Effect of Heat Treatment on the Microstructure and Performance of Cu Nanofoams Processed by Dealloying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Processing of Cu Foams and the Annealing Conditions
2.2. Study of the Microstructure
2.3. Characterization of the Mechanical Behavior by Nanoindentation
2.4. Study of the Electrochemical Performance of the Cu Foams Annealed under Oxidizing Atmospheres
3. Results and Discussion
3.1. Effects of Annealing under Inert Gas Atmospheres on the Microstructural and Mechanical Properties
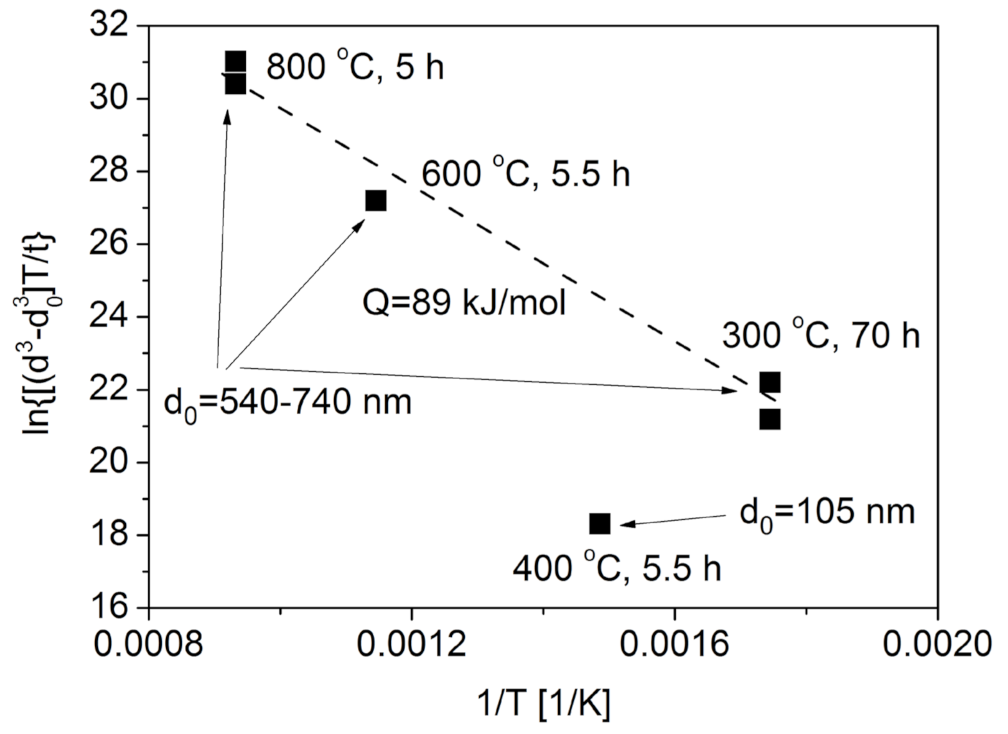
3.1.1. Ligament Coarsening Due to Heat Treatment
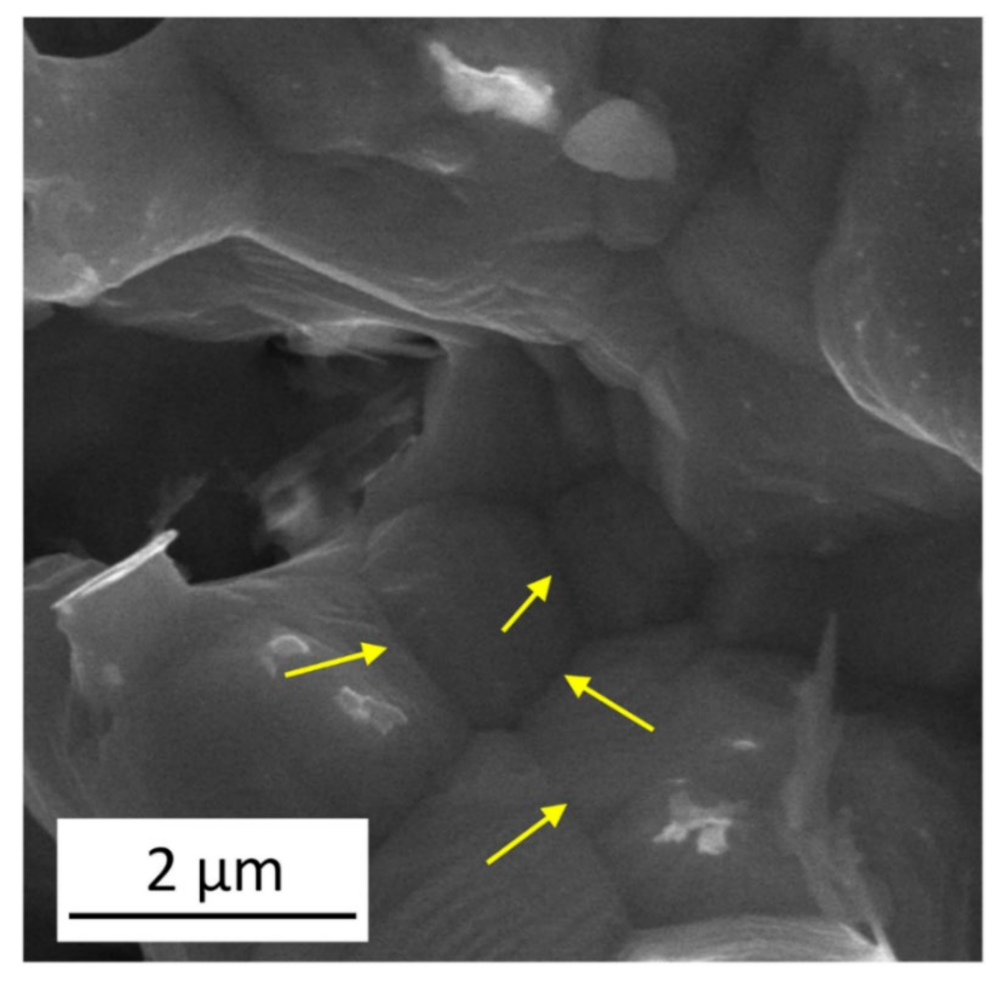
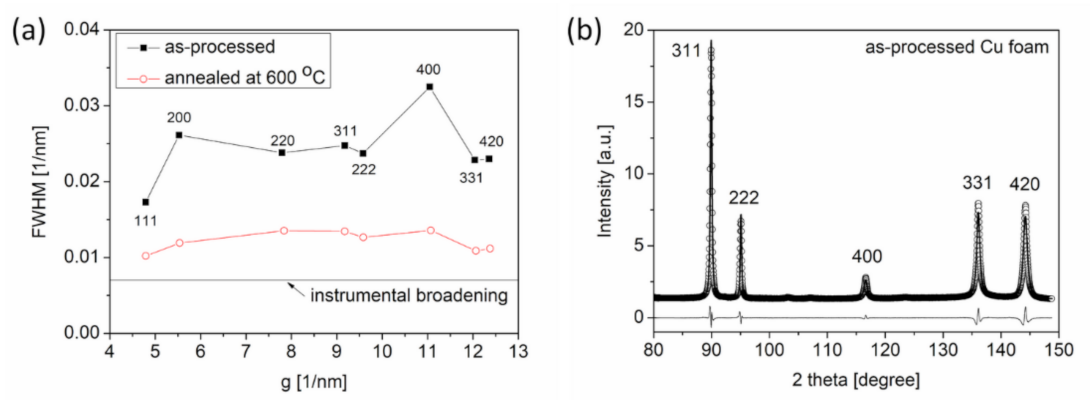
3.1.2. Changes in Defect Density during Annealing
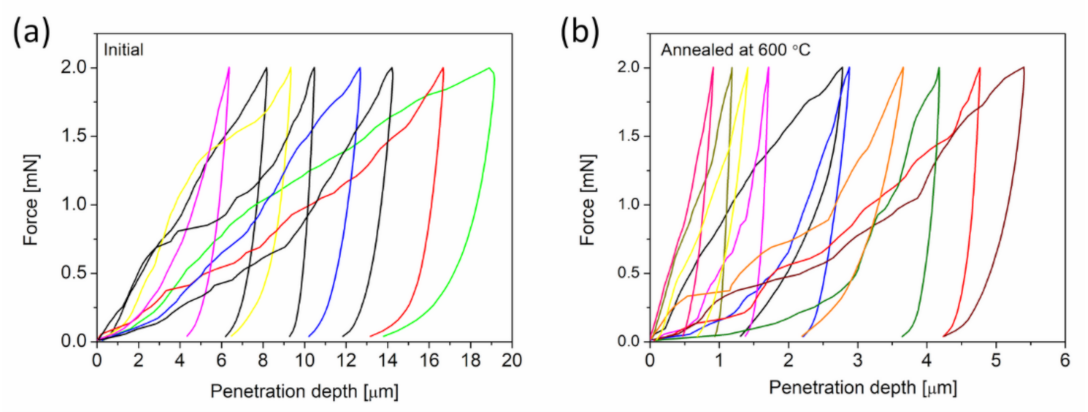
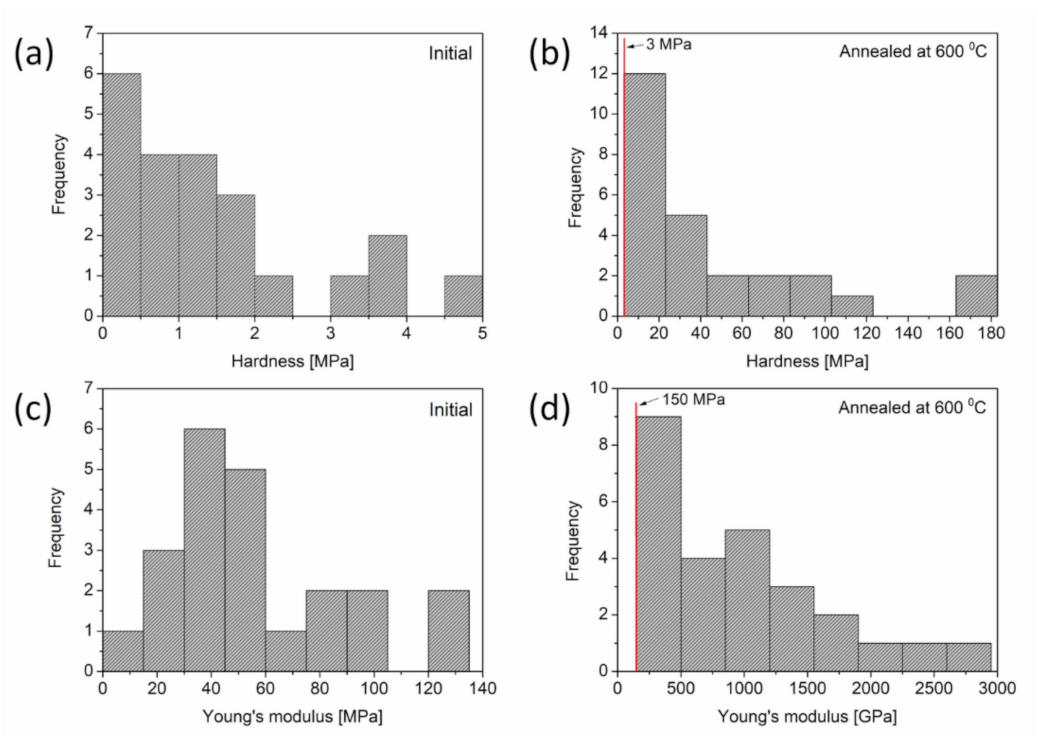
3.1.3. Effect of Heat Treatment on the Mechanical Properties of Cu Foams
3.2. Influence of Oxidizing Heat Treatment on the Microstructure and Electrochemical Performance
4. Conclusions and Future Research Directions
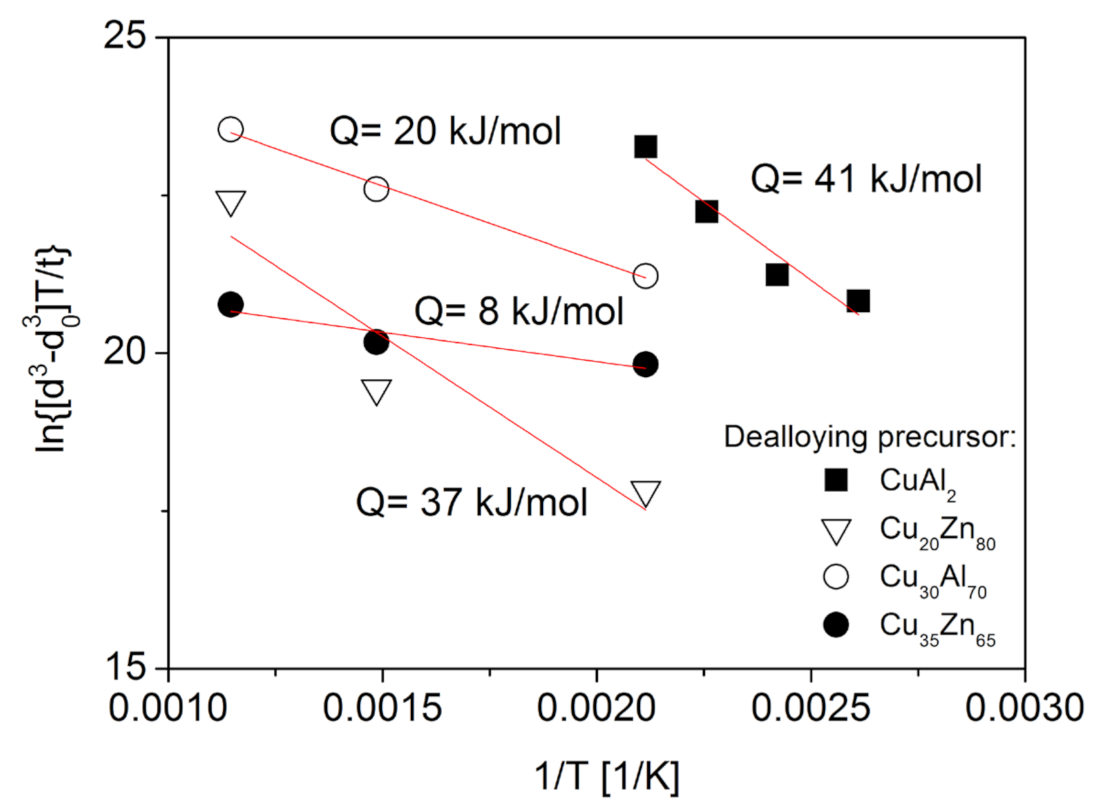
- Heat treatment under inert atmospheres at temperatures between 300 and 800 °C results in significant ligament coarsening. Annealing at the lowest temperature of 300 °C increased the ligament size by only 20–30% versus non-annealed samples, even after 70 h of annealing time. Nonetheless, samples annealed at 800 °C had 700% greater ligament sizes even if the duration of heat treatment was only 5 h. The activation energy of ligament size growth was about 89–103 kJ/mol, suggesting that coarsening is controlled by fast diffusion either on the surface or along lattice defects.
- The density of in-grown lattice defects, such as dislocations and twin faults, in the ligaments decreased significantly during annealing at 600 °C. Softening effects arising from the defect structures were overwhelmed by strengthening caused by the coalescence of the ligaments. Thus, heat treatments considerably improved the hardness and elastic modulus of materials.
- Annealing at low temperatures (110–200 °C) for short (0.5 h) times under oxidizing atmospheres also moderately increased the ligament size. The activation energy of this coarsening process was only about 41 kJ/mol, which is close to the activation energy value of Cu diffusion along the grain boundaries in surface oxide layers. This mechanism is necessary for the growth of the oxide layer.
- Electrochemical analyses demonstrated that oxidized Cu nanofoams can be considered a potential candidate as the anode material in high-performance Li-ion batteries. The CuO/Cu2O/Cu foam anode that was oxidized at 170 °C showed superior cycling stability than the CuO/Cu2O/Cu foam anode oxidized at 200 °C. Furthermore, the discharge and charge behaviors of both the CuO/Cu2O/Cu foam anodes are similar to those reported for other CuO or Cu2O anode materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tappan, B.C.; Huynh, M.H.; Hiskey, M.A.; Chavez, D.E.; Luther, E.P.; Mang, J.T.; Son, S.F. Ultralow-Density Nanostructured Metal Foams: Combustion Synthesis, Morphology, and Composition. J. Am. Chem. Soc. 2006, 128, 6589–6594. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.C.; Thompson, D.T. Catalysis by gold. Catal. Rev. Sci. Eng. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Biener, J.; Wittstock, A.; Zepeda-Ruiz, L.A.; Biener, M.M.; Zielasek, V.; Kramer, D.; Swanath, R.N.; Weissmüller, J.; Baümerand, M.; Hamza, A.V. Surface-chemistry-driven actuation in nanoporous gold. Nat. Mater. 2009, 8, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Wang, X.L.; Parida, S.; Wang, K.; Seo, M.; Weissmüller, J. Nanoporous Au–Pt alloys as large strain electrochemical actuators. Nano Lett. 2010, 10, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, C.; Cai, W.; Ding, Y. Ultralow-platinum-loading high-performance nanoporous electrocatalysts with nanoengineered surface structures. Adv. Mater. 2010, 22, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Shen, P.; Yang, G.; Zhao, L.; Qiu, X.; Wang, H.; Jiang, Q. 3D highly oriented metal foam: A competitive self-supporting anode for high-performance lithium-ion batteries. J. Mater. Sci. 2020, 55, 11462–11476. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. A Mathematical Model of Stress Generation and Fracture in Lithium Manganese Oxide. J. Electrochem. Soc. 2006, 153, A1019. [Google Scholar] [CrossRef]
- Christensen, J. Modeling Diffusion-Induced Stress in Li-Ion Cells with Porous Electrodes. J. Electrochem. Soc. 2010, 157, A36. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, F.; Fang, D. The effects of elastic stiffening on the evolution of the stress field within a spherical electrode particle of lithium-ion batteries. Int. J. Appl. Mech. 2013, 5, 1350040. [Google Scholar] [CrossRef]
- Zhou, W. Effects of external mechanical loading on stress generation during lithiation in Li-ion battery electrodes. Electrochim. Acta 2015, 185, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Omrani, R.; Shabani, B. Gas Diffusion Layers in Fuel Cells and Electrolysers: A Novel Semi-Empirical Model to Predict Electrical Conductivity of Sintered Metal Fibres. Energies 2019, 12, 855. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.; Cho, Y.; Kang, S.; Park, H.; Kim, M.; Lim, J.; Chung, D.; Lee, M.; Choe, H.; Sung, Y. Ordered macroporous platinum electrode and enhanced mass transfer in fuel cells using inverse opal structure. Nat. Commun. 2013, 4, 2473. [Google Scholar] [CrossRef] [Green Version]
- Forty, A.J. Corrosion micromorphology of noble metal alloys and depletion gilding. Nature 1979, 282, 597–598. [Google Scholar] [CrossRef]
- Qi, Z.; Zhao, C.; Wang, X.; Lin, J.; Shao, W.; Zhang, Z.; Bian, X.J. Formation and characterization of monolithic nanoporous copper by chemical dealloying of Al–Cu alloys. Phys. Chem. C 2009, 113, 6694–6698. [Google Scholar] [CrossRef]
- Nam, S.; Jo, H.; Choe, H.; Ahn, D.; Choi, H. Development of Nanoporous Copper Foams by Chemical Dealloying of Mechanically Alloyed AlCu Compounds. Mater. Trans. 2014, 55, 1414–1418. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.; Cho, Y.-H.; Choi, M.; Cho, J.; Um, J.H.; Sung, Y.E.; Choe, H. Novel method of powder-based processing of copper nanofoams for their potential use in energy applications. Mater. Chem. Phys. 2014, 145, 6–11. [Google Scholar] [CrossRef]
- Han, G.; Um, J.H.; Park, H.; Hong, K.; Yoon, W.S.; Choe, H. Hierarchically structured nanoporous copper for use as lithium-ion battery anode. Scripta Mater. 2019, 163, 9–13. [Google Scholar] [CrossRef]
- Jenei, P.; Han, H.P.T.; Choe, H.; Gubicza, J. Influence of pack cementation time on the microstructure of Cu nanofoams processed by dealloying. IOP Conf. Ser. Mater. Sci. Eng. 2020, 903, 01204. [Google Scholar]
- Du, Z.; Zhang, S.; Jiang, T.; Bai, Z. Preparation and characterization of three-dimensional tin thin-film anode with good cycle performance. Electrochim. Acta 2010, 65, 3537–3541. [Google Scholar] [CrossRef]
- Park, H.; Lee, S.; Jo, M.; Park, S.; Kwon, K.; Shobana, M.K.; Choe, H.; Park, H. Nanowire-like copper oxide grown on porous copper, a promising anode material for lithium-ion battery. J. Korean Ceram. Soc. 2017, 54, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Marathey, P.; Khanna, S.; Pati, R.; Mukhopadhyay, I.; Ray, A. Low temperature–controlled synthesis of hierarchical Cu2O/Cu(OH)2/CuO nanostructures for energy applications. J. Mater. Res. 2019, 34, 3173–3185. [Google Scholar] [CrossRef]
- Jenei, P.; Kádár Cs Han, G.; Hung, P.T.; Choe, H.; Gubicza, J. Annealing-induced toughening of a Cu nanofoam processed by dealloying. Metals 2020, 10, 1128. [Google Scholar] [CrossRef]
- Jo, H.; Kim, M.; Choi, H.; Sung, Y.; Choe, H.; Dunand, D.C. Morphological study of directionally freeze-cast nickel foams. Metall. Mater. Trans. E 2016, 3, 46–54. [Google Scholar] [CrossRef]
- Gubicza, J. X-Ray Line Profile Analysis in Materials Science; IGI-Global: Hershey, PA, USA, 2014. [Google Scholar]
- Ribárik, G.; Gubicza, J.; Ungár, T. Correlation between strength and microstructure of ball-milled Al–Mg alloys determined by X-ray diffraction. Mater. Sci. Eng. A 2004, 387, 343–347. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Veshchunov, M.S. Modelling of Grain Growth Kinetics in Porous Ceramic Materials under Normal and Irradiation Conditions. Materials 2009, 2, 1252–1287. [Google Scholar] [CrossRef] [Green Version]
- Bourgeois, L.; Dehaudt, P.; Lemaignan, C.; Fredric, J.P. Pore migration in UO2 and grain growth kinetics. J. Nucl. Mater. 2001, 295, 73–82. [Google Scholar] [CrossRef]
- Chen-Wiegart, Y.K.; Wang, S.; Chu, Y.S.; Liu, W.; McNulty, I.; Voorhees, P.W.; Dunand, D.C. Structural evolution of nanoporous gold during thermal coarsening. Acta Mater. 2012, 60, 4972–4981. [Google Scholar] [CrossRef]
- Baldan, A. Progress in Ostwald ripening theories and their applications to nickel-base superalloys. J. Mater. Sci. 2002, 37, 2171–2202. [Google Scholar] [CrossRef]
- Herring, C. Effect of change of scale on sintering phenomena. J. Appl. Phys. 1950, 21, 301. [Google Scholar] [CrossRef]
- Tsuda, M.; Wada, T.; Kato, H. Kinetics of formation and coarsening of nanoporous α-titanium dealloyed with Mg melt. Jpn. J. Appl. Phys. 2013, 114, 113503. [Google Scholar] [CrossRef]
- Kuwano-Nakatani, S.; Fujita, T.; Uchisawa, K.; Umetsu, D.; Kase, Y.; Kowata, Y.; Chiba, K.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; et al. Environment-sensitive thermal coarsening of nanoporous gold. Mater. Trans. 2015, 56, 468–472. [Google Scholar] [CrossRef] [Green Version]
- Geslin, P.A.; Buchet, M.; Wada, T.; Kato, H. Phase-field investigation of the coarsening of porous structures by surface diffusion. Phys. Rev. Mater. 2019, 3, 083401. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ngô, B.N.D.; Markmann, J.; Weissmüller, J. Topology evolution during coarsening of nanoscale metal network structures. Phys. Rev. Mater. 2019, 3, 076001. [Google Scholar] [CrossRef] [Green Version]
- Rothman, S.J.; Peterson, N.L. Isotope Effect and Divacancies for Self-Diffusion in Copper. Phys. Status Sol. 1969, 35, 305. [Google Scholar] [CrossRef]
- Surholt, T.; Herzig, C. Grain boundary self-diffusion in Cu polycrystals of different purity. Acta Mater. 1997, 45, 3817–3823. [Google Scholar] [CrossRef]
- Divinski, S.; Ribbe, J.; Schmitz, G.; Herzig, C. Grain boundary diffusion and segregation of Ni in Cu. Acta Mater. 2007, 55, 3337–3346. [Google Scholar] [CrossRef]
- Amouyal, Y.; Divinski, S.V.; Klinger, L.; Rabkin, E. Grain boundary diffusion and recrystallization in ultrafine grain copper produced by equal channel angular pressing. Acta Mater. 2008, 56, 5500–5513. [Google Scholar] [CrossRef]
- Karimi, M.; Tomkowski, T.; Vidali, G.; Biham, O. Diffusion of Cu on Cu surfaces. Phys. Rev. B. 1995, 52, 5364–5374. [Google Scholar] [CrossRef]
- Davydov, S.Y. Calculation of the activation energy for surface self-diffusion of transition-metal atoms. Phys. Solid State 1999, 41, 8–10. [Google Scholar] [CrossRef]
- Van Gastel, R.; Somfai, E.; Van Albada, S.B.; Van Saarloos, W.; Frenken, J.W.M. Vacancy diffusion in the Cu(001) surface I: An STM study. Surf. Sci. 2002, 521, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Cheng, I.C.; Hodge, A.M. Morphology, Oxidation, and Mechanical Behavior of Nanoporous Cu Foams. Adv. Eng. Mater. 2012, 14, 219–226. [Google Scholar] [CrossRef]
- Zhu, Y.; Mimura, K.; Isshiki, M. Oxidation Mechanism of Copper at 623–1073 K. Mater. Trans. 2002, 43, 2173–2176. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.P.; Bao, J.L.; Pan, G.L.; Zhu, H.Y.; Huang, P.S.; Wu, F.; Song, D.Y. Preparation and Electrochemical Performance of Polycrystalline and Single Crystalline CuO Nanorods as Anode Materials for Li-ion Battery. J. Phys. Chem. B 2004, 108, 5547–5551. [Google Scholar] [CrossRef]
- Trukawka, M.; Wenelska, K.; Singer, L.; Klingeler, R.; Chen, X.; Mijowska, E. Hollow Carbon Spheres Loaded with Uniform Dispersion of Copper Oxide Nanoparticles for Anode in Lithium- Ion Batteries. J. Alloy Compd. 2021, 853, 156700. [Google Scholar] [CrossRef]
- Yabuki, A.; Tanaka, S. Oxidation behavior of copper nanoparticles at low temperature. Mater. Res. Bull. 2011, 46, 2323–2327. [Google Scholar] [CrossRef]
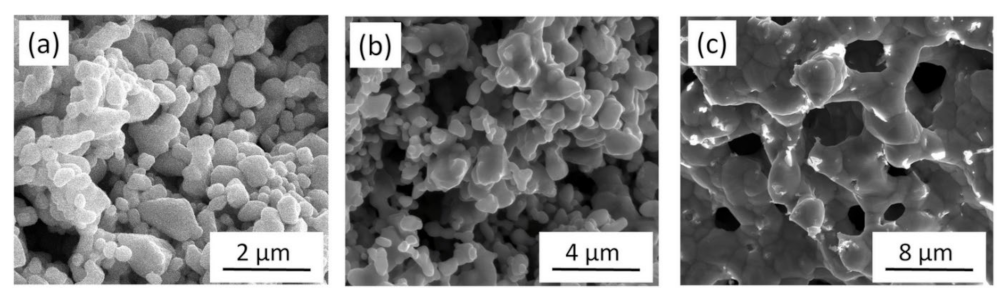





| Processing Conditions | Heat Treatment Conditions | Ligament Size before Annealing [nm] | Ligament Size after Annealing [nm] |
|---|---|---|---|
| Route A * | 400 °C for 6 h | 105 ± 6 | 125 ± 6 |
| Route B ** | 300 °C for 70 h | 640 ± 60 | 770 ± 50 |
| Route B | 800 °C for 5 h | 640 ± 60 | 5180 ± 880 |
| Route C *** | 300 °C for 70 h | 740 ± 50 | 980 ± 60 |
| Route C | 600 °C for 5.5 h | 540 ± 20 | 1620 ± 80 |
| Route C | 800 °C for 5 h | 740 ± 50 | 4220 ± 360 |
| Exponent n | Activation Energy [kJ/mol] | Correlation Coefficient of Fitting |
|---|---|---|
| 3 | 89 ± 5 | 0.99496 |
| 4 | 103 ± 11 | 0.99458 |
| Processing Conditions | Crystallite Size [nm] | Dislocation Density [1014 m2 ] | Twin FaultProbability [%] |
|---|---|---|---|
| Route A * | 18 ± 4 | 4 ± 1 | 2.1 ± 0.2 |
| Route A + annealing at 400 °C for 5.5 h | 142 ± 17 | 6 ± 1 | 1.0 ± 0.1 |
| Route C ** | 60 ± 7 | 11 ± 2 | 1.1 ± 0.1 |
| Route C + annealing at 600 °C for 5.5 h | 90 ± 10 | 0.5 ± 0.2 | 0.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubicza, J.; Jenei, P.; Han, G.; Hung, P.-T.; Song, Y.; Park, D.; Szabó, Á.; Kádár, C.; Kim, J.-H.; Choe, H. Effect of Heat Treatment on the Microstructure and Performance of Cu Nanofoams Processed by Dealloying. Materials 2021, 14, 2691. https://doi.org/10.3390/ma14102691
Gubicza J, Jenei P, Han G, Hung P-T, Song Y, Park D, Szabó Á, Kádár C, Kim J-H, Choe H. Effect of Heat Treatment on the Microstructure and Performance of Cu Nanofoams Processed by Dealloying. Materials. 2021; 14(10):2691. https://doi.org/10.3390/ma14102691
Chicago/Turabian StyleGubicza, Jenő, Péter Jenei, Gigap Han, Pham-Tran Hung, Youngseok Song, Dahye Park, Ábel Szabó, Csilla Kádár, Jae-Hun Kim, and Heeman Choe. 2021. "Effect of Heat Treatment on the Microstructure and Performance of Cu Nanofoams Processed by Dealloying" Materials 14, no. 10: 2691. https://doi.org/10.3390/ma14102691
APA StyleGubicza, J., Jenei, P., Han, G., Hung, P.-T., Song, Y., Park, D., Szabó, Á., Kádár, C., Kim, J.-H., & Choe, H. (2021). Effect of Heat Treatment on the Microstructure and Performance of Cu Nanofoams Processed by Dealloying. Materials, 14(10), 2691. https://doi.org/10.3390/ma14102691









