Hard Dental Tissues Regeneration—Approaches and Challenges
Abstract
:1. Introduction
2. Elements of the Dental Tissue’s Regeneration Process
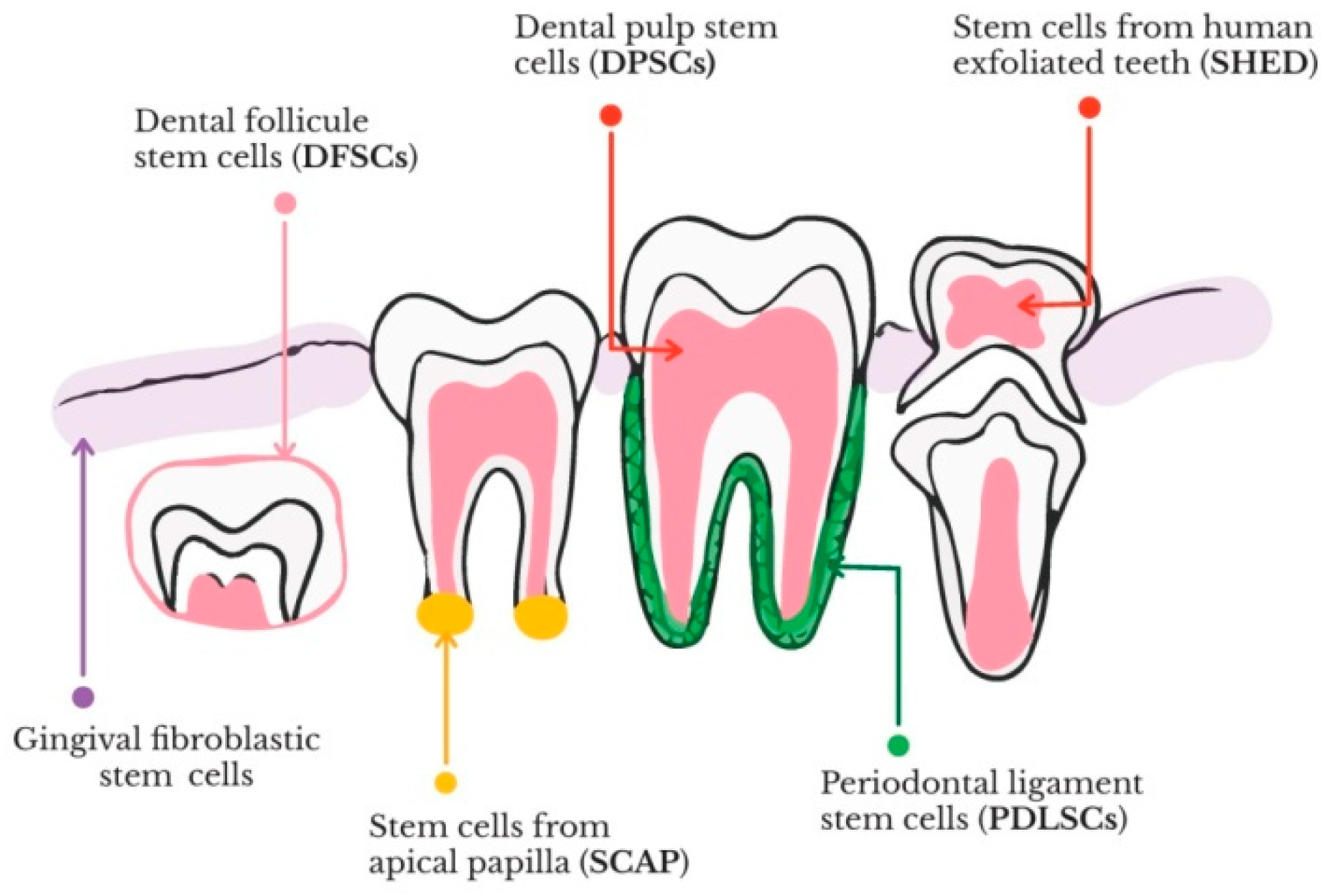
2.1. Stem Cells
2.2. Growth Factors
2.3. Scaffolds for the Regeneration of Hard Dental Tissues
2.3.1. Polymers-Based Scaffolds
2.3.2. Bioactive Ceramic Scaffolds
2.3.3. Composite Scaffolds
3. Enamel Regeneration
4. Dentin Regeneration
5. Cementum Regeneration
5.1. Multiphase Scaffolds for Cementum Regeneration
5.2. 3D Printed Scaffolds for Cementum Regeneration
5.3. Gels and Hydrogels for Cementum Regeneration
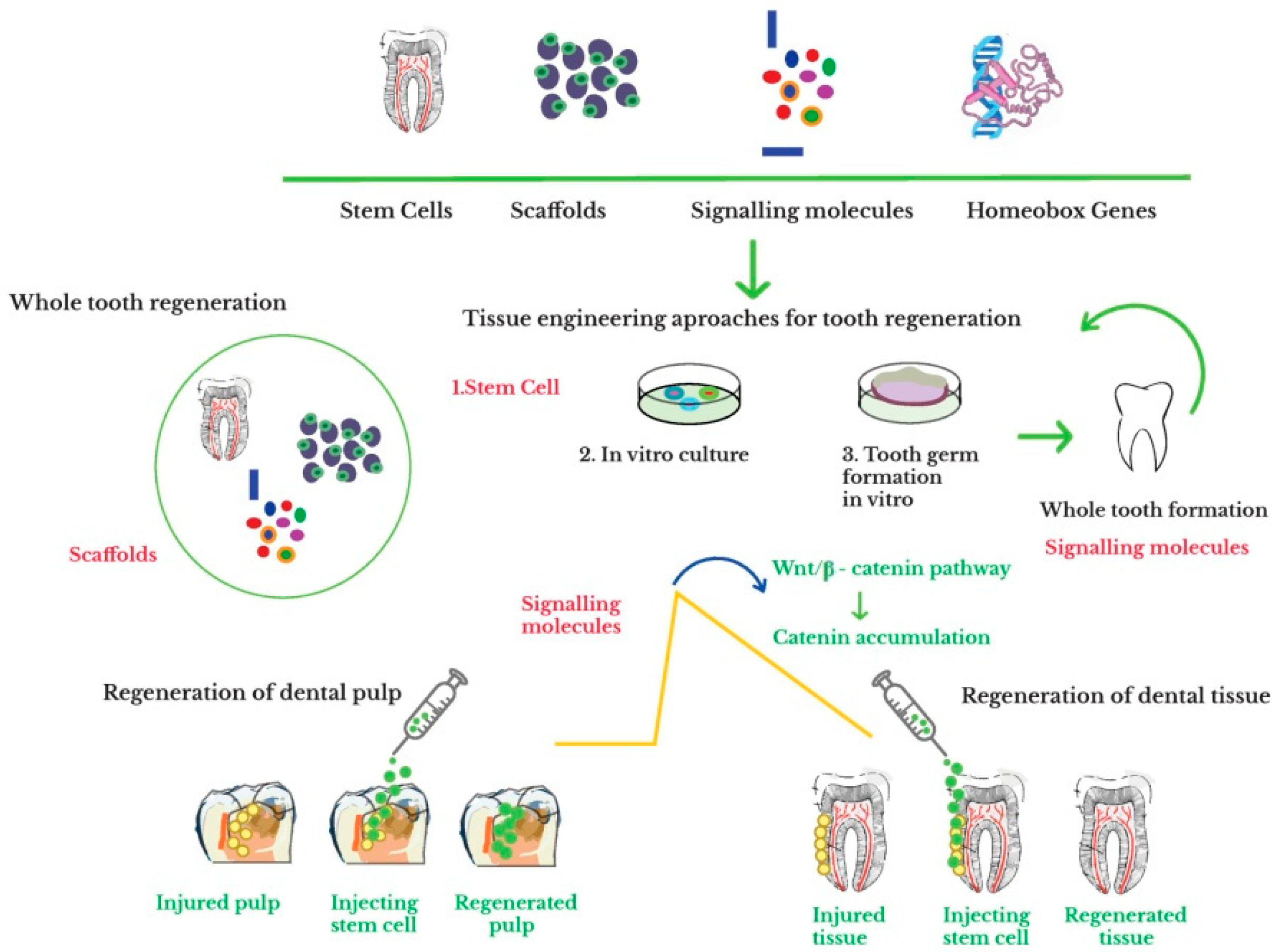
6. Whole Tooth Engineering
6.1. In Situ Tooth Regeneration by Stimulating the Tooth Replacement Ability
6.2. Whole Tooth Regeneration through Bioengineered Organ Germ Method
6.3. Whole Tooth Regeneration through Tissue Engineering Approach
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beniash, E.; Stifler, C.A.; Sun, C.-Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Pupa, U.P.; Gilbert, A. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, M.; Akazawa, T.; Mitsugi, M.; Arafat, M.; Um, I.W.; Minamida, Y.; Kim, K.W.; Kim, Y.K.; Sun, Y.; Qin, C. Autograft of dentin materials for bone regeneration. Adv. Biomater. Sci. Biomed. Appl. 2013, 15, 391–403. [Google Scholar]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Conrads, G.; About, I. Pathophysiology of dental caries. In Caries Excavation, Evolution of Treating Cavitated Carious Lesions; Karger Medical and Scientific Publishers: Berlin, Germany, 2018; pp. 1–10. [Google Scholar]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering, strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef]
- Yen, A.H.; Sharpe, P.T. Stem cells and tooth tissue engineering. Cell Tissue Res. 2008, 331, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Srivastava, D.; Grover, S.; Sharma, V. Biomaterials in tooth tissue engineering, a review. J. Clin. Diagn. Res. JCDR 2014, 8, 309–315. [Google Scholar] [CrossRef]
- Otsu, K.; Kumakami-Sakano, M.; Fujiwara, N.; Kikuchi, K.; Keller, L.; Lesot, H.; Hidemitsu, H. Stem cell sources for tooth regeneration: Current status and future prospects. Front. Physiol. 2014, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Baranova, J.; Büchner, D.; Götz, W.; Schulze, M.; Tobiasch, E. Tooth formation: Are the hardest tissues of human body hard to regenerate? Int. J. Mol. Sci. 2020, 21, 4031. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.M.; Abouauf, E.A.; AbuBakr, N.; Dörfer, C.E.; El-Sayed, K.F. Tissue engineering approaches for enamel, dentin, and pulp regeneration: An update. Stem Cells Int. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Park, C.H. Tooth-supporting hard tissue regeneration using biopolymeric material fabrication strategies. Molecules 2020, 25, 4802. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in pluripotent stem cells: History, mechanisms, technologies, and applications. Stem Cell Rev. 2019, 16, 3–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; He, J.; Zhang, C.; Xu, J.; Wang, Y. Strategies for derivation of endothelial lineages from human stem cells. Stem Cell Res. Ther. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Orbay, H.; Tobita, M.; Mizuno, H. Mesenchymal stem cells isolated from adipose and oTher. tissues: Basic biological properties and clinical applications. Stem Cells Int. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental tissue-derived human mesenchymal stem cells and their potential in therapeutic application. Stem Cells Int. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2018, 13, 152–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y. Bioengineering of a human whole tooth: Progress and challenge. Cell Regen. 2014, 3, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Sahab-Negah, S.; Hajali, V.; Moradi, H.R.; Gorji, A. The impact of estradiol on neurogenesis and cognitive functions in Alzheimer’s disease. Cell. Mol. Neurobiol. 2020, 40, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Feki, A.; Papaccio, G.; Catón, J. Dental pulp stem cells, niches, and notch signaling in tooth injury. Adv. Dent. Res. 2011, 23, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from oTher. sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L.; Xiao, L.; Zhang, D. Recycle the dental fairy’s package: Overview of dental pulp stem cells. Stem Cell Res. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Okiji, T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit. Anom. 2016, 56, 144–153. [Google Scholar] [CrossRef]
- Ikeda, E.; Nakagawa, M.; Ogawa, M.; Takeo, M.; Tsuji, T. Functional tooth regeneration as a next-generation therapy. J. Dent. Oral Disord. 2020, 6, 114. [Google Scholar]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function, 8th ed.; Mosby: Maryland Heights, MO, USA, 2003; p. 400. [Google Scholar]
- Stevens, M.M. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Chen, F.-M.; An, Y.; Zhang, R.; Zhang, M. New insights into and novel applications of release technology for periodontal reconstructive therapies. J. Control. Release 2011, 149, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.F.; Butler, P.E.; Seifalian, A.M.; Kalaskar, D.M. Control of stem cell fate by engineering their micro and nanoenvironment. World J. Stem Cells 2015, 26, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Aurrekoetxea, M.; Irastorza, I.; García-Gallastegui, P.; Jiménez-Rojo, L.; Nakamura, T.; Yamada, Y.; Ibarretxe, G.; Unda, F.J. Wnt/β-catenin regulates the activity of epiprofin/Sp6, SHH, FGF, and BMP to coordinate the stages of odontogenesis. Front. Cell Dev. Biol. 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seppala, M.; Fraser, G.; Birjandi, A.; Xavier, G.; Cobourne, M. Sonic hedgehog signaling and development of the dentition. J. Dev. Biol. 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saygin, N.E.; Tokiyasu, Y.; Giannobile, W.V.; Somerman, M.J. Growth factors regulate expression of mineral associated genes in cementoblasts. J. Periodontol. 2000, 71, 1591–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, V.A.; Jung, J.-Y.; Koh, J.-T.; Oh, W.-M.; Hwang, Y.-C.; Lee, B.-N. Leptin induces odontogenic differentiation and angiogenesis in human dental pulp cells via activation of the mitogen-activated protein kinase signaling pathway. J. Endod. 2018, 44, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Luder, H.U.; Graf, D. Bone morphogenetic protein 2 coordinates early tooth mineralization. J. Dent. Res. 2018, 97, 835–843. [Google Scholar]
- Ishikawa, Y.; Nakatomi, M.; Ida-Yonemochi, H.; Ohshima, H. Quiescent adult stem cells in murine teeth are regulated by Shh signaling. Cell Tissue Res. 2017, 369, 497–512. [Google Scholar] [CrossRef]
- Tonk, C.; Witzler, M.; Schulze, M.; Tobiasch, E. Mesenchymal stem cells. In Essential Current Concepts in Stem Cell Biology; Brand-Saberi, B., Ed.; Springer: Berlin, Germany, 2020; pp. 21–39. [Google Scholar]
- Witzler, M.; Alzagameem, A.; Bergs, M.; Khaldi-Hansen, B.E.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-derived biomaterials for drug release and tissue engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef] [Green Version]
- Khaldi-Hansen, E.B.; El-Sayed, F.; Tobiasch, E.; Witzleben, S.; Schulze, M. Functionalized 3D scaffolds for template- mediated biomineralization in bone regeneration. Front. Stem Cell Regen. Med. Res. 2017, 4, 3–58. [Google Scholar]
- Leiendecker, A.; Witzleben, S.; Schulze, M.; Tobiasch, E. Template-mediated biomineralization for bone tissue engineering. Curr. Stem Cell Res. T 2016, 12, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Bains, P.S.; Sidhu, S.S.; Bahraminasab, M.; Prakash, C. Materials Horizons: From Nature to Nanomaterials; Springer Nature Pte Ltd.: Singapore, 2019. [Google Scholar]
- Horst, O.V.; Chavez, M.G.; Jheon, A.H.; Desai, T.; Klein, O.D. Stem cell and biomaterials reseArch. in dental tissue engineering and regeneration. Dent. Clin. N. Am. 2012, 56, 495–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ritchie, A.C.; Everitt, N.M. Comparison of glutaraldehyde and procyanidin cross-linked scaffolds for soft tissue engineering. Mat. Sci. Eng. C 2017, 80, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Coyac, B.R.; Chicatun, F.; Hoac, B.; Nelea, V.; Chaussain, C.; Nazhat, S.N.; McKee, M.D. Mineralization of dense collagen hydrogel scaffolds by human pulp cells. J. Dent. Res. 2013, 92, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Cheema, U.; Knowles, J.C.; Brown, R.A.; Nazhat, S.N. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter 2006, 2, 986. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseinie, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio. 2019, 1, 100008. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.; Pins, G. Cell growth on collagen: A review of tissue engineering using scaffolds containing extracellular matrix. J. Long Term Eff. Med. Implant. 1992, 2, 67–80. [Google Scholar]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-F.; Wang, C.-Y.; Wan, P.; Wang, S.-G.; Wang, X.-M. Comparison of bone regeneration in alveolar bone of dogs on mineralized collagen grafts with two composition ratios of nano-hydroxyapatite and collagen. Regen. Biomater. 2015, 3, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Sancilio, S.; Gallorini, M.; Di Nisio, C.; Marsich, E.; Di Pietro, R.; Schweikl, H. Alginate/hydroxyapatite-based nanocomposite scaffolds for bone tissue engineering improve dental pulp biomineralization and differentiation. Stem Cells Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohamy, K.M.; Mabrouk, M.; Soliman, I.E.; Beherei, H.H.; Aboelnasr, M.A. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. MacroMol. 2018, 112, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Paolleti, S. Alginate/hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Farzin, A.; Bahrami, N.; Mohamadnia, A.; Mousavi, S.; Gholami, M.; Ai, J.; Moayeri, R.S. Scaffolds in dental tissue engineering: A review. Arch. NeuroSci. 2019, 7, e97014. [Google Scholar] [CrossRef] [Green Version]
- Masuda, K.; Sah, R.L.; Hejna, M.J.; Thonar, E.J. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: The alginate-recovered-chondrocyte (ARC) method. J. Orthop. Res. 2003, 21, 139–148. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Al Kayal, T.; Losi, P.; Pierozzi, S.; Soldani, G. A new method for fibrin-based electrospun/sprayed scaffold fabrication. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litvinov, R.I.; Weisel, J.W. Fibrin mechanical properties and their structural origins. Matrix Biol. 2017, 60–61, 110–123. [Google Scholar] [CrossRef]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and fibrin in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e13–e21. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.S.; Feng, Z.H.; Wu, G.F.; Bai, S.-Z.; Dong, Y.; Chen, F.-M.; Zhao, Y.-M. The use of platelet-rich fibrin combined with periodontal ligament and jaw bone mesenchymal stem cell sheets for periodontal tissue engineering. Sci. Rep. 2016, 6, 28126. [Google Scholar] [CrossRef] [PubMed]
- Sybil, D.; Sawai, M.; Faisal, M.; Singh, S.; Jain, V. Platelet-rich fibrin for hard- and soft-tissue healing in mandibular third molar extraction socket. Ann. Maxillofac. Surg. 2020, 10, 102–107. [Google Scholar] [PubMed]
- Bujoli, B.; Scimeca, J.C.; Verron, E. Fibrin as a multipurpose physiological platform for bone tissue engineering and targeted delivery of bioactive compounds. Pharmaceutics 2019, 11, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Ma, L.; Gao, C.; Wang, J.; Shen, J. Fabrication and properties of injectable β-tricalcium phosphate particles/fibrin gel composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2009, 29, 836–842. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, W.; Weir, M.D.; Xu, H.H.K. Biofunctionalized calcium phosphate cement to enhance the attachment and osteodifferentiation of stem cells released from fast-degradable alginate-fibrin microbeads. Tissue Eng. Part A 2012, 18, 1583–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Zhou, H.; Weir, M.D.; Bao, C.; Xu, H.H.K. Umbilical cord stem cells released from alginate–fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement for bone regeneration. Acta Biomater. 2012, 8, 2297–2306. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Xiu, P.; Tan, J.; Jia, Z.; Cai, H.; Liu, Z. Enhanced angiogenesis and osteogenesis in critical bone defects by the controlled release of BMP-2 and VEGF: Implantation of electron beam melting-fabricated porous Ti6Al4V scaffolds incorporating growth factor-doped fibrin glue. BioMed. Mater. 2015, 10, 035013. [Google Scholar] [CrossRef]
- Kopf, B.S.; Schipanski, A.; Rottmar, M.; Berner, S.; Maniura-Weber, K. Enhanced differentiation of human osteoblasts on Ti surfaces pre-treated with human whole blood. Acta Biomater. 2015, 19, 180–190. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, X.; Bai, S.; Li, B.; Liu, H.; Wu, G.; Liu, S.; Zhao, Y. Fabrication of gelatin methacrylate/nanohydroxyapatite microgel arrays for periodontal tissue regeneration. Int. J. Nanomed. 2016, 11, 4707–4718. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; França, C.M.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Chronopoulou, L.; Palocci, C.; Amalfitano, A.; Cantiani, M.; Cordaro, M.; Lajolo, C.; Calla, C.; Boninsegna, A.; Lucchetti, D.; et al. Controlled release of 18-β-glycyrrhetic acid by nanodelivery systems increases cytotoxicity on oral carcinoma cell line. Nanotechnology 2018, 29, 285101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Krishnamoorti, R. Nanocomposites: Structure, phase behavior, and properties. Annu. Rev. Chem. BioMol. Eng. 2010, 1, 37–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keles, H.; Naylor, A.; Clegg, F.; Sammon, C. Investigation of factors influencing the hydrolytic degradation of single PLGA microparticles. Polym. Degrad. Stab. 2015, 119, 228–241. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Xu, C.; Wu, G.; Ye, Q.; Wang, C. Poly(lactic-co-glycolic acid): Applications and future prospects for periodontal tissue eegeneration. Polymers 2017, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [Green Version]
- Arnold, M.M.; Gorman, E.M.; Schieber, L.J.; Munson, E.J.; Berkland, C. Nano Cipro encapsulation in monodisperse large porous PLGA microparticles. J. Control. Release 2007, 121, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Narayan, D.; Venkatraman, S.S. Effect of pore size and interpore distance on endothelial cell growth on polymers. J. BioMed. Mater. Res. Part A 2008, 87, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Spalazzi, J.P.; Vyner, M.C.; Jacobs, M.T.; Moffat, K.L.; Lu, H.H. Mechanoactive scaffold induces tendon remodeling and expression of fibrocartilage markers. Clin. Orthop. Relat. Res. 2008, 466, 1938–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbarzadeh, E.; Starnes, T.; Khan, Y.M.; Jiang, T.; Wirtel, A.J.; Deng, M.; Lv, Q.; Nair, L.S.; Doty, S.B.; Laurencin, C.T. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: A combined gene therapy-cell transplantation approach. Proc. Natl. Acad. Sci. USA 2008, 105, 11099–11104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.J.; Kim, I.K.; Kim, T.G.; Park, T.G. Highly Open Porous Biodegradable Microcarriers: In Vitro Cultivation of Chondrocytes for Injectable Delivery. Tissue Eng. Part A 2008, 14, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Lee, L.Y.; Jackson, J.S.H.; Tong, Y.W.; Wang, C.-H. Characterization of porous poly (D,L-lactic-co-glycolic acid) sponges fabricated by supercritical CO2 gas-foaming method as a scaffold for three-dimensional growth of Hep3B cells. Biotechnol. BioEng. 2008, 100, 998–1009. [Google Scholar] [CrossRef]
- Ge, Z.; Wang, L.; Heng, C.B.; Tian, X.-F.; Lu, K.; Fan, V.T.W.; Yeo, J.F.; Cao, T.; Tan, E. Proliferation and differentiation of human osteoblasts within 3D printed poly-lactic-co-glycolic acid scaffolds. J. Biomater. Appl. 2009, 23, 533–547. [Google Scholar]
- Bashur, C.A.; Dahlgren, L.A.; Goldstein, A.S. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly (d,l-lactic-co-glycolic acid) meshes. Biomaterials 2006, 27, 5681–5688. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [Green Version]
- Moffat, K.L.; Kwei, A.S.-P.; Spalazzi, J.P.; Doty, S.B.; Levine, W.N.; Lu, H.H. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng. Part A 2009, 15, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Aviss, K.; Gough, J.; Downes, S. Aligned electrospun polymer fibRes. for skeletal muscle regeneration. Eur. Cells Mater. 2010, 19, 193–204. [Google Scholar] [CrossRef]
- Yoon, J.J.; Chung, H.J.; Lee, H.J.; Park, T.G. Heparin-immobilized biodegradable scaffolds for local and sustained release of angiogenic growth factor. J. BioMed. Mater. Res. Part A 2006, 79, 934–942. [Google Scholar] [CrossRef]
- Perron, J.K.; Naguib, H.E.; Daka, J.; Chawla, A.; Wilkins, R. A study on the effect of degradation media on the physical and mechanical properties of porous PLGA 85/15 scaffolds. J. BioMed. Mater. Res. Part B Appl. Biomater. 2009, 91, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Chan, C.; Baek, S.; Naguib, H. Comparison of morphology and mechanical properties of PLGA bioscaffolds. BioMed. Mater. 2008, 3, 025006. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; John, T.; Endres, M.; Rosen, C.; Kaps, C.; Kohl, B.; Sittinger, M.; Ertel, W.; Schulze-Tanzil, G. Extracellular matrix expression of human tenocytes in three-dimensional air-liquid and PLGA cultuRes. compared with tendon tissue: Implications for tendon tissue engineering. J. Orthop. Res. 2010, 28, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, K.A.; McKean, R.; Canton, I.; Freeman, C.O.; Franklin, K.L.; Cole, D.; Brook, J.; Farthing, P.; Rimmer, S.; Haycock, J.W.; et al. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials 2008, 29, 3091–3104. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Ambrosio, L. The synergic effect of polylactide fiber and calcium phosphate particle reinforcement in poly ε-caprolactone-based composite scaffolds. Acta Biomater. 2008, 4, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-J.; Bae, W.-J.; Kim, J.-M.; Kim, J.-J.; Lee, E.-J.; Kim, H.-W.; Kim, E.-C. Mineralized polycaprolactone nanofibrous matrix for odontogenesis of human dental pulp cells. J. Biomater. Appl. 2013, 28, 1069–1078. [Google Scholar] [CrossRef]
- Park, C.; Kim, K.-H.; Lee, Y.-M.; Seol, Y.-J. Advanced engineering strategies for periodontal complex regeneration. Materials 2016, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, G.Y.; Ryu, T.-K.; Choi, Y.R.; Park, J.R.; Lee, M.J.; Choi, S.-W. Fabrication and optimization of nanodiamonds-composited poly(ε-caprolactone) fibrous matrices for potential regeneration of hard tissues. Biomater. Res. 2018, 22, 16. [Google Scholar] [CrossRef]
- Fuchs, A.; Youssef, A.; Seher, A.; Hartmann, S.; Brands, R.C.; Müller-Richter, U.D.A.; Kübler, A.C.; Linz, C. A new multilayered membrane for tissue engineering of oral hard- and soft tissue by means of melt electrospinning writing and film casting—An in vitro study. J. Craniomaxillofac. Surg. 2019, 47, 695–703. [Google Scholar] [CrossRef]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef]
- Sequeira, D.B.; Oliveira, A.R.; Catarina, M.S.; Palma, P.J.; Ramos, C.; Figueiredo, M.H.; Santos, A.C.; Cardoso, A.L.; Peça, J.; Santos, J.M. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: In vivo. Clin. Oral Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Neo, M.; Kotani, S.; Nakamura, T.; Yamamuro, T.; Ohtsuki, C.; Kokubo, T.; Bando, Y. A comparative study of ultrastructuRes. of the interfaces between four kinds of surface-active ceramic and bone. J. BioMed. Mater. Res. 1992, 26, 1419–1432. [Google Scholar] [CrossRef]
- Cacciotti, I. Multisubstituted hydroxyapatite powders and coatings: The influence of the codoping on the hydroxyapatite performances. Int. J. Appl. Ceram. Technol. 2019, 16, 1864–1884. [Google Scholar] [CrossRef]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Nam, S.; Won, J.-E.; Kim, C.-H.; Kim, H.-W. Odontogenic differentiation of human dental pulp stem cells stimulated by the calcium phosphate porous granules. J. Tissue Eng. 2011, 2011, 812547. [Google Scholar] [CrossRef]
- Zhang, L.; Morsi, Y.; Wang, Y.; Li, Y.; Ramakrishna, S. Review scaffold design and stem cells for tooth regeneration. Jpn. Dent. Sci. Rev. 2013, 49, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Fielding, G.A.; Bandyopadhyay, A.; Bose, S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent. Mater. 2012, 28, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Tonomura, A.; Mizuno, D.; Hisada, A.; Kuno, N.; Ando, Y.; Sumit, Y.; Kagami, H. Differential effect of scaffold shape on dentin regeneration. Ann. BioMed. Eng. 2010, 38, 1664–1671. [Google Scholar] [CrossRef]
- Hench, L.L.; Jones, J.R.; Sepulveda, P. Bioactive materials for tissue engineering scaffolds. In Future Strategies for Tissue and Organ Replacement; Imperial College Press: London, UK, 2002; pp. 3–24. [Google Scholar]
- Fu, L.; Engqvist, H.; Xia, W. Glass–ceramics in dentistry: A review. Materials 2020, 13, 1049. [Google Scholar] [CrossRef] [Green Version]
- El-Gendy, R.; Yang, X.B.; Newby, P.J.; Boccaccini, A.R.; Kirkham, J. Osteogenic differentiation of human dental pulp stromal cells on 45S5 bioglass® based scaffolds in vitro and in vivo. Tissue Eng. Part A 2013, 19, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, R.A.; Won, J.-E.; Knowles, J.C.; Kim, H.-W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. BioMed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-F.; Sfeir, C.; Kumta, P.N. Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Philos. Trans. A Math Phys. Eng. Sci. 2010, 368, 1981–1997. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodru, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. NanoMed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Yang, F.; Shen, H.; Hu, X.; Mochizuki, C.; Sato, M.; Wang, S.; Zhang, Y. The effect of composition of calcium phosphate composite scaffolds on the formation of tooth tissue from human dental pulp stem cells. Biomaterials 2011, 32, 7053–7059. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Aparicio, C.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Polylactic acid-based porous scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for biomedical application. Mater. Sci. Eng. C 2018, 82, 163–181. [Google Scholar] [CrossRef]
- Ho, C.C.; Fang, H.Y.; Wang, B.; Huang, T.H.; Shie, M.Y. The effects of biodentine/polycaprolactone three-dimensional-scaffold with odontogenesis properties on human dental pulp cells. Int. Endod. J. 2018, 51, e291–e300. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yan, X.; Pandya, M.; Luan, X.; Diekwisch, T.G.H. Daughters of the enamel organ: Development, fate, and function of the stratum intermedium, stellate reticulum, and outer enamel epithelium. Stem Cells Dev. 2016, 25, 1580–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandya, M.; Lin, T.; Li, L.; Allen, M.J.; Jin, T.; Luan, X.; Diekwisch, T.G.H. Posttranslational amelogenin processing and changes in matrix assembly during enamel development. Front. Physiol. 2017, 8, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandya, M.; Diekwisch, T.G.H. Enamel biomimetics—fiction or future of dentistry. Int. J. Oral Sci. 2019, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Harada, H.; Saito, M.; Taniguchi, A. Induction of enamel matrix protein expression in an ameloblast cell line co-cultured with a mesenchymal cell line in vitro. In Vitro Cell Dev. Biol. Anim. 2010, 47, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.J.; Shinmura, Y.; Shinohara, Y. Enamel tissue engineering using subcultured enamel organ epithelial cells in combination with dental pulp cells. Cells Tissues Organs 2009, 189, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.J.; Shimodaira, T.; Ogaeri, T.; Shinohara, Y.; Hata, K.; Ueda, M. A novel culture system for porcine odontogenic epithelial cells using a feeder layer. Arch. Oral Biol. 2006, 51, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, M.; Ishikawa, M.; Nakamura, T.; Iwamoto, T.; Yamada, A.; Fukumoto, E.; Saito, M.; Otsu, K.; Harada, H.; Yamada, Y.; et al. Role of epithelial-stem cell interactions during dental cell differentiation. J. Biol. Chem. 2012, 287, 10590–10601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, S.; Morotomi, T.; Toyono, T.; Nakamura, N.; Uchida, T.; Ohishi, M.; Toyoshima, K.; Harada, H. Establishment of dental epithelial cell line (HAT-7) and the cell differentiation depenDent. on notch signaling pathway. Connect. Tissue Res. 2002, 43, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Bori, E.; Guo, J.; Rácz, R.; Burghardt, B.; Földes, A.; Kerémi, B.; Harada, H.; Steward, M.C.; Besten, P.D.; Bronckers, A.L.J.J.; et al. Evidence for bicarbonate secretion by ameloblasts in a novel cellular model. J. Dent. Res. 2016, 95, 588–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Chiba, Y.; Naruse, M.; Saito, K.; Harada, H.; Fukumoto, S. Globoside accelerates the differentiation of dental epithelial cells into ameloblasts. Int. J. Oral Sci. 2016, 8, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakata, A.; Kameda, T.; Nagai, H.; Ikegami, K.; Duan, Y.; Terada, K.; Sugiyama, T. Establishment and characterization of a spontaneously immortalized mouse ameloblast-lineage cell line. BioChem. Biophys. Res. Commun. 2003, 308, 834–839. [Google Scholar] [CrossRef]
- Sidaly, R.; Landin, M.A.; Suo, Z.; Snead, M.L.; Lyngstadaas, S.P.; Reseland, J.E. Hypoxia increases the expression of enamel genes and cytokines in an ameloblast-derived cell line. Eur. J. Oral Sci. 2015, 123, 335–340. [Google Scholar] [CrossRef]
- Huang, Z.; Sargeant, T.D.; Hulvat, J.F.; Mata, A.; Bringas, P.; Koh, C.-Y.; Stupp, S.I.; Snead, M.L. Bioactive nanofibers instruct cells to proliferate and differentiate during enamel regeneration. J. Bone Miner. Res. 2008, 23, 1995–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, J.; Simanian, E.J.; Tuggy, S.Y.; Bartlett, J.D.; Snead, M.L.; Sugiyama, T.; Paine, M.L. Comparison of two mouse ameloblast-like cell lines for enamel-specific gene expression. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Newcomb, C.J.; Zhou, Y.; Lei, Y.P.; Bringas, P., Jr.; Stupp, S.I.; Snead, M.L. The role of bioactive nanofibers in enamel regeneration mediated through integrin signals acting upon C/EBPα and c-Jun. Biomaterials 2013, 34, 3303–3314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Zhang, Y.; Liu, P.; Chen, S.; Wu, X.; Sun, Y.; Li, A.; Huang, K.; Luo, R.; Wang, L.; et al. Generation of tooth-like structuRes. from integration-free human urine induced pluripotent stem cells. Cell Regen. 2013, 2, 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Jiang, M.; Hao, W.; Liu, W.; Tang, L.; Liu, H.; Jin, Y. Skin epithelial cells as possible substitutes for ameloblasts during tooth regeneration. J. Tissue Eng. Regen. Med. 2012, 7, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, Y.; Tsuchiya, S.; Hata, K.; Honda, M.J. Quiescent epithelial cell rests of Malassez can differentiate into ameloblast-like cells. J. Cell Physiol. 2008, 217, 728–738. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Du, S.; Liu, C.; Lin, X.; Chen, Y.; Zhang, Y. Induction of human keratinocytes into enamel-secreting ameloblasts. Dev. Biol. 2010, 344, 795–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavez, M.G.; Hu, J.; Seidel, K.; Li, C.; Jheon, A.; Naveau, A.; Horst, O.; Klein, O.D. Isolation and culture of dental epithelial stem cells from the adult mouse incisor. J. Vis. Exp. 2014, 87, 51266. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef] [Green Version]
- Sakai, V.T.; Zhang, Z.; Dong, Z.; Neiva, K.G.; Machado, M.A.A.M.; Shi, S.; Santos, S.S.; Nor, J.E. SHED differentiate into functional odontoblasts and endothelium. J. Dent. Res. 2010, 89, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/progenitor cell–mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-J.; Zhao, Y.-H.; Zhao, Y.-J.; Liu, N.-X.; Lv, X.; Li, Q.; Chen, F.-M.; Zheng, M. Potential dental pulp revascularization and odonto-/osteogenic capacity of a novel transplant combined with dental pulp stem cells and platelet-rich fibrin. Cell Tissue Res. 2015, 361, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Ruangsawasdi, N.; Zehnder, M.; Weber, F.E. Fibrin gel improves tissue ingrowth and cell differentiation in human immature premolars implanted in rats. J. Endod. 2014, 40, 246–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng. Part A 2011, 17, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galler, K.M.; Hartgerink, J.D.; Cavender, A.C.; Schmalz, G.; D’Souza, R.N. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng. Part A 2012, 18, 176–184. [Google Scholar] [CrossRef]
- Rosa, V.; Zhang, Z.; Grande, R.H.M.; Nör, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef] [Green Version]
- Galler, K.M.; Cavender, A.C.; Koeklue, U.; Suggs, L.J.; Schmalz, G.; D’Souza, R.N. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen. Med. 2011, 6, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Perard, M.; Zanini, M.; Smith, A.J.; Charpentier, E.; Djole, S.X.; Lumley, P.J. Should pulp chamber pulpotomy be seen as a permanent treatment? Some preliminary thoughts. Int. Endod. J. 2012, 46, 79–87. [Google Scholar] [CrossRef]
- Taha, N.A.; Khazali, M.A. Partial pulpotomy in mature permanent teeth with clinical signs indicative of irreversible pulpitis: A randomized clinical trial. J. Endod. 2017, 43, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Qudeimat, M.A.; Alyahya, A.; Hasan, A.A.; Barrieshi-Nusair, K.M. Mineral trioxide aggregate pulpotomy for permanent molars with clinical signs indicative of irreversible pulpitis: A preliminary study. Int. Endod. J. 2016, 50, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Mandakhbayar, N.; El-Fiqi, A.; Lee, J.-H.; Kim, H.-W. Evaluation of strontium-doped nanobioactive glass cement for dentin–pulp complex regeneration therapy. ACS Biomater-Sci. Eng. 2019, 5, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
- Rad, R.M.; Atila, D.; Akgün, E.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Evaluation of human dental pulp stem cells behavior on a novel nanobiocomposite scaffold prepared for regenerative endodontics. Mater. Sci. Eng. C 2019, 100, 928–948. [Google Scholar]
- Kontonasaki, E.; Bakopoulou, A.; Theocharidou, A.; Theodorou, G.S.; Papadopoulou, L.; Kantiranis, N.; Bousnaki, M.; Chatzichristou, C.; Papachristou, E.; Paraskevopoulos, K.M.; et al. Effective cell growth potential of Mg-based bioceramic scaffolds towards targeted dentin regeneration. Balk. J. Dent. Med. 2015, 19, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Soares, D.G.; Rosseto, H.L.; Basso, F.G.; Scheffel, D.S.; Hebling, J.; Costa, C.A.d.S. Chitosan-collagen biomembrane embedded with calcium-aluminate enhances dentinogenic potential of pulp cells. Braz. Oral Res. 2016, 30, e54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, H.L.B.; Doan, V.N. Human dental pulp stem cells cultured onto dentin derived scaffold can regenerate dentin-like tissue in vivo. Cell Tissue Bank. 2015, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Neves, V.C.M.; Babb, R.; Chandrasekaran, D.; Sharpe, P.T. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci. Rep. 2017, 7, 39654. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nor, J.E. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef]
- Ouchi, T.; Nakagawa, T. Mesenchymal stem cell-based tissue regeneration therapies for periodontitis. Regen. Ther. 2020, 14, 72–78. [Google Scholar] [CrossRef]
- Yang, Y.; Rossi, F.M.V.; Putnins, E.E. Periodontal regeneration using engineered bone marrow mesenchymal stromal cells. Biomaterials 2010, 31, 8574–8582. [Google Scholar] [CrossRef] [PubMed]
- Gay, I.; Chen, S.; MacDougall, M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod. Craniofac. Res. 2007, 10, 149–160. [Google Scholar] [CrossRef]
- Han, C.; Yang, Z.; Zhou, W.; Jin, F.; Song, Y.; Wang, Y.; Huo, N.; Chen, L.; Qian, H.; Hou, R.; et al. Periapical follicle stem cell: A promising candidate for cementum/periodontal ligament regeneration and bio-root engineering. Stem Cells Dev. 2010, 19, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, J.; Sanz-Blasco, S.; Vignoletti, F.; Muñoz, F.; Arzate, H.; Villalobos, C.; Nuñez, L.; Caffesse, R.G.; Sanz, M. Periodontal regeneration following implantation of cementum and periodontal ligament-derived cells. J. Periodont. Res. 2011, 47, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Arzate, H.; Zeichner-David, M.; Mercado-Celis, G. Cementum proteins: Role in cementogenesis, biomineralization, periodontium formation and regeneration. Periodontol. 2000 2014, 67, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Hoz, L.; Romo, E.; Zeichner-David, M.; Sanz, M.; Nuñez, J.; Gaitán, L.; Mercado, G.; Arzate, H. Cementum protein 1 (CEMP1) induces differentiation by human periodontal ligament cells under three-dimensional culture conditions. Cell Biol. Int. 2011, 36, 129–136. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Schroeder, H.E. Cementogenesis reviewed: A comparison between human premolars and rodent molars. Anat. Rec. 1996, 245, 267–292. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, M. Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Chen, G.; Li, J.; Zou, Q.; Xie, D.; Chen, Y.; Wang, H.; Zheng, X.; Long, J.; Tang, W.; et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix—Based scaffold. Biomaterials 2012, 33, 2449–2461. [Google Scholar] [CrossRef]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.E.; Vaquette, C.; Klein, T.J.; Hutmacher, D.W. Perspectives in multiphasic osteochondral tissue engineering. Anat. Rec. 2013, 297, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akizuki, T.; Oda, S.; Komaki, M.; Tsuchioka, H.; Kawakatsu, N.; Kikuchi, A.; Yamato, M.; Okano, T.; Ishikawa, I. Application of periodontal ligament cell sheet for periodontal regeneration: A pilot study in beagle dogs. J. Periodontal Res. 2005, 40, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Gomez Flores, M.; Hasegawa, M.; Yamato, M.; Takagi, R.; Okano, T.; Ishikawa, I. Cementum-periodontal ligament complex regeneration using the cell sheet technique. J. Periodontal Res. 2008, 43, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Yamato, M.; Kikuchi, A.; Okano, T.; Ishikawa, I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005, 11, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Iwata, T.; Washio, K.; Okano, T.; Nagasawa, T.; Iwasaki, K.; Ando, T. Cell sheet engineering and oTher. novel cell-based approaches to periodontal regeneration. Periodontol. 2000 2009, 51, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamato, M.; Tsuchioka, H.; Takagi, R.; Mukobata, S.; Washio, K.; Okano, T.; Ishikawa, I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 2009, 30, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamato, M.; Zhang, Z.; Mukobata, S.; Washio, K.; Ando, T.; Feijen, J.; Ishikawa, I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J. Clin. Periodontol. 2010, 37, 1088–1099. [Google Scholar] [CrossRef]
- Flores, M.G.; Yashiro, R.; Washio, K.; Yamato, M.; Okano, T.; Ishikawa, I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J. Clin. Periodontol. 2008, 35, 1066–1072. [Google Scholar] [CrossRef]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef]
- Anusaksathien, O.; Jin, Q.; Zhao, M.; Somerman, M.J.; Giannobile, W.V. Effect of sustained gene delivery of platelet-derived growth factor or its antagonist (PDGF-1308) on tissue-engineered cementum. J. Periodontol. 2004, 75, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Jin, Q.-M.; Zhao, M.; Webb, S.A.; Berry, J.E.; Somerman, M.J.; Giannobile, W.V. Cementum engineering with three-dimensional polymer scaffolds. J. BioMed. Mater. Res. A 2003, 67, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Rios, H.F.; Jin, Q.; Bland, M.E.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials 2010, 31, 5945–5952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Rios, H.F.; Taut, A.D.; Padial-Molina, M.; Flanagan, C.L.; Pilipchuk, S.P.; Hollister, S.J.; Giannobile, W.V. Image-based, fiber guiding scaffolds: A platform for regenerating tissue interfaces. Tissue Eng. Part C Methods 2014, 20, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. Part C Methods A 2014, 20, 1342–1351. [Google Scholar] [CrossRef]
- Cho, H.; Tarafder, S.; Fogge, M.; Kao, K.; Lee, C.H. Periodontal ligament stem/progenitor cells with protein-releasing scaffolds for cementum formation and integration on dentin surface. Connect. Tissue Res. 2016, 57, 488–495. [Google Scholar] [CrossRef]
- Mao, L.X.; Liu, J.; Zhao, J.; Xia, L.; Jiang, L.; Wang, X.; Lin, K.; Fang, B. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. Int. J. Nanomed. 2015, 10, 7031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chronopoulou, L.; Cacciotti, I.; Amalfitano, A.; Di Nitto, A.; D’Arienzo, V.; Nocca, G.; Paloci, C. Biosynthesis of innovative calcium phosphate/hydrogel composites: Physicochemical and biological characterisation. Nanotechnology 2020, 32, 095102. [Google Scholar] [CrossRef]
- Takayama, S.; Murakami, S.; Shimabukuro, Y.; Kitamura, M.; Okada, H. Periodontal Regeneration by FGF-2 (bFGF) in Primate Models. J. Dent. Res. 2001, 80, 2075–2079. [Google Scholar] [CrossRef]
- Murakami, S.; Takayama, S.; Kitamura, M.; Shimabukuro, Y.; Yanagi, K.; Ikezawa, K.; Okada, H. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J. Periodontal Res. 2003, 38, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-layered nanocomposite hydrogel scaffold for the concurrent regeneration of cementum, periodontal ligament, and alveolar bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Yelick, P.C. Advances and perspectives in tooth tissue engineering. J. Tissue Eng. Regen. Med. 2016, 11, 2443–2461. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tang, Q.; Wang, A.; Chen, Y. Regrowing a tooth: In vitro and in vivo approaches. Curr. Opin. Cell Biol. 2019, 61, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.; Srijaya, T.C.; Sukumaran, P.; Zain, R.B.; Abu Kasim, N.H. Homeobox genes and tooth development: Understanding the biological pathways and applications in regenerative dental science. Arch. Oral Biol. 2018, 85, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-F.; Lee, J.-M.; Jung, H.-S. Molecular and engineering approaches to regenerate and repair teeth in mammals. Cell. Mol. Life Sci. 2013, 71, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, T.; Li, M.; Fu, N.; Fu, Y.; Ba, K.; Deng, S.; Jiang, Y.; Hu, J.; Peng, Q.; et al. Electrospun fibers for dental and craniofacial applications. Curr. Stem Cell Res. T 2014, 9, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kiso, H.; Saito, K.; Togo, Y.; Tsukamoto, H.; Huang, B.; Bessho, K. Feasibility of gene therapy for tooth regeneration by stimulation of a third dentition. In Gene Therapy—Tools and Potential Applications; InTech: London, UK, 2013. [Google Scholar]
- Hu, X.; Lee, J.-W.; Zheng, X.; Zhang, J.; Lin, X.; Song, Y.; Wang, B.; Hu, X.; Chang, H.-H.; Chen, Y.; et al. Efficient induction of functional ameloblasts from human keratinocyte stem cells. Stem Cell Res. Ther. 2018, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Lechguer, A.N.; Kuchler-Bopp, S.; Hu, B.; Haïkel, Y.; Lesot, H. Vascularization of engineered teeth. J. Dent. Res. 2008, 87, 1138–1143. [Google Scholar] [CrossRef]
- Lechguer, A.N.; Couble, M.L.; Labert, N.; Kuchler-Bopp, S.; Keller, L.; Magloire, H.; Bleicher, F.; Lesot, H. Cell differentiation and matrix organization in engineered teeth. J. Dent. Res. 2011, 90, 583–589. [Google Scholar] [CrossRef]
- Hu, B.; Nadiri, A.; Bopp-Küchler, S.; Perrin-Schmitt, F.; Lesot, H. Dental epithelial histomorphogenesis in vitro. J. Dent. Res. 2005, 84, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Nadiri, A.; Kuchler-Bopp, S.; Perrin-Schmitt, F.; Peters, H.; Lesot, H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006, 12, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.J.; Fong, H.; Iwatsuki, S.; Sumita, Y.; Sarikaya, M. Tooth-forming potential in embryonic and postnatal tooth bud cells. Med. Mol. Morphol. 2008, 41, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, P.C.J.; Rücklin, M. The ins and outs of the evolutionary origin of teeth. Evol. Dev. 2014, 18, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abduweli, D.; Baba, O.; Tabata, M.J.; Higuchi, K.; Mitani, H.; Takano, Y. Tooth replacement and putative odontogenic stem cell niches in pharyngeal dentition of medaka (Oryziaslatipes). Microscopy 2014, 63, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Jernvall, J.; Thesleff, I. Tooth shape formation and tooth renewal: Evolving with the same signals. Development 2012, 139, 3487–3497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juuri, E.; Jussila, M.; Seidel, K.; Holmes, S.; Wu, P.; Richman, J.; Heikinheimo, K.; Chuong, C.-M.; Arnold, K.; Hochedlinger, K.; et al. Sox2 marks epithelial competence to generate teeth in mammals and reptiles. Development 2013, 140, 1424–1432. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Wu, X.; Jiang, T.-X.; Elsey, R.M.; Temple, B.L.; Divers, S.J.; Glenn, T.C.; Yuan, K.; Chen, M.H.; Widelitz, R.B.; et al. Specialized stem cell niche enables repetitive renewal of alligator teeth. Proc. Natl. Acad. Sci. USA 2013, 110, E2009–E2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Nadiri, A.; Bopp-Kuchler, S.; Perrin-Schmitt, F.; Wang, S.; Lesot, H. Dental epithelial histo-morphogenesis in the mouse: Positional information versus cell history. Arch. Oral Biol. 2005, 50, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Tsuji, T. Growing bioengineered teeth from single cells: Potential for dental regenerative medicine. Expert Opin. Biol. Ther. 2008, 8, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Murofushi, M.; Nakao, K.; Morita, R.; Ogawa, M.; Tsuji, T. The regulation of tooth morphogenesis is associated with epithelial cell proliferation and the expression of Sonic hedgehog through epithelial–mesenchymal interactions. BioChem. Biophys. Res. Commun. 2011, 405, 455. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Lin, C.; Shen, B.; Ruan, N.; Guan, Z.; Zhang, Y. Conserved odontogenic potential in embryonic dental tissues. J. Dent. Res. 2014, 93, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part II: Clinical applications. J. Prosthodont. Res. 2012, 56, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Ohazama, A.; Modino, S.A.C.; Miletich, I.; Sharpe, P.T. Stem-cell-based tissue engineering of murine teeth. J. Dent. Res. 2004, 83, 518–522. [Google Scholar] [CrossRef]
- Modino, S.A.C.; Sharpe, P.T. Tissue engineering of teeth using adult stem cells. Arch. Oral Biol. 2005, 50, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Sone, P.P.; Zaw, S.Y.M.; Sueyama, Y.; Zaw, Z.C.T.; Okada, Y.; Murano, H.; Gu, B.; Okiji, T. In vivo fate of bone marrow mesenchymal stem cells implanted into rat pulpotomized molars. Stem Cell Res. 2019, 38, 101457. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, F.; Zhang, W.; Li, Y.; Yu, M.; Nan, X.; Chen, L.; Yue, X.; Xu, X.; Pei, X. Application of induced pluripotent stem cells in generation of a tissue-engineered tooth-like. Tissue Eng. Part A 2012, 18, 1677–1685. [Google Scholar] [CrossRef] [Green Version]
- Otsu, K.; Kishigami, R.; Oikawa-Sasaki, A.; Fukumoto, S.; Yamada, A.; Fujiwara, N.; Ishizeki, K.; Harada, H. Differentiation of induced pluripotent stem cells into dental mesenchymal cells. Stem Cells Dev. 2012, 21, 1156–1164. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.-F.; Zhang, J.; Duan, Y.-Z.; Jin, Y. Ameloblasts serum-free conditioned medium: Bone morphogenic protein 4-induced odontogenic differentiation of mouse induced pluripotent stem cells. J. Tissue Eng. Regen. Med. 2013, 10, 466–474. [Google Scholar] [CrossRef]
- Smith, E.E.; Zhang, W.; Schiele, N.R.; Khademhosseini, A.; Kuo, C.K.; Yelick, P.C. Developing a biomimetic tooth bud model. J. Tissue Eng. Regen. Med. 2017, 11, 3326–3336. [Google Scholar] [CrossRef]
- Khayat, A.; Monteiro, N.; Smith, E.E.; Pagni, S.; Zhang, W.; Khademhosseini, A.; Yelik, P.C. GelMA-encapsulated hDPSCs and HUVECs for dental pulp regeneration. J. Dent. Res. 2016, 96, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.E.; Yelick, P.C. Bioengineering tooth bud constructs using GelMA hydrogel. Methods Mol. Biol. 2019, 1922, 139–150. [Google Scholar] [PubMed]
- Monteiro, N.; Smith, E.E.; Angstadt, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. Dental cell sheet biomimetic tooth bud model. Biomaterials 2016, 106, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.E.; Angstadt, S.; Monteiro, N.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. Bioengineered tooth buds exhibit featuRes. of natural tooth buds. J. Dent. Res. 2018, 97, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, S.; Honda, M.J.; Harada, H.; Ueda, M. Cell proliferation in teeth reconstructed from dispersed cells of embryonic tooth germs in a three-dimensional scaffold. Eur. J. Oral Sci. 2006, 114, 310–317. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Yang, B.; Li, L.; Luo, X.; Zhang, X.; Feng, L.; Jiang, Z.; Yu, M.; Guo, W.; et al. Combination of aligned PLGA/gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials 2015, 52, 56–70. [Google Scholar] [CrossRef]
- Xu, W.P.; Zhang, W.; Asrican, R.; Kim, H.J.; Kaplan, D.L.; Yelick, P.C. Accurately shaped tooth bud cell-derived mineralized tissue formation on silk scaffolds. Tissue Eng. Part A 2008, 14, 549–557. [Google Scholar] [CrossRef]
- Traphagen, S.B.; Fourligas, N.; Xylas, J.F.; Sengupta, S.; Kaplan, D.L.; Georgakoudi, I.; Yelik, P.C. Characterization of natural, decellularized and reseeded porcine tooth bud matrices. Biomaterials 2012, 33, 5287–5296. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Vazquez, B.; Oreadi, D.; Yelick, P.C. Decellularized tooth bud scaffolds for tooth regeneration. J. Dent. Res. 2017, 96, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Sumita, Y.; Honda, M.J.; Ohara, T.; Tsuchiya, S.; Sagara, H.; Kagami, H.; Ueda, M. Performance of collagen sponge as a 3D scaffold for tooth-tissue engineering. Biomaterials 2006, 27, 3238–3248. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Takimoto, K.; Jeon, M.; Vadakekalam, J.; Ruparel, N.B.; Diogenes, A. Decellularized human dental pulp as a scaffold for regenerative endodontics. J. Dent. Res. 2017, 96, 640–646. [Google Scholar] [CrossRef] [PubMed]
| Biomaterials | Type | Fabrication Method | Forms of Delivery | Advantages | Limitations | References |
|---|---|---|---|---|---|---|
| Type-I collagen | Natural biopolymer | Plastic compression of hydrogels, multiple unconfined plastic compression, microextrusion, electrospinning | (3D) scaffolds, nanofibrous membranes, gels, sponges | Resemblance with extracellular matrix structure, low cytotoxicity and immunogenicity, high biocompatibility, enzymatic biodegradability, delivery of bioactive molecules for the regeneration of mineralized tissues, stimulation of osteoblasts differentiation, adeptness and efficiency to form many shapes, high tensile strength | High complexity structure | [48,49,50,51,52,53,54] |
| Alginate | Natural biopolymer | Freeze drying, freeze-casting, dehydrothermal treatment | Scaffolds, gels | High biocompatibility, low toxicity, easy chemical modification, easy gelling, relatively inert aqueous medium, easy encapsulation at room temperature without organic solvents, high gel porosity with high diffusion rate, suitable substrate for the release of encapsulated transforming growth factor-beta, capacity to control porosity by simple coatings, dissolution and biodegradation under normal physiological conditions, slow gelling time after the addition of Ca2+ divalent cations, osteoconductive and/or bioactive components promoting osteogenic differentiation, calcium deposition, biomineralization and sustaining the natural regeneration of mineral matrix | Poor mechanical properties, lack of cellular interactions, uncontrollable degradation, sterilization inducing degradation, non-degradable in mammals | [55,56,57,58,59] |
| Fibrin matrices | Natural biopolymer | Electrospinning, inkjet printing, magnetically influenced self-assembly, oil-stirring mixture | 3D scaffolds, injectable hydrogels, beads or microbeads encapsulating stem cells, coating agents, nanoparticles, nanofibers, microfibers, microspheres | Appropriate environment for angiogenesis, formability to 3D structures, injectability, transforming of growth factor-beta, controlling pro-angiogenic growth factors release, excellent cytocompatibility, non-toxicity of the degradation products | Weak mechanical properties, fast degradation, high shrinkage | [58,60,61,62,63,64,65,66,67,68,69,70,71,72] |
| Methacrylated gelatin | Natural biopolymer | Electrospun, blending, photopatterning, photolithography microfabrication technique | Scaffolds, microgel arrays | Excellent cellular compatibility, cell encapsulation at human body temperature, promoting cell viability and proliferation | Low mechanical strength, inappropriate for applications where superior tunability as regards cell adhesion, migration and degradation mediated by cells are required | [73,74,75] |
| Poly(lactic-co-glycolic acid) (PLGA) | Synthetic polymer | Porogen leaching, gas foaming, polymer printing, electrospinning, combination of these methods, self-assembly | (3D) scaffolds, membranes, hydrogels, sponges, micro- and nanoparticles | Biocompatibility, tunable biodegradability, non-toxicity, high cell adhesion and proliferation, appropriate mechanical properties | Incomplete solvent removal upon evaporation, lack of open-pore structure and interconnectivity | [76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96] |
| Poly-ε-caprolactone (PCL) | Synthetic polymer | Porogen leaching, electrospun fibers, stereolithography, solvent casting particle leaching | Scaffolds with adhered microspheres, porous networks | Non-toxicity, biodegradable, low melting point, good solubility in organic solvents, high drug permeability, ability of mineralized PCL scaffolds with apatite to promote the dental pulp cells growth and differentiation | Low in vivo degradation, hydrophobicity | [97,98,99,100,101,102,103] |
| Platelet-rich plasma (PRP) | Mixture containing proteins, i.e., natural polymers of amino acids | One-step centrifugation, two-step centrifugation | Scaffold | Ingrowth of vascularized connective tissues from endodontically disinfected root canals, evidence of dentin-like tissues when SCAPs were embedded in a PRP scaffold | Minimal evidence of dentin development in most of the published articles, controversial results as regards PRP therapeutic efficacy in periodontal regenerative procedures | [104,105] |
| Biomaterials | Cell Types | Advantages | Limitations | References |
|---|---|---|---|---|
| Strontium-free and strontium-doped calcium silicate mesoporous nanobioactive glass cements | Dental pulp stem cells | High biocompatibility and strong odontogenic potential for the nanobiocements containing strontium, more degradable and more hydroxyapatite deposition with strontium substitution, absence of cytotoxicity, rapid release of therapeutic ions, clinically appropriate teeth defect model for dentin−pulp complex regeneration | - | [158] |
| Bioactive glass nanoparticles modified with boron and containing 3D scaffolds based on cellulose acetate, oxidized pullulan and gelatin | Human dental pulp stem cells (hDPSCs) | High porosity, good mechanical strength, proliferation and differentiation of hDPSCs into odontoblasts in vitro conditions, improved mechanical properties, lack of cytotoxicity | - | [159] |
| Magnesium-based glass ceramic scaffolds with copper and zinc ions | Dental Pulp Stem Cells (DPSCs) | Attachment and growth of dental pulp stem cell for bioceramic scaffolds doped with zinc, formation of a mineralized tissue for all copper-doped scaffolds and only for zinc-doped ones exposed to lower temperatures | Cytotoxicity effect of all bioceramic scaffolds doped with copper | [160] |
| Porous hydroxyapatite, β-tricalcium phosphate, powdered hydroxyapatite and polyglycolic acid bioceramics | Dental pulp stem cells (DPSCs) | Osteoconductivity for the scaffolds containing porous hydroxyapatite and β-tricalcium phosphate, biocompatibility, resemblance with the mineralized tissues, positive for type I collagen, osteonectin, and dentin markers | Brittleness | [113] |
| Collagen/chitosan biomembrane with calcium-aluminate microparticles | Human dental pulp cells | Stimulation of odontoblastic differentiation, deposition of mineralized matrix, enhanced mechanical properties, cytocompatibility | - | [161] |
| Human treated dentin | Human dental pulp stem cells (DPSCs, stem cells from human exfoliated deciduous teeth (SHED), periodontal ligament stem cells (PDLSCs), dental follicle progenitor cells (DFPCs), stem cells from apical papilla (SCAP) | Regeneration of dentin-like tissues in in vivo conditions, differentiation of DPSCs into odontoblasts, appropriate mechanical properties, non-immunogenicity | - | [162] |
| Collagen sponges with small amounts of glycogen synthase kinase inhibitors | Resident mesenchymal stem | Natural formation of dentine via delivery of Wnt signalling agonists | - | [163] |
| Poly(lactide-co-glycolide, PLGA), composite scaffolds containing 50 wt.% poly(lactide-co-glycolide) combined with hydroxyapatite, tricalcium phosphate or calcium carbonate hydroxyapatite | Human dental pulp stem cells (DPSCs) | Generation of dentin- and pulp-like structure, high cell affinity | Pure PLGA scaffold inhibits DPSCs proliferation, lack of enamel structure for all composite scaffolds | [122] |
| PolyL-lactic acid (PLLA) scaffolds | Stem cells from human exfoliated deciduous teeth (SHED), primary human endothelial cells | Formation of a microvascular network and influx of nutrients and oxygen when SHEDs were co-implanted with human endothelial cells, SHED differentiation into odontoblast-like and blood vessel-forming cells in vivo conditions | Low pH locally generated due to the degradation of PLLA-based scaffold | [164] |
| Biomaterials | Type of Cells | Advantages | Limitations | References |
|---|---|---|---|---|
| Canine periodontal defect model filled with collagen and β-tricalcium phosphate mixture | Periodontal ligament derived multipotent mesenchymal stromal cells (PDL-MSC) | Substantial regeneration of the newly formed cementum tissue, periodontal tissue regeneration without any side effects | Decrease of cell viability as a consequence of lentiviral transduction, the size of the periodontal defect is too large to deliver proper nutrients and blood | [175] |
| Hyaluronic acid carrier | Periodontal ligament containing stem cells | Formation of a new cementum, regeneration of periodontal tissues, adherence and proliferation of periodontal ligament cells | A partial regeneration was obtained | [176] |
| Poly(N-isopropylacrylamide) | Human periodontal ligament (HPDL) cells | Regeneration of periodontal ligament tissues, enhanced cell proliferation, cell migration and differentiation toward mineralized tissues upon the addition of ascorbic acid | - | [177] |
| Polyglycolic acid | Canine periodontal ligament (PDL)derived cells | Ability of osteoblastic differentiation, formation of a cementum tissue connected with oriented collagen fibers, appropriate orientation of cementum and periodontal ligaments in the experimental group | - | [178] |
| Trypsin/ethylenediaminetetraacetic acid, collagenase/dispase | Human PDL (hPDL) cells, human adipose-derived stem cells (hADSCs), gingival fibroblasts (hGFs), bone marrow-derived mesenchymal stem cells (hBMMSCs) | Osteogenic potential in vivo and in vitro conditions, promotion of calcium deposition and rapid proliferation of hPDL cells | Low chondrogenic and adipogenic potentials of hPDL cells, lack of calcified tissues for hPDL cells | [179] |
| Gore-Tex membrane | Periodontal ligament (PDL) cells | Osteogenic differentiation expressing osteopontin (OPN) and bone sialoprotein (BSP), appearance of cementum-like tissues in vivo conditions, including PDL fibers and Sharpey’s fibers, in the presence of an osteogenic differentiation medium | - | [180] |
| Fibrin gel | Multilayered human periodontal ligament cells | Formation of undeveloped cementum-like tissues and periodontal ligaments resembling Sharpey’s fibers in an osteodifferentiation medium, increased calcium deposition and alkaline phosphatase activity | The appearance of a cementum–periodontal ligament complex was not observed in all experimental samples | [181] |
| Polycaprolactone with β-tricalcium phosphate | Multiple periodontal ligament (PDL) cells | Increased cell sheets stability on dentine surface, appearance of a discontinuous cementum-like tissue | Frequent cell monitoring | [182] |
| Poly(lactic-co-glycolic acid) (PLGA) | Immortalized cementoblasts (OCCM) transduced with antagonist of platelet-derived growth factor (PDGF) signaling (ADGF-1308), adenovirus encoding PDGF (PDGF-A), control virus (GFP) | Formation of well differentiated cementoblasts, cells attachment to PLGA scaffolds | Inhibitory effect of PDGF-A on cementogenesis | [183] |
| Poly(glycolic acid) (PGA), polycaprolactone (PCL) | Primary human gingival fibroblast (hGF) cells | Appearance of a human tooth dentin–ligament–bone complex in porcine mandibulae with surgically created defects | Lack of symmetric design and adequate mechanical properties for hybrid scaffolds only | [184] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaru, M.; Sachelarie, L.; Calin, G. Hard Dental Tissues Regeneration—Approaches and Challenges. Materials 2021, 14, 2558. https://doi.org/10.3390/ma14102558
Olaru M, Sachelarie L, Calin G. Hard Dental Tissues Regeneration—Approaches and Challenges. Materials. 2021; 14(10):2558. https://doi.org/10.3390/ma14102558
Chicago/Turabian StyleOlaru, Mihaela, Liliana Sachelarie, and Gabriela Calin. 2021. "Hard Dental Tissues Regeneration—Approaches and Challenges" Materials 14, no. 10: 2558. https://doi.org/10.3390/ma14102558
APA StyleOlaru, M., Sachelarie, L., & Calin, G. (2021). Hard Dental Tissues Regeneration—Approaches and Challenges. Materials, 14(10), 2558. https://doi.org/10.3390/ma14102558





.jpg)


