Novel Cast Polyurethanes Obtained by Using Reactive Phosphorus-Containing Polyol: Synthesis, Thermal Analysis and Combustion Behaviors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyurethanes
2.3. Methods
3. Results and Discussion
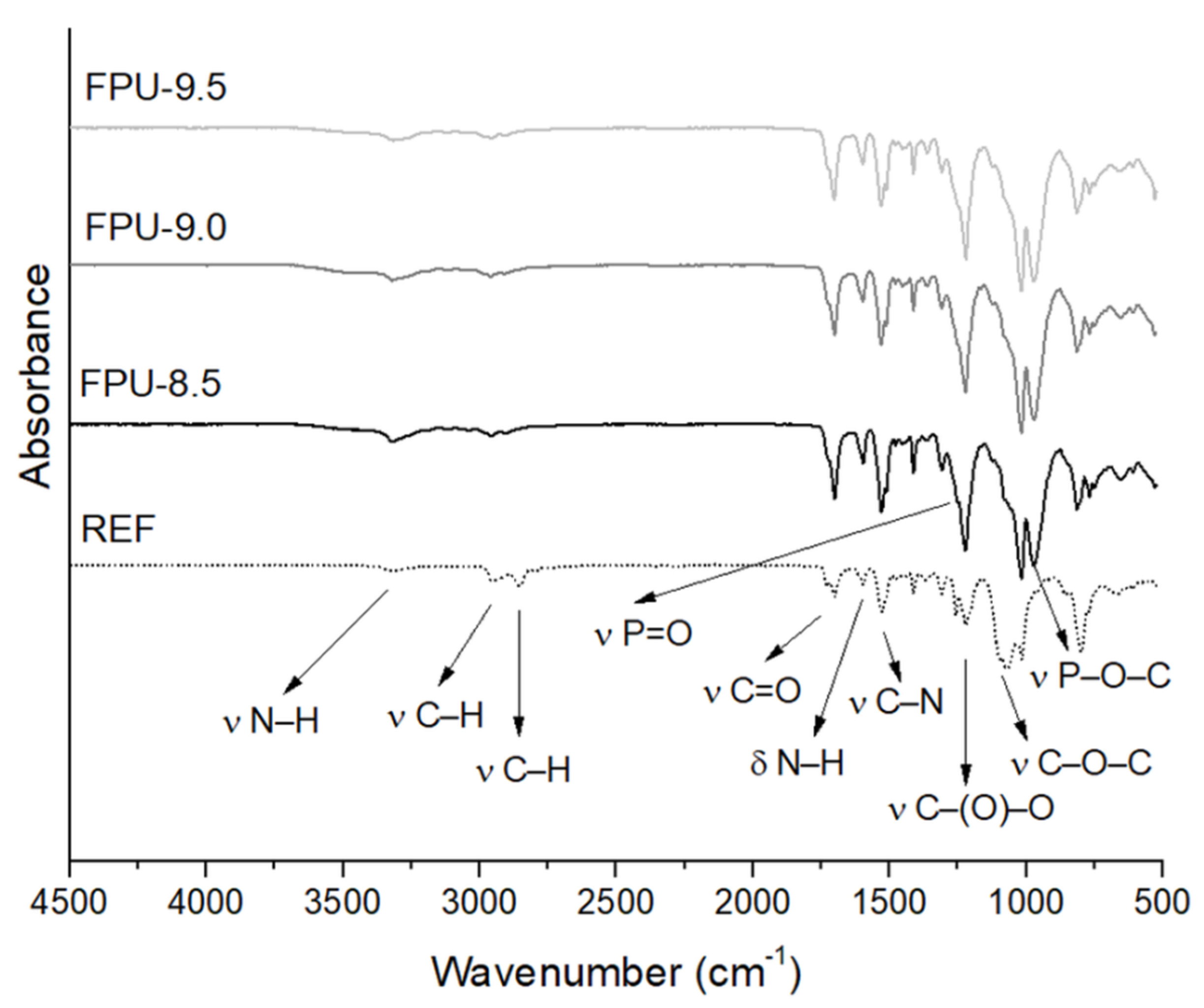
3.1. Fourier Transform Infrared Spectroscopy Results (FTIR)
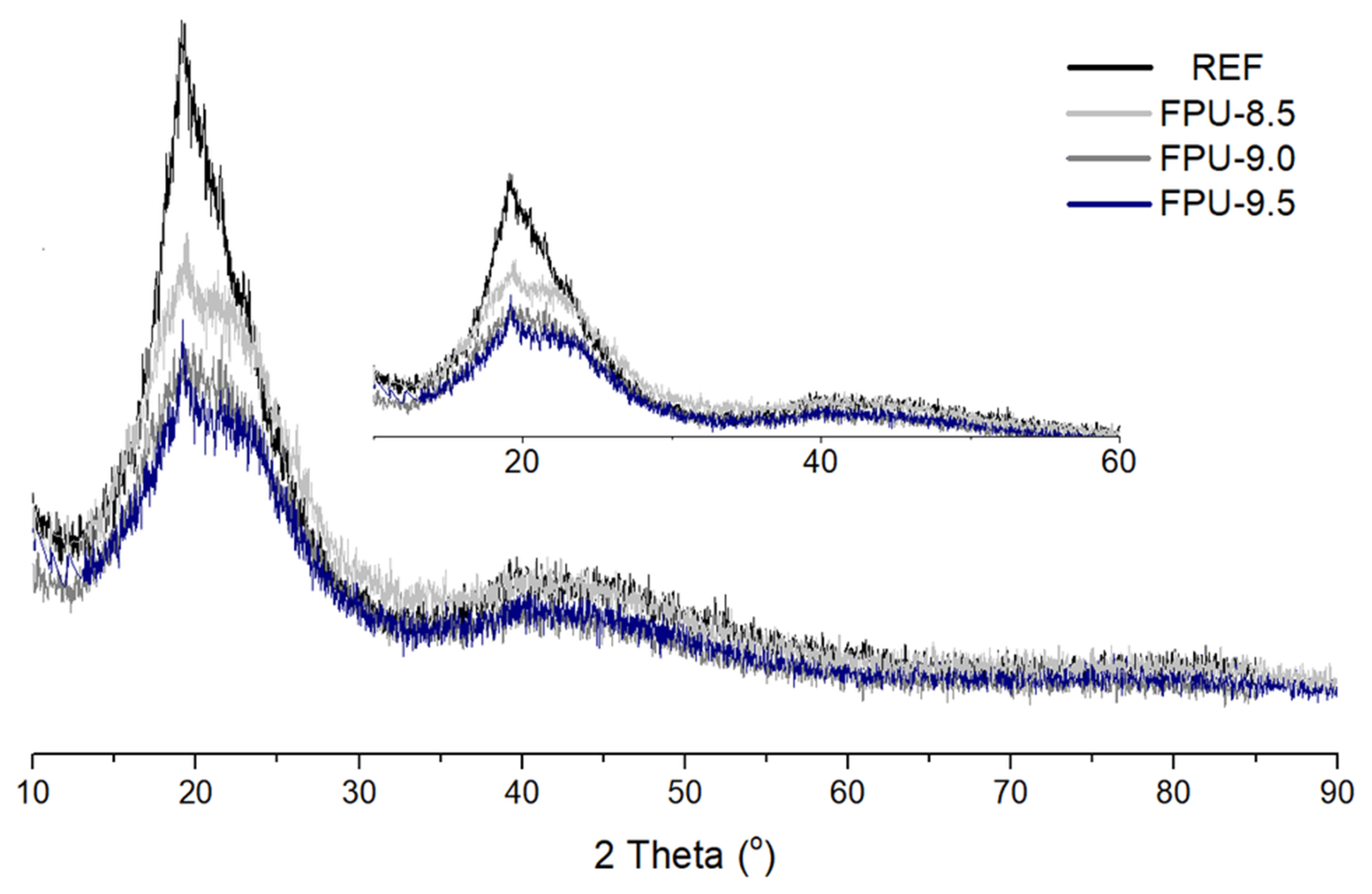
3.2. X-ray Diffraction (XRD)
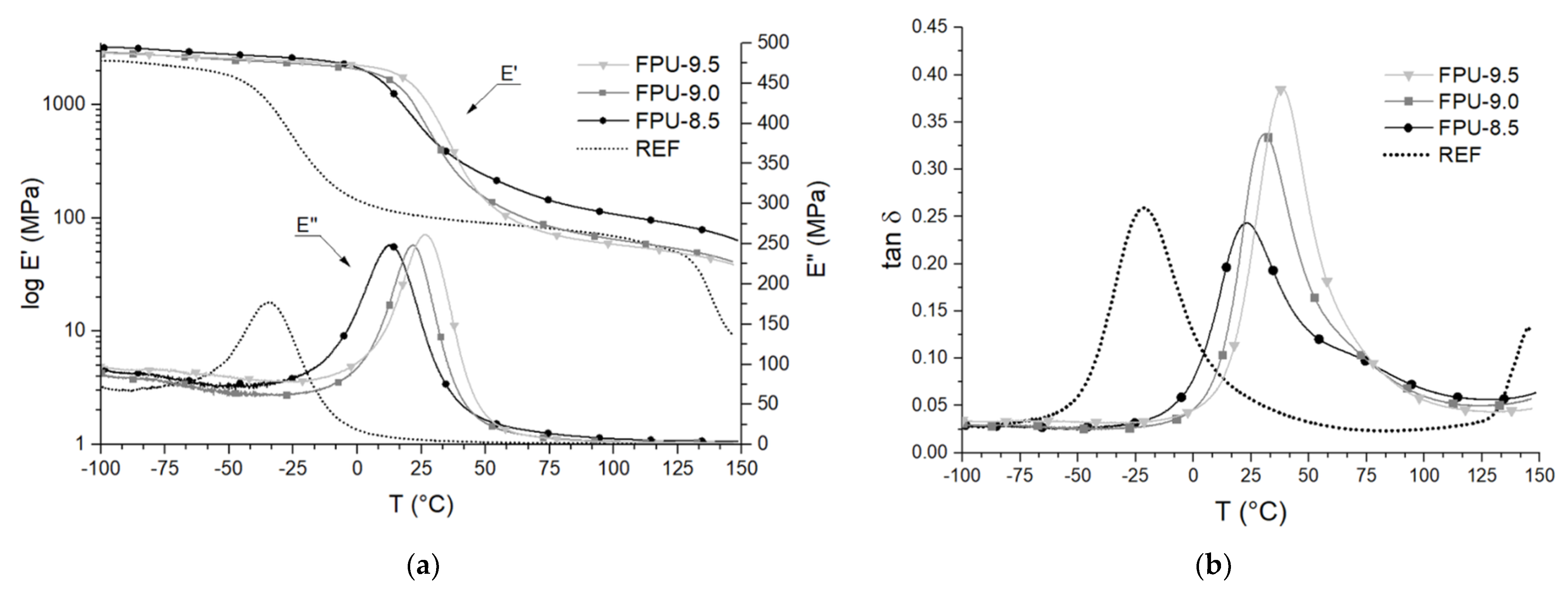
3.3. Dynamic Mechanical Thermal Analysis (DMTA)
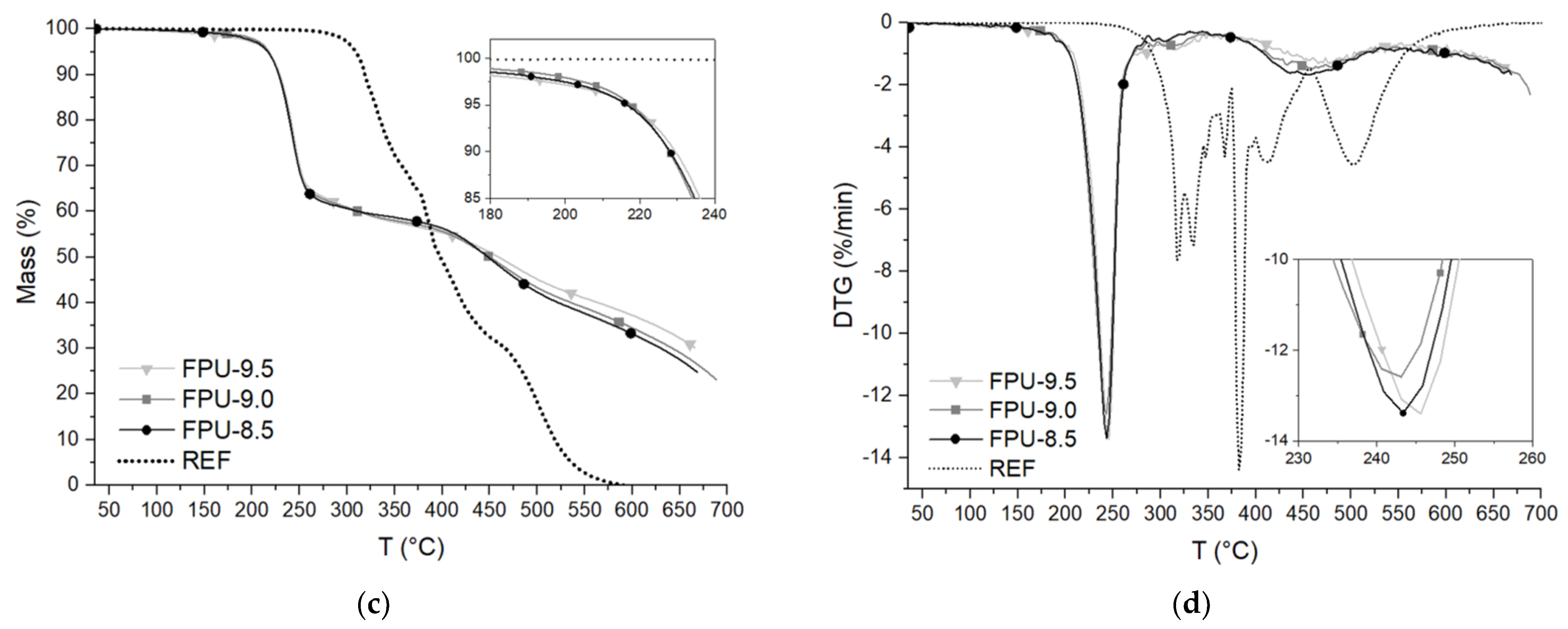
3.4. Thermogravimetric Analysis (TGA)
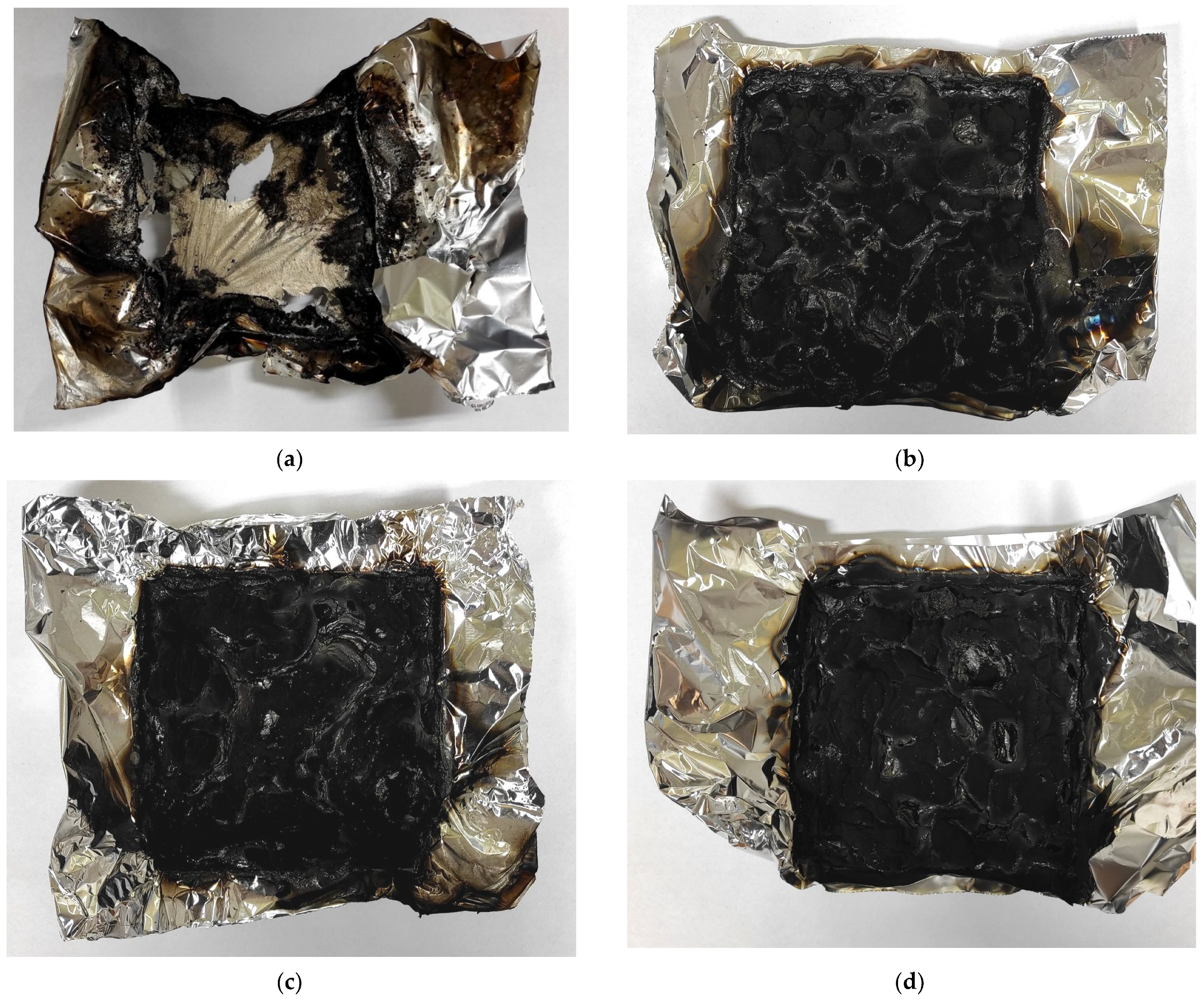
3.5. The UL-94 Vertical Burning Test and LOI Results
3.6. Cone Calorimeter (CC)
3.7. Hardness, Density and Tensile Properties
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parcheta, P.; Głowińska, E.; Datta, J. Effect of bio-based components on the chemical structure, thermal stability and mechanical properties of green thermoplastic polyurethane elastomers. Eur. Polym. J. 2020, 123, 109422. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J.; Włoch, M.; Różańska, M. The influence of chemical structure on mechanical and thermomechanical properties of cast biopolyurethane elastomers. Elastomery 2018, 22, 40–49. [Google Scholar]
- Clemitson, I.R. Castable Polyurethane Elastomers; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ray, S.S.; Kurkuma, M. Halogen-Free Flame-Retardants Polymers. Next-generation Fillers for Polymer Nanocomposite Applications. Springer Ser. Mater. Sci. 2020, 294, 113. [Google Scholar]
- Liu, X.; Hao, J.; Gaan, S. Recent studies of decomposition and strategies of smoke and toxicity suppression for polyurethane based materials. RSC Adv. 2016, 6, 74742–74756. [Google Scholar] [CrossRef] [Green Version]
- Błażek, K.; Datta, J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 173–211. [Google Scholar] [CrossRef]
- Janowska, G.; Przygocki, W.; Włochowicz, A. Palność Polimerów i Materiałów Polimerowych; WNT: Warszawa, Poland, 2007. [Google Scholar]
- Węgrzyn, M. Development of the flame retardants used in polymers. Zesz. Nauk. Sgsp 2018, 65, 37–53. [Google Scholar]
- Cai, W.; Zhan, J.; Feng, X.; Yuan, B. Facile Construction of Flame-Retardant-Wrapped Molybdenum Disulfide Nanosheets for Properties Enhancement of Thermoplastic Polyurethane. Ind. Eng. Chem. Res. 2017, 56, 25. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Zhou, K.; Yuen, R. Novel CuCo2O4/graphitic carbon nitride nanohybrids: Highly effective catalysts for reducing CO generation and fire hazards of thermoplastic polyurethane nanocomposites. J. Hazard. Mater. 2015, 293, 87–96. [Google Scholar] [CrossRef]
- Sut, A.; Metzsch-Zilligen, E.; Groβhauser, M. Synergy between melamine cyanurate, melamine polyphosphate and aluminum diethylphosphinate in flame retarded thermoplastic polyurethane. Polym. Test. 2019, 74, 196–204. [Google Scholar] [CrossRef]
- Wang, J.; Zang, Y.; Cai, W.; Yao, C. Construction of multifunctional boron nitride nanosheet towards reducing toxic volatiles (CO and HCN) generation and fire hazard of thermoplastic polyurethane. J. Hazard. Mater. 2018, 362, 482–494. [Google Scholar] [CrossRef]
- Szołyga, M.; Dutkiewicz, M.; Marciniec, B. Polyurethane composites based on silsesquioxane derivatives of different structures. J. Anal. Calorim. 2018, 132, 1693–1706. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Jin, Y.; Lai, S.; Shen, Y.; Pan, J. Urethane-silica functionalized graphene oxide for enhancing mechanical property and fire safety of waterborne polyurethane composites. Appl. Surf. Sci. 2019, 492, 298–308. [Google Scholar] [CrossRef]
- Wang, S.; Gao, R.; Zhou, K. The influence of cerium dioxide functionalized reduced graphene oxide on reducing fire hazards of thermoplastic polyurethane nanocomposites. J. Colloid Interface Sci. 2019, 536, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, W.; Chen, R.; Li, W. Synergistic effect between zeolitic imidazolate framework-8 and expandable graphite to improve the flame retardancy and smoke suppression of polyurethane elastomer. J. Appl. Polym. Sci. 2019, 137, 48048. [Google Scholar] [CrossRef]
- Ji, X.; Chen, D.; Wang, Q.; Shen, J. Synergistic effect of flame retardants and carbon nanotubes on flame retarding and electromagnetic shielding properties of thermoplastic polyurethane. Compos. Sci. Technol. 2018, 163, 49–55. [Google Scholar] [CrossRef]
- Atagur, M.; Demiroglu, S. Flame retardancy of Composites and Nanocomposites Based on PU Polymers. Polyurethane Polym. 2017, 17, 499–524. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Radojcic, D.; Kahol, P.K. Highly flame-retardant bio-based polyurethanes using novel reactive polyols. J. Appl. Polym. Sci. 2017, 135, 46027. [Google Scholar] [CrossRef]
- Savas, L.A.; Deniz, T.K.; Tayfun, U.; Dogan, M. Effect of microencapsulated red phosphorus on flame retardant, thermal and mechanical properties of thermoplastic polyurethane composites filled with huntite & hydromagnesite mineral. Polym. Degrad. Stab. 2017, 135, 121–129. [Google Scholar] [CrossRef]
- Chen, X.; Lai, Y.; Gu, Y.; Jaio, C.; Li, S. Effect of Functionalized Carbon Microspheres Combined with Ammonium Polyphosphate on Fire Safety Performance of Thermoplastic Polyurethane. ACS Omega 2020, 5, 6051–6061. [Google Scholar] [CrossRef]
- Yang, A.H.; Deng, C.; Chen, H. A novel Schiff-base polyphosphate ester: Highly-efficient flame retardant for polyurethane elastomer. Polym. Degrad. Stab. 2017, 144, 70–82. [Google Scholar] [CrossRef]
- Rao, W.H.; Zhu, Z.M.; Wang, S.X.; Wang, T.; Wang, Y.Z. A reactive phosphorus-containing polyol incorporated into flexible polyurethane foam: Self-extinguishing behavior and mechanism. Polym. Degrad. Stab. 2018, 153, 192–200. [Google Scholar] [CrossRef]
- Chen, H.; Luo, Y.; Chai, C.; Wang, J. Synthesis and Characterization of Phosphorus-Containing Waterborne Polyurethanes: Effects of the Organophosphonate Content on the Flame Retardancy, Morphology and Film Properties. J. Appl. Polym. Sci. 2008, 110, 3107–3115. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Datta, J. Novel bio-based thermoplastic poly(ether-urethane)s. Correlation between the structure, processing and properties. Polymer 2019, 160, 1–10. [Google Scholar] [CrossRef]
- Chiu, S.H.; Wu, C.L.; Lee, H.Y. Synthesis and characterization of novel flame retardant polyurethanes containing designer phosphorus units. J. Polym. Res. 2016, 23, 205. [Google Scholar] [CrossRef]
- International Organization for Standardization. Plastics—Determination of Dynamic Mechanical Properties—Part 1: General Principles; ISO 6721-1:2019-07; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- Polski Komitet Normalizacyjny. Badanie Zagrożenia Ogniowego—Część 11-10: Płomienie Probiercze—Metody Badania Płomieniem Probierczym 50 W przy Poziomym i Pionowym Ustawieniu Próbki; PN-EN 60695-11-10:2014-02; Polski Komitet Normalizacyjny: Warsaw, Poland, 2014. [Google Scholar]
- Polski Komitet Normalizacyjny. Tworzywa Sztuczne—Oznaczanie Zapalności Metodą Wskaźnika Tlenowego—Część 2: Badanie w Temperaturze Pokojowej; PN-EN ISO 4589-2:2017-06; Polski Komitet Normalizacyjny: Warsaw, Poland, 2017. [Google Scholar]
- International Organization for Standardization. Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement); ISO 5660-1:2015; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Wu, N.; Niu, F.; Lang, W.; Yu, J.; Fu, G. Synthesis of reactive phenylphosphoryl glycol ether oligomer and improved flame retardancy and mechanical property of modified rigid polyurethane foams. Mater. Des. 2019, 181, 107929. [Google Scholar] [CrossRef]
- Polski Komitet Normalizacyjny. Tworzywa Sztuczne—Oznaczanie Właściwości Mechanicznych przy Statycznym Rozciąganiu—Część 1: Zasady Ogólne; PN-EN ISO 527-1:2020-01; Polski Komitet Normalizacyjny: Warsaw, Poland, 2020. [Google Scholar]
- Polski Komitet Normalizacyjny. Tworzywa Sztuczne i Ebonit—Oznaczanie Twardości Metodą Wciskania z Zastosowaniem Twardościomierza (Twardość Metodą Shore’a); PN-EN ISO 868:2005; Polski Komitet Normalizacyjny: Warsaw, Poland, 2005. [Google Scholar]
- Polski Komitet Normalizacyjny. Tworzywa Sztuczne—Metody Oznaczania Gęstości Tworzyw Sztucznych Nieporowatych--Część 1: Metoda Zanurzeniowa, Metoda Piknometru Cieczowego i Metoda Miareczkowa; PN-EN ISO 1183-1:2019-05; Polski Komitet Normalizacyjny: Warsaw, Poland, 2019. [Google Scholar]
- Kasprzyk, P.; Sadowska, E.; Datta, J. Investigation of Thermoplastic Polyurethanes Synthesized via Two Different Prepolymers. J. Polym. Environ. 2019, 27, 2588–2599. [Google Scholar] [CrossRef] [Green Version]
- Głowińska, E.; Datta, J.; Rodriguez Romero, J.F. Novel cast polyurethanes based on dispersed polymeric polyols. Polym. Test. 2018, 68, 340–349. [Google Scholar] [CrossRef]
- Piszczyk, Ł. Synteza i Właściwości Nowych Uniepalnionych Pianek Poliuretanowych Otrzymanych z Użyciem Surowców Odnawialnych. Ph.D. Thesis, Gdansk University of Technology, Gdansk, Poland, 2011. [Google Scholar]
- Gosz, K.; Haponiuk, J.; Piszczyk, Ł. The influence of Substitution of a Phosphorus-Containing Polyol with the Bio-polyol on the Properties of Bio-based PUR/PIR Foams. J. Polym. Environ. 2018, 26, 3877–3888. [Google Scholar] [CrossRef] [Green Version]
- Fuensanta, M.; Martin-Martinez, J.M. Influence of the hard segments content on the structure, viscoelastic and adhesion properties of thermoplastic polyurethane pressure sensitive adhesives. J. Adhes. Sci. Tech. 2020, 34, 2652–2671. [Google Scholar] [CrossRef]
- Hu, X.; Su, T.; Pan, W.; Li, P.; Wang, Z. Difference in solid-state properties and enzymatic degradation of three kinds of poly(butylene succinate)/cellulose blends. RSC Adv. 2017, 7, 35496. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.C. Chemical composition and XRD analyses for alkaline composite PVA polymer electrolyte. Mater. Lett. 2004, 58, 33–38. [Google Scholar] [CrossRef]
- Nagane, S.S.; Verma, S.; Tawade, B.V.; Sane, P.S. Aromatic polyesters containing pendant azido groups: Synthesis, characterization, chemical modification and thermal cross-linking. Eur. Polym. J. 2019, 116, 180–189. [Google Scholar] [CrossRef]
- Zhao, X.; Shou, T.; Liang, R.; Hu, S. Bio-based thermoplastic polyurethane derived from polylactic acid with high-damping performance. Ind. Crop. Prod. 2020, 154, 112619. [Google Scholar] [CrossRef]
- Datta, J.; Kasprzyk, P. Thermoplastic polyurethanes derived from petrochemical of renewable resources: A comprehensive review. Polym. Eng. Sci. 2018, 58, E14–E35. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.P.; Mishra, A.; Kumar, N. Evaluation of thermal, morphological and flame retardant properties of thermoplastic polyurethane polyphosphazene blends. Polym. Bull. 2018, 75, 2415–2430. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J.; Parcheta, P. Effect of sisal fiber filler on thermal properties of bio-based polyurethane composites. J. Anal. Calorim. 2017, 130, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Li, J.; Luo, Y. Flame Retardancy and Thermal Degradation Mechanism of a Novel Post-chain Extension Flame Retardant Waterborne Polyurethane. Polym. Degrad. Stab. 2016, 123, 36–46. [Google Scholar] [CrossRef]
- Huang, W.-K.; Yeh, J.-T.; Chen, K.-J.; Chen, K.-N. Flame retardation improvement of aqueos-based polyurethane with aziridinyl phosphazene curing system. J. Appl. Polym. Sci. 2000, 79, 662–673. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Ramos, M.J.; Klein, R. Thermal degradation and fire behaviour of novel polyurethanes based on phosphate polyols. Polym. Degrad. Stab. 2014, 101, 40–51. [Google Scholar] [CrossRef]
- Pau, D.S.W.; Fleischmann, C.M.; Delichatsios, M.A. Thermal decomposition of flexible polyurethane foams in air. Fire Saf. J. 2020, 11, 102925. [Google Scholar] [CrossRef]
- Phan, H.T.Q.; Nguyen, B.T.; Pham, L.H. Excellent Fireproof Characteristic and High Thermal Stability of Rice Husk-Filled Polyurethane with Halogen-Free Flame Retardant. Polymers 2019, 11, 1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Haro, J.C.; López-Pedrajas, D.; Perez, A.; Rodriguez, J.F. Synthesis of rigid polyurethane foams from phoshorylated biopolyols. Environ. Sci. Pollut. Res. 2019, 26, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Patel, R.H. Processing and characterizations: Effect of PPG molecular weight on properties of phosphate based polyurethanes. Prog. Org. Coat. 2020, 147, 105868. [Google Scholar] [CrossRef]
- Toldy, A.; Harakaly, G.; Szolnoki, B. Flame retardancy of thermoplastic polyurethanes. Polym. Degrad. Stab. 2012, 97, 2524–2530. [Google Scholar] [CrossRef]
- Sałasińska, K.; Borucka, M.; Leszczyńska, M.; Zatorski, W. Analysis of flammability and smoke emission of rigid polyurethane foams modified with nanoparticles and halogen-free fire retardants. J. Anal. Calorim. 2017, 130, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, W.; Jiao, C. A recycled environmental friendly flame retardant by modifying para-aramid fiber with phosphorus acid for thermoplastic polyurethane elastomer. J. Hazard. Mater. 2017, 331, 257–264. [Google Scholar] [CrossRef]
- Amado, J.C.Q. Thermal Resistance Properties of Polyurethanes and Its Composites. In Thermosoftening Plastics; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Zhang, T.; Zou, B.; Hu, W. Synthesis of a novel liquid phosphorus-containing flame retardant for flexible polyurethane foam: Combustion behaviors and thermal properties. Polym. Degrad. Stab. 2020, 171, 109029. [Google Scholar] [CrossRef]
- Hirzin, R.; Azzahari, A.; Yahya, R. The behavior of semi-rigid polyurethane film based on funcionalized rubber by one-shot and two-shot metod preparation. J. Mater. Sci. 2018, 53, 13280–13290. [Google Scholar] [CrossRef]
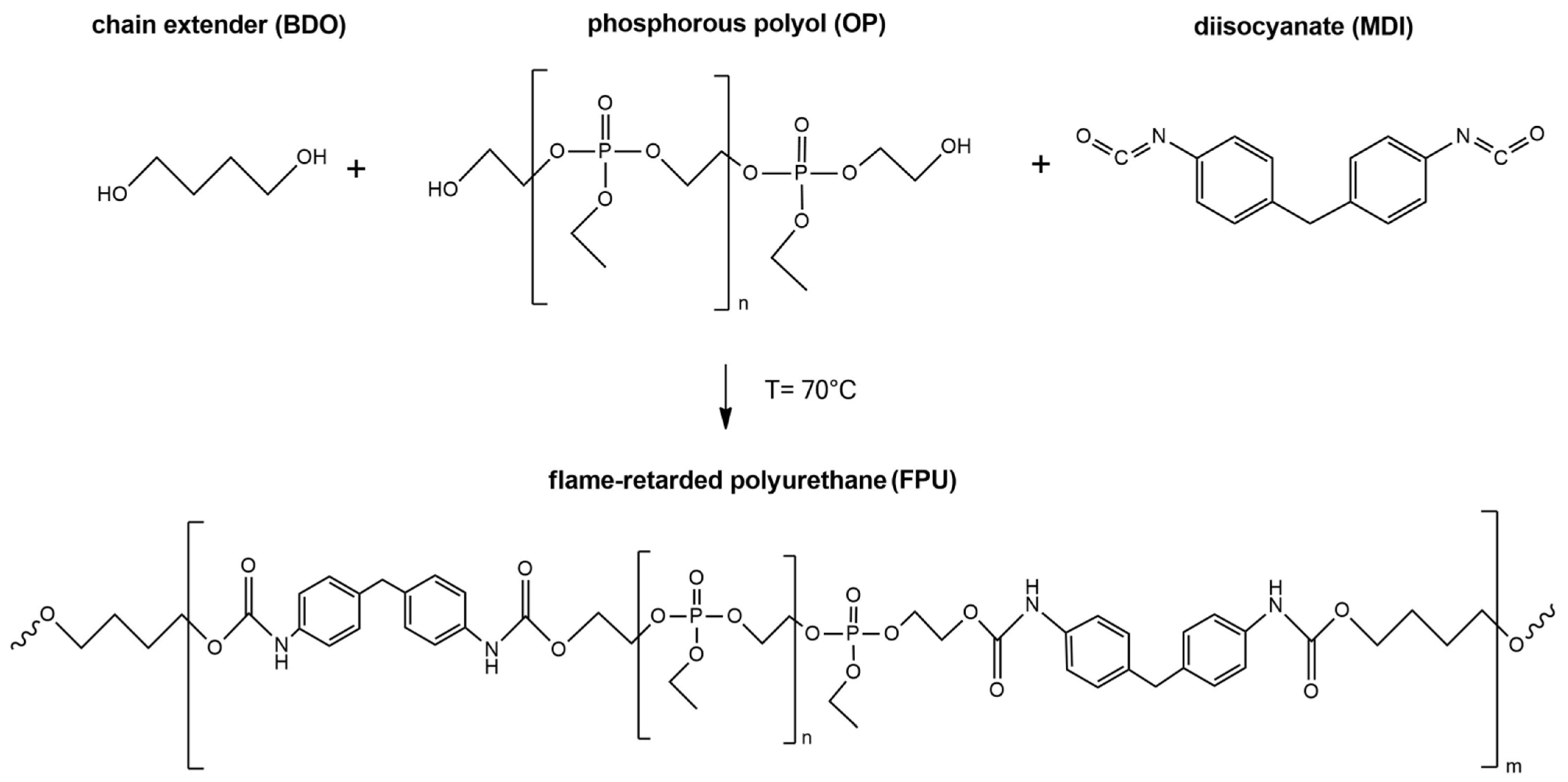


| PU Materials | PTHF (wt%) | OP (wt%) | MDI (wt%) | BDO (wt%) | Phosphorus (wt%) | Molar Ratio MDI:polyol:BDO |
|---|---|---|---|---|---|---|
| REF | 55.59 | - | 36.58 | 7.83 | 0.00 | 1.00:0.38:0.60 |
| FPU-8.5 | - | 50.81 | 41.36 | 7.83 | 8.63 | 1.00:0.45:0.53 |
| FPU-9.0 | - | 53.34 | 39.73 | 6.93 | 9.07 | 1.00:0.50:0.49 |
| FPU-9.5 | - | 55.87 | 38.15 | 5.98 | 9.50 | 1.00:0.54:0.44 |
| PU Materials | HS (%) | SS (%) | R | DPS | DPM |
|---|---|---|---|---|---|
| REF | 44.41 | 55.59 | 1.48 | 0.60 | 0.40 |
| FPU-8.5 | 49.19 | 50.81 | 2.03 | 0.70 | 0.30 |
| FPU-9.0 | 46.66 | 53.34 | 1.79 | 0.64 | 0.36 |
| FPU-9.5 | 44.13 | 55.87 | 1.74 | 0.64 | 0.36 |
| PU Materials | tan δ | TgSS (°C) | E’SS (MPa) | E’23°C (MPa) | E’’ (MPa) | ∆T (°C) | S |
|---|---|---|---|---|---|---|---|
| REF | 0.26 | −21.2 | 394 | 93 | 176.6 | - | 13.48 |
| FPU-8.5 | 0.24 | 25.1 | 624 | 719 | 248.3 | - | 15.74 |
| FPU-9.0 | 0.33 | 30.5 | 471 | 927 | 248.3 | 11.46 | 17.00 |
| FPU-9.5 | 0.39 | 38.4 | 354 | 1398 | 261.7 | 16.75 | 18.06 |
| PU Materials | T5% (°C) | T50% (°C) | T70% (°C) | TOP max (°C) | THS max (°C) | TSS max (°C) | Y (%) |
|---|---|---|---|---|---|---|---|
| REF | 304.7 | 319.7 | 424.7 | - | 319.7 | 422.3 | 7.28 |
| FPU-8.5 | 218.4 | 285.7 | 393.2 | 246.3 | 293.4 | 310.4 | 23.91 |
| FPU-9.0 | 220.6 | 288.8 | 408.2 | 246.3 | 294.2 | 308.4 | 24.94 |
| FPU-9.5 | 212.7 | 297.8 | 513.3 | 246.6 | 329.5 | 358.7 | 24.37 |
| PU Materials | T5% (°C) | T50% (°C) | T70% (°C) | T1 max (°C) | T2 max (°C) | T3 max (°C) | T4 max (°C) | T5 max (°C) | T6 max (°C) | T7 max (°C) | Y (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| REF | 307.3 | 397.3 | 464.8 | 317.3 | 334.8 | 347.3 | 367.3 | 382.3 | 409.8 | 502.3 | 0.00 |
| FPU-8.5 | 216.6 | 448.4 | 628.4 | 243.3 | 306.4 | 451.3 | - | - | - | - | 25.16 |
| FPU-9.0 | 217.7 | 449.9 | 640.7 | 242.6 | 317.5 | 458.8 | - | - | - | - | 26.92 |
| FPU-9.5 | 217.2 | 459.2 | 665.7 | 245.2 | 316.5 | 476.9 | - | - | - | - | 30.14 |
| PU Materials | Rating | Char Formation | The Burning Time of the Material after Removing the Fire Source | Dripping | LOI (%) | |
|---|---|---|---|---|---|---|
| I Flame Application | II Flame Application | |||||
| REF | V-2 | no | 5 s | 2 s | yes | 22.4 |
| FPU-8.5 | V-0 | yes | 1 s | 1 s | no | 25.4 |
| FPU-9.0 | V-0 | yes | 1 s | 1 s | no | 25.4 |
| FPU-9.5 | V-0 | yes | 1 s | 1 s | no | 25.2 |
| PU Materials | TTI (s) | pHRR (kW/m2) | FSR (kW/(m2s)) | THR (MJ/m2) | MAHRE (kW/m2) | pMLR (g/s) | TTF (s) | TOC (g) | EHC (MJ/kg) | pSPR (m2/s) | R (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| REF | 24 | 2232 | 93.0 | 132.2 | 677.9 | 0.72 | 591 | 100.2 | 20.74 | 0.22 | 0.0 |
| FPU-8.5 | 16 | 648 | 40.5 | 73.8 | 433.7 | 0.55 | 244 | 56.3 | 14.01 | 0.31 | 26.9 |
| FPU-9.0 | 15 | 664 | 44.3 | 52.0 | 454.1 | 0.42 | 186 | 39.9 | 14.13 | 0.29 | 20.7 |
| FPU-9.5 | 19 | 643 | 33.8 | 47.5 | 402.6 | 0.47 | 154 | 36.3 | 12.99 | 0.40 | 27.9 |
| PU Materials | Rm (MPa) | ε (%) | εt (%) | H (ShD) | ρ (g/cm3) |
|---|---|---|---|---|---|
| REF | 36.7 ± 1.7 | 720 ± 5 | 28.1 ± 0.6 | 37.9 ± 3.2 | 1.1243 ± 0.0003 |
| FPU-8.5 | 16.3 ± 1.8 | 57 ± 2 | 18.7 ± 3.7 | 52.3 ± 5.7 | 1.3267 ± 0.0006 |
| FPU-9.0 | 19.6 ± 2.2 | 63 ± 6 | 24.0 ± 3.4 | 53.8 ± 5.5 | 1.3336 ± 0.0003 |
| FPU-9.5 | 17.6 ± 0.6 | 73 ± 1 | 31.6 ± 3.6 | 45.7 ± 5.7 | 1.3342 ± 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagożdżon, I.; Parcheta, P.; Datta, J. Novel Cast Polyurethanes Obtained by Using Reactive Phosphorus-Containing Polyol: Synthesis, Thermal Analysis and Combustion Behaviors. Materials 2021, 14, 2699. https://doi.org/10.3390/ma14112699
Zagożdżon I, Parcheta P, Datta J. Novel Cast Polyurethanes Obtained by Using Reactive Phosphorus-Containing Polyol: Synthesis, Thermal Analysis and Combustion Behaviors. Materials. 2021; 14(11):2699. https://doi.org/10.3390/ma14112699
Chicago/Turabian StyleZagożdżon, Izabela, Paulina Parcheta, and Janusz Datta. 2021. "Novel Cast Polyurethanes Obtained by Using Reactive Phosphorus-Containing Polyol: Synthesis, Thermal Analysis and Combustion Behaviors" Materials 14, no. 11: 2699. https://doi.org/10.3390/ma14112699







