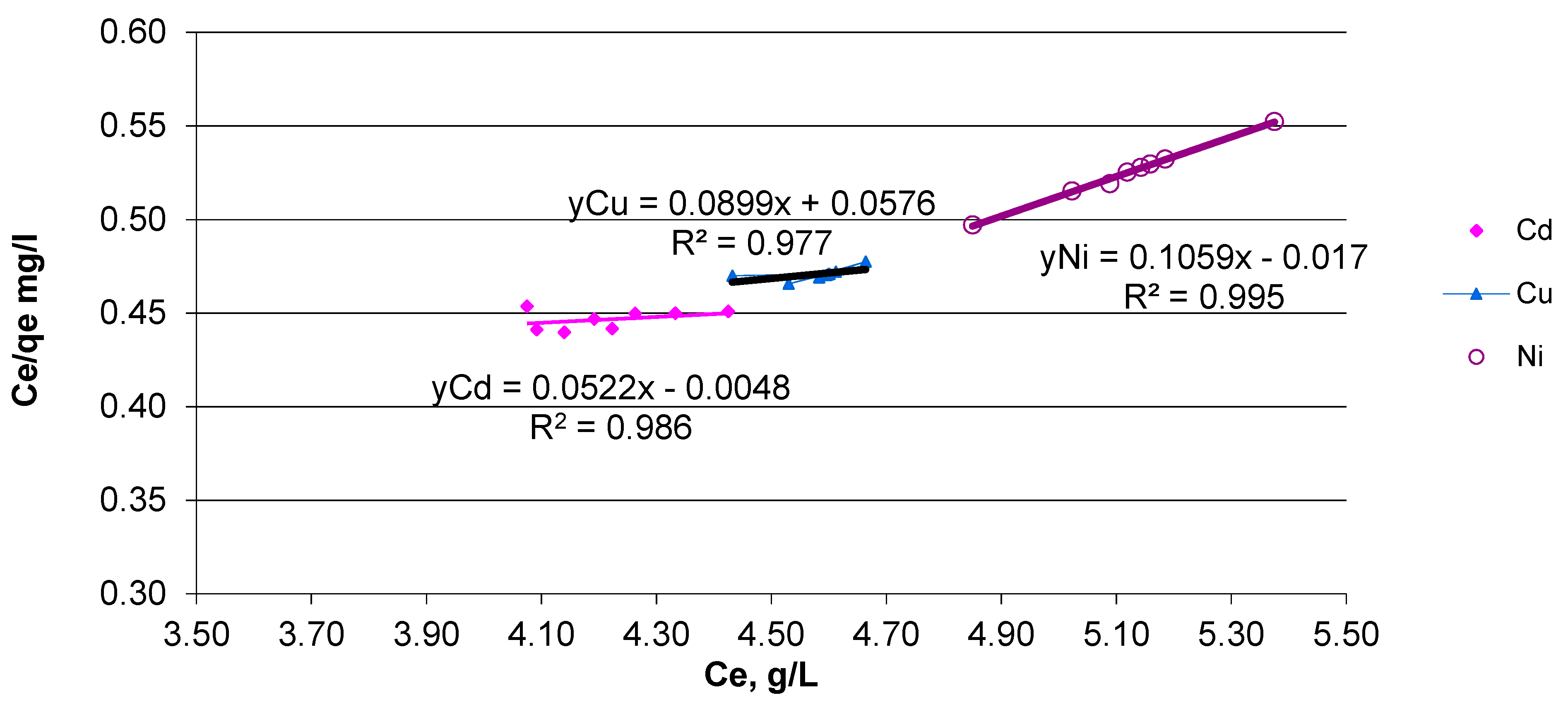
3.5. Adsorption Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, N.; Houser, J.B.; Wood, L.A. Production of cleaner mill scale by dynamic separation of the mill scale from the fast-moving flume water at a hot rolling mill. J. Clean. Prod. 2018, 176, 889–894. [Google Scholar] [CrossRef]
- Giri, S.; Das, N.; Pradhan, G. Magnetite powder and kaolinite derived from waste iron ore tailings for environmental applications. Powder Technol. 2011, 214, 513–518. [Google Scholar] [CrossRef]
- Eissa, M.; Ahmed, A.; El-Fawkhry, M. Conversion of Mill Scale Waste into Valuable Products via Carbothermic Reduction. J. Met. 2015, 2015, 926028. [Google Scholar] [CrossRef][Green Version]
- Paswan, M.D.; Malathi, R.; Minj, K.; Bandopadhyay, D. Mill Scale: A Potential Raw Material for Iron and Steel Making. Steelworld 2015, 21, 3. [Google Scholar]
- Sanin, V.N.; Ikornikov, D.M.; Andreev, D.E.; Sachkova, N.V.; Yukhvid, V.I. Mill scale recycling by SHS metallurgy for production of cast ferrosilicon and ferrosilicoaluminium. IOP Conf. Ser. Mater. Sci. Eng. 2019, 558, 012041. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J. Metal Recovery from Stainless Steel Mill Scale by Microwave Heating. Met. Mater. Int. 2008, 14, 193–196. [Google Scholar] [CrossRef]
- Skvára, F.; Kastánek, F.; Pavelková, I.; Solcová, O.; Maléterová, Y.; Schneider, P. Solidification of waste steel foundry dust with Portland cement. J. Hazard. Mater. 2002, 89, 67–81. [Google Scholar] [CrossRef]
- Danielewski, M. Gaseous Corrosion Mechanisms; ASM International: Novelty, OH, USA, 2013; Volume 13A. [Google Scholar]
- Azad, A.M.; Kesavan, S.; Al-Batty, S. Redemption of microscale mill waste into commercial nanoscale asset. In Innovation in Materials Science; Sekhar, J.A., Dismukes, J.P., Eds.; Key Engineering Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2008; Volume 380, pp. 229–255. [Google Scholar]
- El-Hussiny, N.A.; Mohamed, F.M.; Shalabi, M.E.H. Recycling of mill scale in sintering process. Sci. Sinter. 2011, 43, 21–31. [Google Scholar] [CrossRef]
- Gaballah, N.M.; Zikry, A.F.; Khalifa, M.G.; Farag, A.B.; El-Hussiny, N.A.; Shalabi, M.E.H. Production of Iron from Mill Scale Industrial Waste via Hydrogen. Open J. Inorg. Non-Met. Mater. 2013, 3, 23–28. [Google Scholar] [CrossRef][Green Version]
- Legodi, M.; De Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dye. Pigment. 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Spiliotis, X.; Ntampegliotis, K.; Kasiteropoulou, D.; Lamprakopoulos, S.; Lolos, K.; Karayannis, V.; Papapolymerou, G. Valorization of Mill Scale Waste by its Incorporation in Fired Clay Bricks. Key Eng. Mater. 2014, 608, 8–13. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Silva, R.D.A.; Castro, C.D.; Vigânico, E.M.; Petter, C.O.; Schneider, I.A.H. Selective precipitation/UV production of magnetite particles obtained from the iron recovered from acid mine drainage. Miner. Eng. 2012, 29, 22–27. [Google Scholar] [CrossRef]
- Al-Anber, Z.A.; Al-Anber, M.A.; Matouq, M.; Al-Ayed, O.; Omari, N.M. Defatted Jojoba for the removal of methylene blue from aqueous solution: Thermodynamic and kinetic studies. Desalination 2011, 276, 169–174. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Arica, M.Y. Kinetics of mercury ions removal from synthetic aqueous solutions using by novel magnetic p(GMA-MMA-EGDMA) beads. J. Hazard. Mater. 2007, 144, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Keyvani, F.; Rahpeima, S.; Javanbakht, V. Synthesis of EDTA-modified magnetic activated carbon nanocomposite for removal of permanganate from aqueous solutions. Solid State Sci. 2018, 83, 31–42. [Google Scholar] [CrossRef]
- Nowruzi, R.; Heydari, M.; Javanbakht, V. Synthesis of a chitosan/polyvinyl alcohol/activate carbon biocomposite for removal of hexavalent chromium from aqueous solution. Int. J. Biol. Macromol. 2020, 147, 209–216. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, Y.; Zeng, G.; Xu, W.; Yang, C.; Zhang, J. Biosorption of copper(II) by immobilizing Saccharomyces cerevisiae on the surface of chitosan-coated magnetic nanoparticles from aqueous solution. J. Hazard. Mater. 2010, 177, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H. Simultaneous removal of heavy-metal ions in wastewater samples using nano-alumina modified with 2,4-dinitrophenylhydrazine. J. Hazard. Mater. 2010, 181, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Izatt, R.M.; Christensen, J.J.; Rytting, J.H. Sites and thermodynamic quantities associated with proton and metal ion interaction with ribonucleic acid, deoxyribonucleic acid, and their constituent bases, nucleosides, and nucleotides. Chem. Rev. 1971, 71, 439–481. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-M.; Man, C.; Hu, Z.-B. Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Dryden, D.M.; Sun, T.; McCormick, R.; Hickey, R.; Vidu, R.; Stroeve, P. Anomalous Deposition of Co-Ni Alloys in Film and Nanowire Morphologies from Citrate Baths. Electrochim. Acta 2016, 220, 595–600. [Google Scholar] [CrossRef]
- Quach, D.V.; Vidu, R.; Groza, J.R.; Stroeve, P. Electrochemical Deposition of Co−Sb Thin Films and Nanowires. Ind. Eng. Chem. Res. 2010, 49, 11385–11392. [Google Scholar] [CrossRef]
- Quinlan, F.T.; Vidu, R.; Predoana, L.; Zaharescu, M.; Gartrner, M.; Groza, J.; Stroeve, P. Lithium Cobalt Oxide (LiCoO2) Nanocoatings by Sol−Gel Methods. Ind. Eng. Chem. Res. 2004, 43, 2468–2477. [Google Scholar] [CrossRef]
- Hua, C.C.; Zakaria, S.; Farahiyan, R.; Khong, L.T.; Nguyen, K.L.; Abdullah, M.; Ahmad, S. Size-controlled Synthesis and Characterization of Fe3O4 Nanoparticles by Chemical Coprecipitation Method. Sains Malays. 2008, 37, 389–394. [Google Scholar]
- Jaramillo Tabares, B.; Zuleta Gil, A.A.; Jaramillo Isaza, F. Effects of the Synthetic Method on the Particle Size and Purity of Magnetite. Revista Facultad de Ingeniería Universidad de Antioquia Medelin Colombia 2009, 50, 9–16. [Google Scholar]
- Khan, U.S.; Manan, A.; Khan, N.; Mahmood, A.; Rahim, A. Transformation mechanism of magnetite nanoparticles. Mater. Sci. 2015, 33, 278–285. [Google Scholar] [CrossRef]
- Khodabakhshi, M.A.; Amin, M.; Mozaffari, M. Synthesis of Magnetite Nanoparticles and Evaluation of Its Efficiency for Arsenic Removal from Simulated Industrial Wastewater. Iran. J. Environ. Health Sci. Eng. 2011, 8, 12. [Google Scholar]
- Demirbas, A. Agricultural based activated carbons for the removal of dyes from aqueous solutions: A review. J. Hazard. Mater. 2009, 167, 1–9. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Rios, R.; Fabris, J.D.; Garg, V.; Sapag, K.; Lago, R.M. Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water. Carbon 2002, 40, 2177–2183. [Google Scholar] [CrossRef]
- Gnanaprakash, G.; Mahadevan, S.; Jayakumar, T.; Kalyanasundaram, P.; Philip, J.; Raj, B. Effect of initial pH and temperature of iron salt solutions on formation of magnetite nanoparticles. Mater. Chem. Phys. 2007, 103, 168–175. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.-L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Predescu, A.M.; Matei, E.; Berbecaru, A.C.; Pantilimon, C.; Drăgan, C.; Vidu, R.; Predescu, C.; Kuncser, V. Synthesis and characterization of dextran-coated iron oxide nanoparticles. R. Soc. Open Sci. 2018, 5, 171525. [Google Scholar] [CrossRef]
- Tang, S.C.; Lo, I.M. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef] [PubMed]
- Van Fan, Y.; Varbanov, P.S.; Klemeš, J.J.; Romanenko, S.V. Urban and industrial symbiosis for circular economy: Total EcoSite Integration. J. Environ. Manag. 2021, 279, 111829. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Nomura, T.; Mizoe, K. A new synthesis route from spent sulfuric acid pickling solution to ferrite nanoparticles. Hydrometallurgy 2004, 74, 57–65. [Google Scholar] [CrossRef]
- López-Delgado, A.; Alguacil, F.; López, F. Recovery of iron from bio-oxidized sulphuric pickling waste water by precipitation as basic sulphates. Hydrometallurgy 1997, 45, 97–112. [Google Scholar] [CrossRef]
- Tang, B.; Yuan, L.; Shi, T.; Yu, L.; Zhu, Y. Preparation of nano-sized magnetic particles from spent pickling liquors by ultrasonic-assisted chemical co-precipitation. J. Hazard. Mater. 2009, 163, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Dehsari, H.S.; Ksenofontov, V.; Möller, A.; Jakob, G.; Asadi, K. Determining Magnetite/Maghemite Composition and Core–Shell Nanostructure from Magnetization Curve for Iron Oxide Nanoparticles. J. Phys. Chem. C 2018, 122, 28292–28301. [Google Scholar] [CrossRef]
- Nauer, G.; Strecha, P.; Brinda-Konopik, N.; Liptay, G. Spectroscopic and thermoanalytical characterization of standard substances for the identification of reaction products on iron electrodes. J. Therm. Anal. Calorim. 1985, 30, 813–830. [Google Scholar] [CrossRef]
- Matei, E.; Covaliu, C.I.; Predescu, A.; Coman, G.; Drăgan, C.; Nițu, C.V.; Predescu, C. Synthesis of a magnetic composite for environmental engineering application. Rev. Rom. Mater. 2019, 49, 8. [Google Scholar]
- Matei, E.; Predescu, A.M.; Râpă, M.; Tarcea, C.; Pantilimon, C.M.; Favier, L.; Berbecaru, A.C.; Sohaciu, M.; Predescu, C. Removal of Chromium(VI) from Aqueous Solution Using a Novel Green Magnetic Nanoparticle–Chitosan Adsorbent. Anal. Lett. 2019, 52, 2416–2438. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, H.; Shi, J.X.; Langrish, T.A.G. Adsorption of Chromium(VI) from Aqueous Solutions Using Cross-Linked Magnetic Chitosan Beads. Ind. Eng. Chem. Res. 2009, 48, 2646–2651. [Google Scholar] [CrossRef]
- Lasheen, M.; El-Sherif, I.Y.; Tawfik, M.E.; El-Wakeel, S.; El-Shahat, M. Preparation and adsorption properties of nano magnetite chitosan films for heavy metal ions from aqueous solution. Mater. Res. Bull. 2016, 80, 344–350. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Nagpure, A.; Gupta, R.K. Bacterial Chitinases: Properties and Potential. Crit. Rev. Biotechnol. 2007, 27, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Matei, E.; Predescu, A.M.; Predescu, C.; Sohaciu, M.G.; Berbecaru, A.; Covaliu, C.I. Characterization and Application Results of Two Magnetic Nanomaterials. J. Environ. Qual. 2013, 42, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Mazari, S.A.; Nizamuddin, S.; Karri, R.R. Magnetic nanoadsorbents’ potential route for heavy metals removal—A review. Environ. Sci. Pollut. Res. 2020, 27, 24342–24356. [Google Scholar] [CrossRef] [PubMed]
- Predescu, C.; Matei, E.; Predescu, A.M.; Berbecaru, A.C.; Vidu, R. Magnetic Nanostructures and Device Implementing Same. U.S. Patent US469555B2, 18 October 2016. [Google Scholar]
- Ilyushenkov, D.S.; Kozub, V.I.; Yavsin, D.A.; Kozhevin, V.M.; Yassievich, I.N.; Nguyen, T.T.; Bruck, E.H.; Gurevich, S.A. Magnetic properties of self-assembled nanostructure films on the base of amorphous Ni granules. J. Magn. Magn. Mater. 2009, 321, 343–347. [Google Scholar] [CrossRef]
- Tombacz, E.; Majzik, A.; Horvat, E.; Illes, E. Magnetite in aqueous medium: Coating its surface and surface coated with it. Rom. Rep. Phys. 2006, 58, 6. [Google Scholar]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- van Loon, G.W.; Duffy, S.J. Environmental Chemistry: A Global Perspective, 2nd ed.; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]







| Element | Average Content, wt.% |
|---|---|
| Fe | 77.06 ± 0.04 |
| O | 13.35 ± 0.15 |
| Ca | 4.50 ± 0.26 |
| Si | 1.90 ± 0.88 |
| Mn | 0.83 ± 0.41 |
| Na | 0.71 ± 5.72 |
| Al | 0.64 ± 1.87 |
| Mg | 0.22 ± 4.40 |
| P | 0.18 ± 2.51 |
| Cr | 0.04 ± 2.26 |
| Ti | 0.03 ± 4.01 |
| Fe Concentration, mg/L | Analysis Method |
|---|---|
| Fe tot = 242.93 | AAS (liquid) |
| Fe tot = 247.92 | XRF (liquid) |
| Fe(II) = 50.80 | Spectrophotometry (liquid) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predescu, A.M.; Matei, E.; Berbecaru, A.C.; Râpă, M.; Sohaciu, M.G.; Predescu, C.; Vidu, R. An Innovative Method of Converting Ferrous Mill Scale Wastes into Superparamagnetic Nanoadsorbents for Water Decontamination. Materials 2021, 14, 2539. https://doi.org/10.3390/ma14102539
Predescu AM, Matei E, Berbecaru AC, Râpă M, Sohaciu MG, Predescu C, Vidu R. An Innovative Method of Converting Ferrous Mill Scale Wastes into Superparamagnetic Nanoadsorbents for Water Decontamination. Materials. 2021; 14(10):2539. https://doi.org/10.3390/ma14102539
Chicago/Turabian StylePredescu, Andra Mihaela, Ecaterina Matei, Andrei Constantin Berbecaru, Maria Râpă, Mirela Gabriela Sohaciu, Cristian Predescu, and Ruxandra Vidu. 2021. "An Innovative Method of Converting Ferrous Mill Scale Wastes into Superparamagnetic Nanoadsorbents for Water Decontamination" Materials 14, no. 10: 2539. https://doi.org/10.3390/ma14102539
APA StylePredescu, A. M., Matei, E., Berbecaru, A. C., Râpă, M., Sohaciu, M. G., Predescu, C., & Vidu, R. (2021). An Innovative Method of Converting Ferrous Mill Scale Wastes into Superparamagnetic Nanoadsorbents for Water Decontamination. Materials, 14(10), 2539. https://doi.org/10.3390/ma14102539









