Performance Improvement of PVDF–HFP-Based Gel Polymer Electrolyte with the Dopant of Octavinyl-Polyhedral Oligomeric Silsesquioxane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVDF–HFP Polymer Membrane with OVAPOSS
2.3. Preparation of Positive Plate and Assembly of Button Cell
2.4. Characterization of Polymer Membrane
2.5. Electrochemical Performance Test
3. Results and Discussions
3.1. Physical Characterization of Polymer Membrane
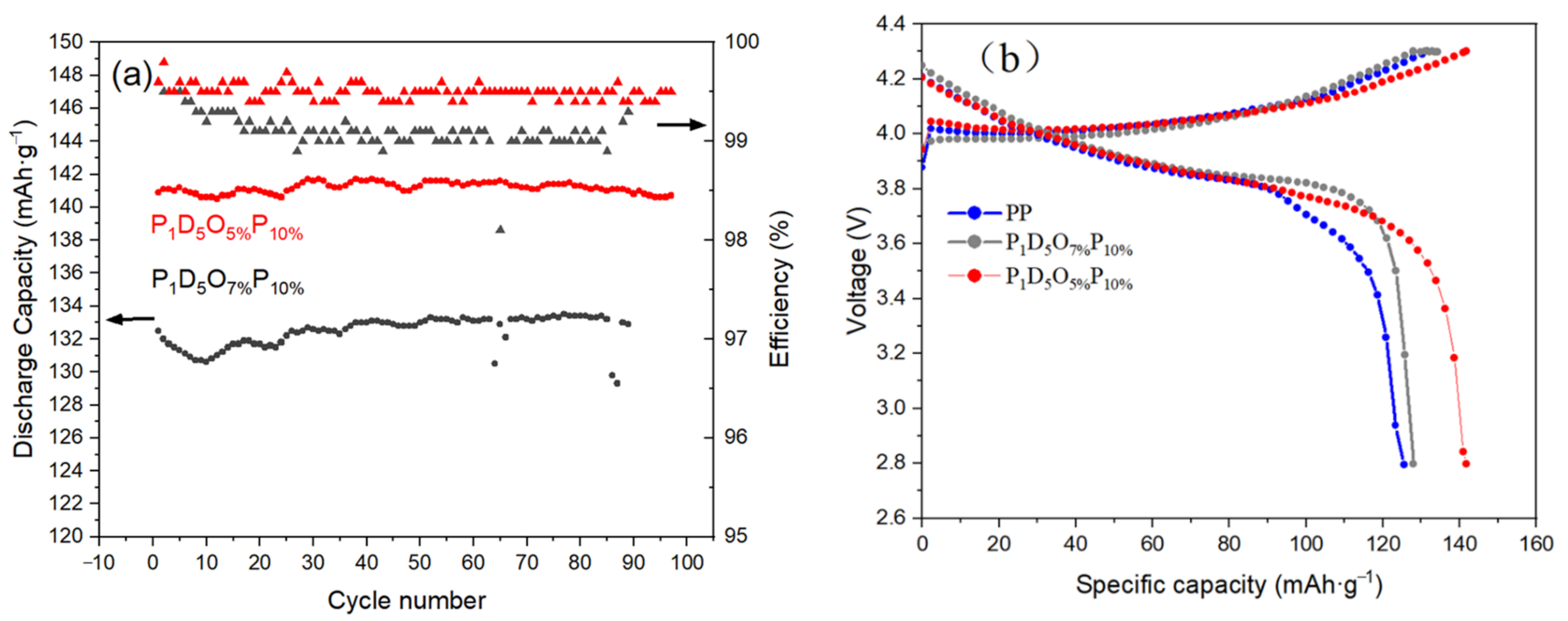
3.2. Electrochemical Characterization of Polymer Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Mauger, A.; Julien, C.M.; Goodenough, J.B.; Zaghib, K. Tribute to Michel Armand: From Rocking Chair—Li-ion to Solid-State Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070507. [Google Scholar] [CrossRef]
- Yu, D.; Li, X.; Xu, J. Safety regulation of gel electrolytes in electrochemical energy storage devices. Sci. China-Mater. 2019, 62, 1556–1573. [Google Scholar] [CrossRef]
- Waqas, M.; Ali, S.; Feng, C.; Chen, D.; Han, J.; He, W. Recent Development in Separators for High-Temperature Lithium-Ion Batteries. Small 2019, 15, e1901689. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Yang, M.; Hou, J. Membranes in Lithium Ion Batteries. Membranes 2012, 2, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Manuel Stephan, A. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Michot, T.; Nishimoto, A.; Watanabe, M. Electrochemical properties of polymer gel electrolytes based on poly (vinylidene fluoride) copolymer and homopolymer. Electrochim. Acta 2000, 45, 1347–1360. [Google Scholar] [CrossRef]
- Song, M.-K.; Kim, Y.-T.; Cho, J.-Y.; Cho, B.W.; Popov, B.N.; Rhee, H.W. Composite polymer electrolytes reinforced by non-woven fabrics. J. Power Sources 2004, 125, 10–16. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. SC-CO2-assisted process for a high energy density aerogel supercapacitor: The effect of GO loading. Nanotechnology 2017, 28, 204001. [Google Scholar] [CrossRef]
- Luo, K.; Shao, D.; Yang, L.; Liu, L.; Chen, X.; Zou, C.; Wang, D.; Luo, Z.; Wang, X. Semi-interpenetrating gel polymer electrolyte based on PVDF-HFP for lithium-ion batteries. J. Appl. Polym. Sci. 2021, 138, 49993. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Yoo, D.J.; Nahm, K.S.; Gerbaldi, C.; Stephan, A.M. MgAl2SiO6-incorporated poly (ethylene oxide)-based electrolytes for all-solid-state lithium batteries. Ionics 2014, 20, 151–156. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, X.; Cao, C.; Li, J.; Zhu, Y. Poly (vinylidene fluoride)/SiO2 composite membranes prepared by electrospinning and their excellent properties for nonwoven separators for lithium-ion batteries. J. Power Sources 2014, 251, 423–431. [Google Scholar] [CrossRef]
- Xie, H.; Liao, Y.; Sun, P.; Chen, T.; Rao, M.; Li, W. Investigation on polyethylene-supported and nano-SiO2 doped poly (methyl methacrylate-co-butyl acrylate) based gel polymer electrolyte for high voltage lithium-ion battery. Electrochim. Acta 2014, 127, 327–333. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; He, J.; Wu, D.; Meng, J.; Ni, P. Effects of fluorinated SiO2 nanoparticles on the thermal and electrochemical properties of PP nonwoven/PVDF–HFP composite separator for Li-ion batteries. J. Membr. Sci. 2014, 455, 368–374. [Google Scholar] [CrossRef]
- Saikia, D.; Chen-Yang, Y.W.; Chen, Y.T.; Li, Y.K.; Lin, S.I. Investigation of ionic conductivity of composite gel polymer electrolyte membranes based on P(VDF-HFP), LiClO4 and silica aerogel for lithium-ion battery. Desalination 2008, 234, 24–32. [Google Scholar] [CrossRef]
- Yan, P.; Huang, Z.; Lin, Y.; Wu, X.; Yang, Y.; Wang, D.; Chen, F.; Zhang, C.; He, D. Composite-porous polymer membrane with reduced crystalline for lithium–ion battery via non-solvent evaporate method. Ionics 2014, 21, 593–599. [Google Scholar] [CrossRef]
- Wei, N.; Hu, J.; Zhang, M.; He, J.; Ni, P. Cross-linked porous polymer separator using vinyl-modified aluminum oxide nanoparticles as cross-linker for lithium-ion batteries. Electrochim. Acta 2019, 307, 495–502. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, M.; He, J.; Ni, P. A porous cross-linked gel polymer electrolyte separator for lithium-ion batteries prepared by using zinc oxide nanoparticle as a foaming agent and filler. Electrochim. Acta 2018, 292, 769–778. [Google Scholar] [CrossRef]
- Shang, D.; Fu, J.; Lu, Q.; Chen, L.; Yin, J.; Dong, X.; Xu, Y.; Jia, R.; Yuan, S.; Chen, Y.; et al. A novel polyhedral oligomeric silsesquioxane based ionic liquids (POSS-ILs) polymer electrolytes for lithium-ion batteries. Solid State Ion. 2018, 319, 247–255. [Google Scholar] [CrossRef]
- Jung, G.-Y.; Choi, J.H.; Lee, J.K. Thermal Behavior and Ion Conductivity of Polyethylene Oxide/Polyhedral Oligomeric Silsesquioxane Nanocomposite Electrolytes. Adv. Polym. Technol. 2015, 34. [Google Scholar] [CrossRef]
- Yin, K.; Zhang, Z.; Li, X.; Yang, L.; Tachibana, K.; Hirano, S.I. Polymer electrolytes based on dicationic polymeric ionic liquids: Application in lithium metal batteries. J. Mater. Chem. A 2015, 3, 170–178. [Google Scholar] [CrossRef]
- Cong, C.; Cui, C.; Meng, X.; Zhou, Q. Stability of POSS crosslinks and aggregates in tetrafluoroethylene-propylene elastomers/OVPOSS composites exposed to hydrochloric acid solution. Polym. Degrad. Stab. 2014, 100, 29–36. [Google Scholar] [CrossRef]
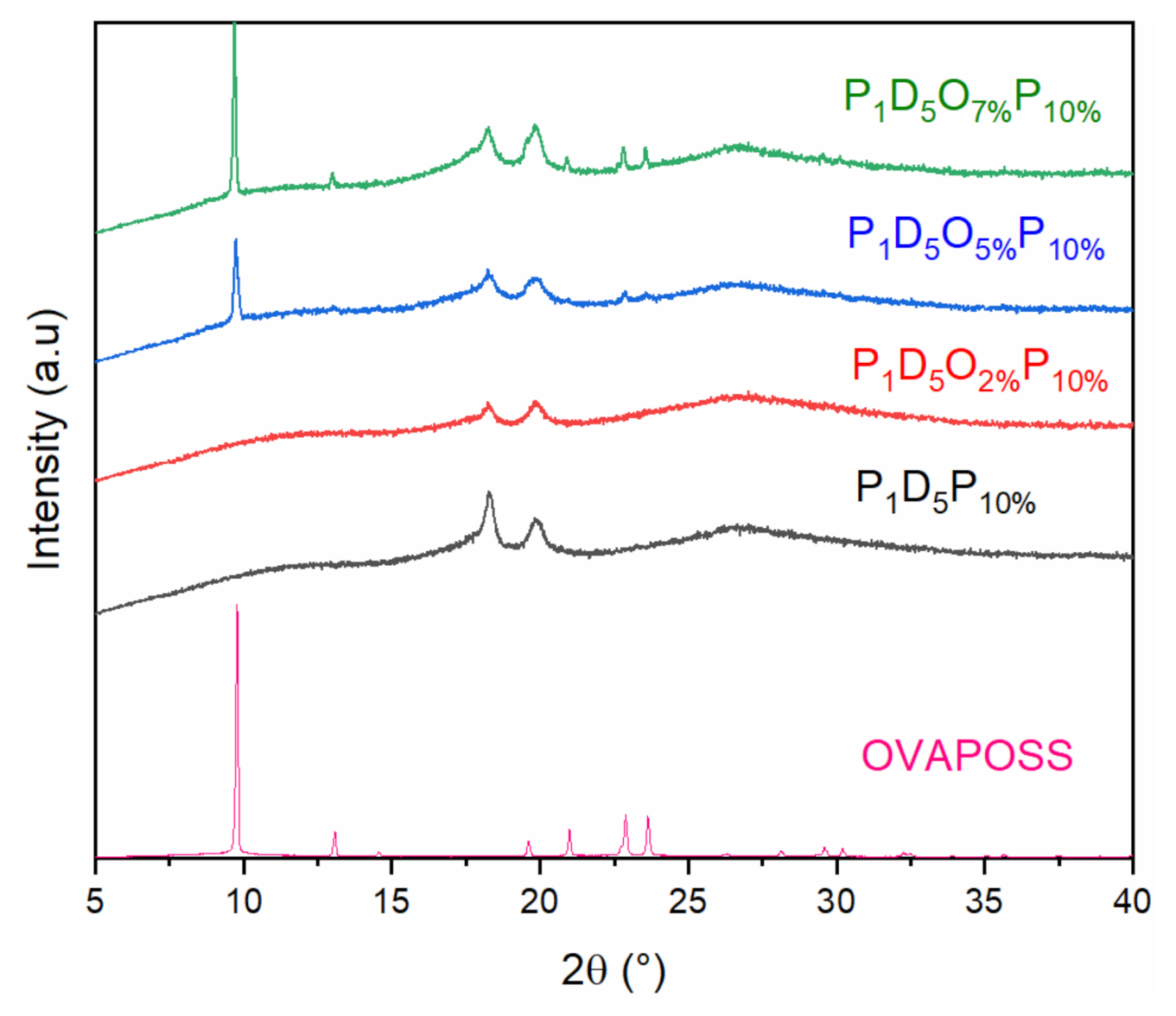
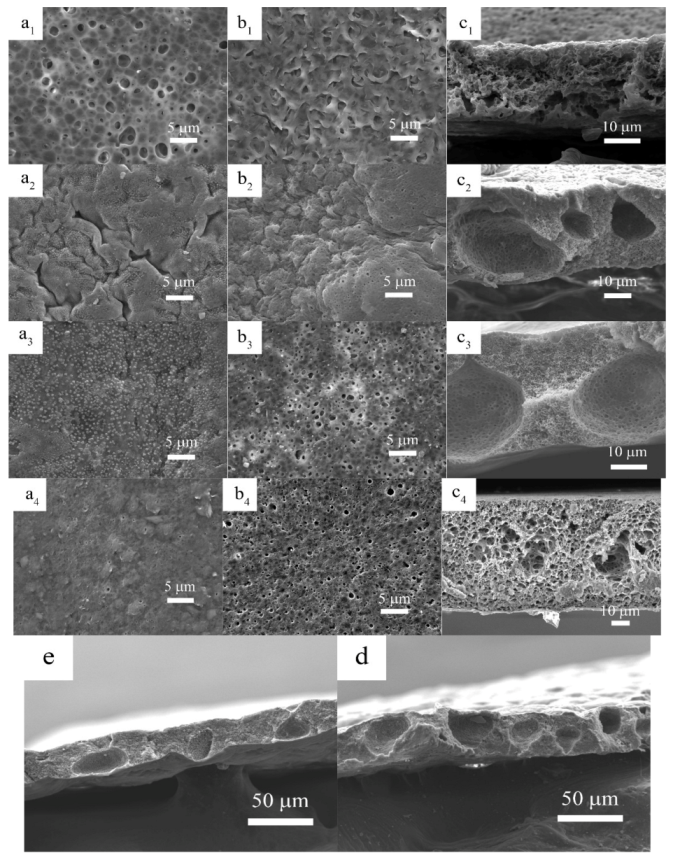

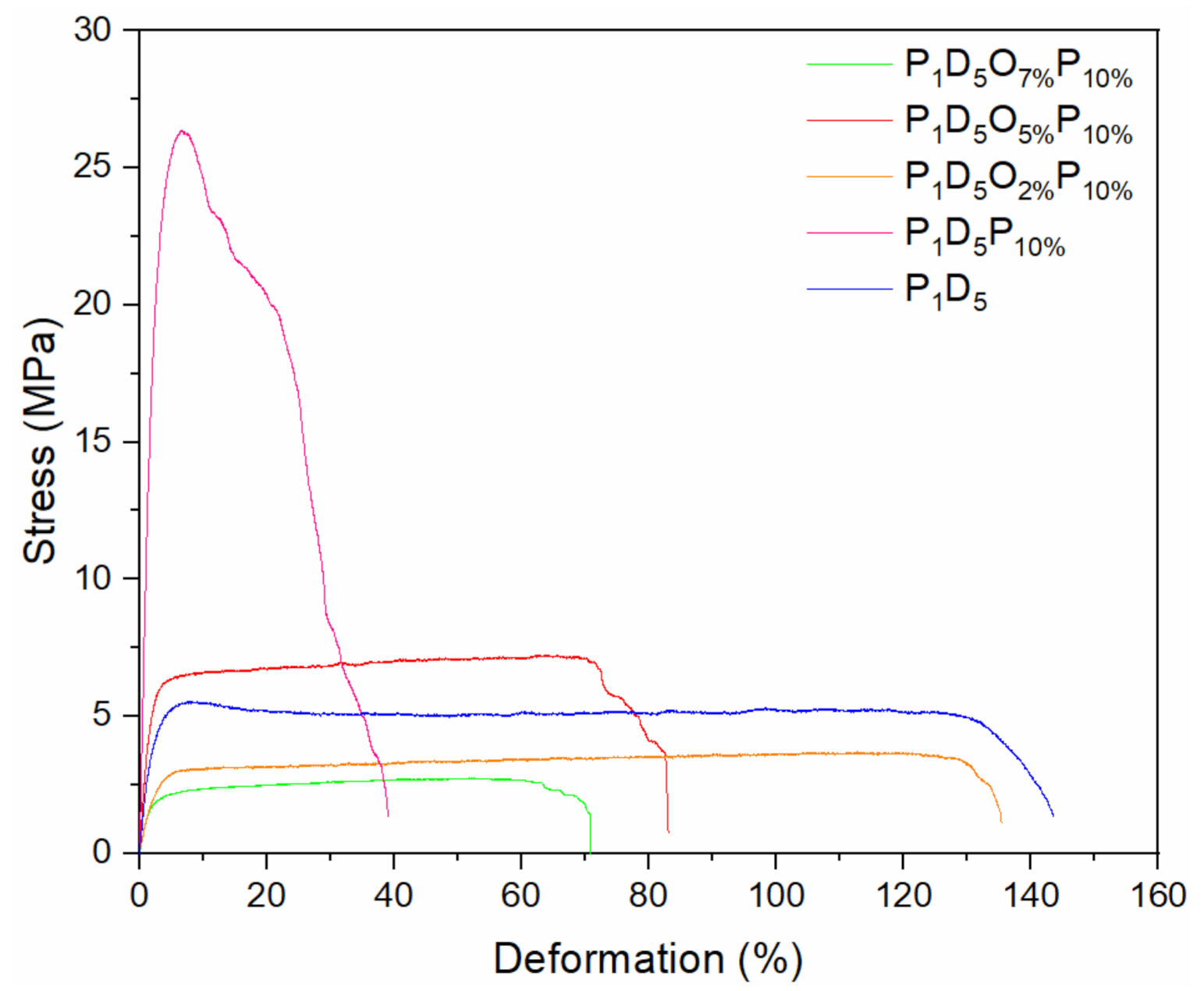
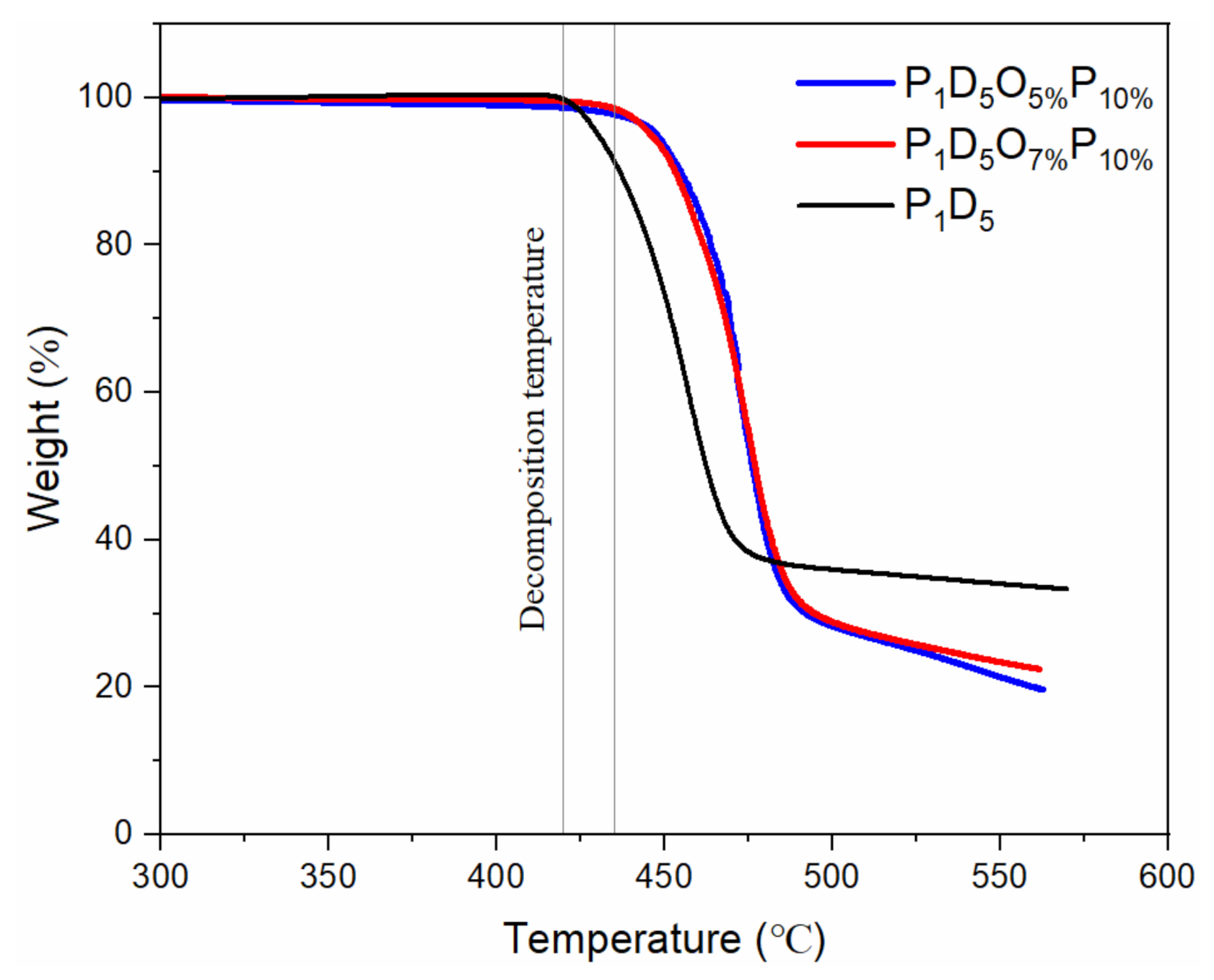
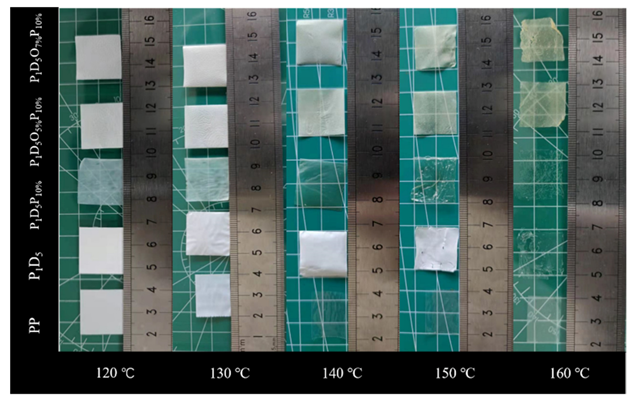

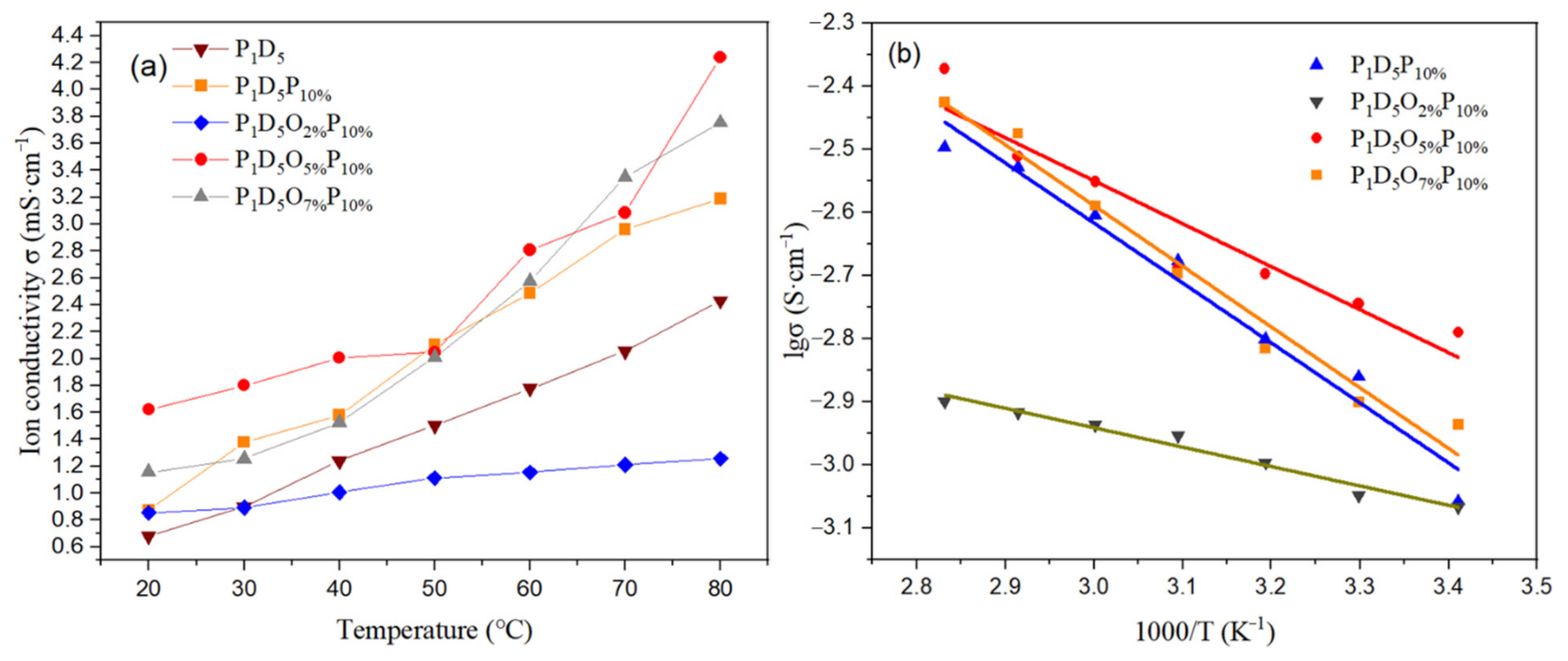
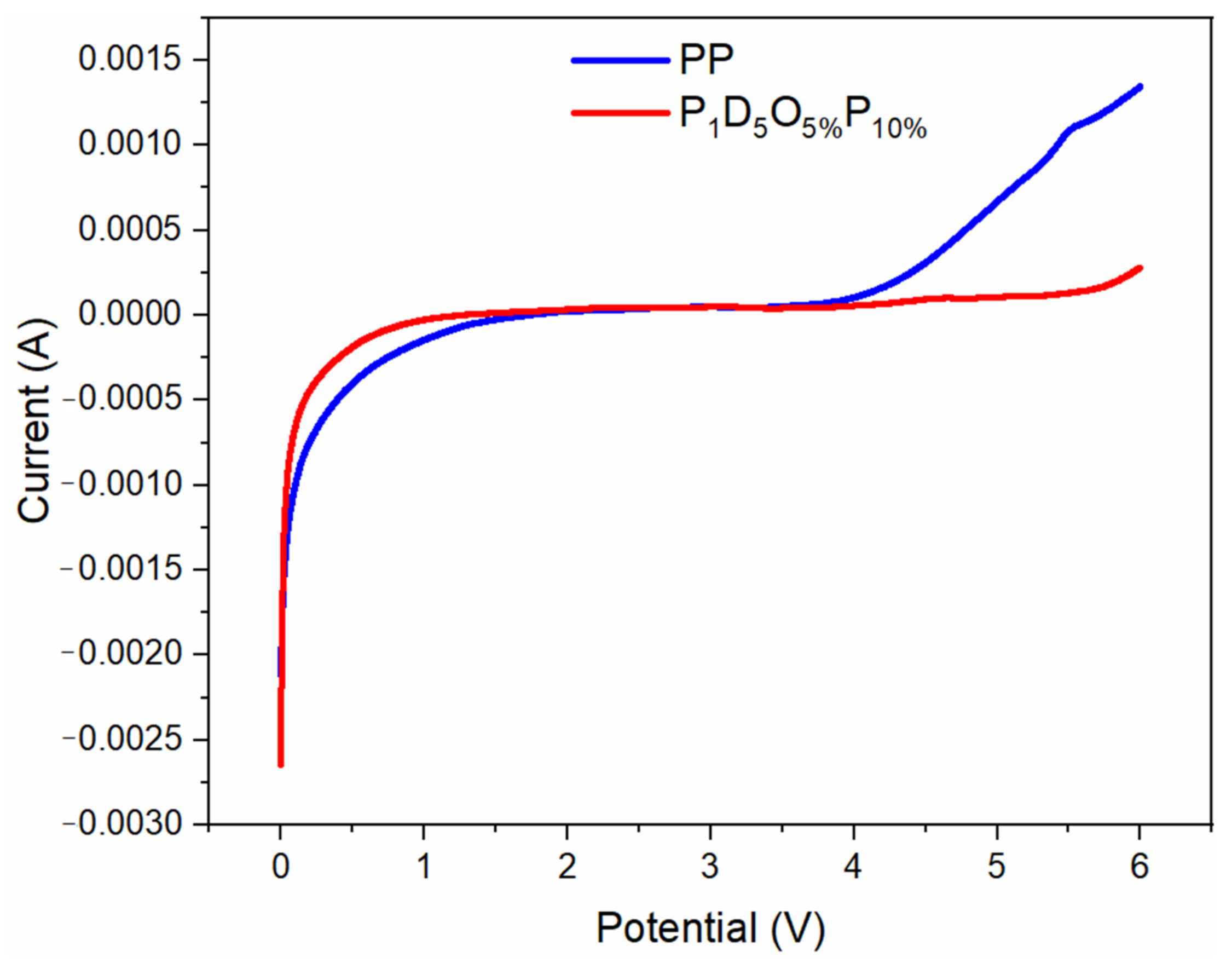
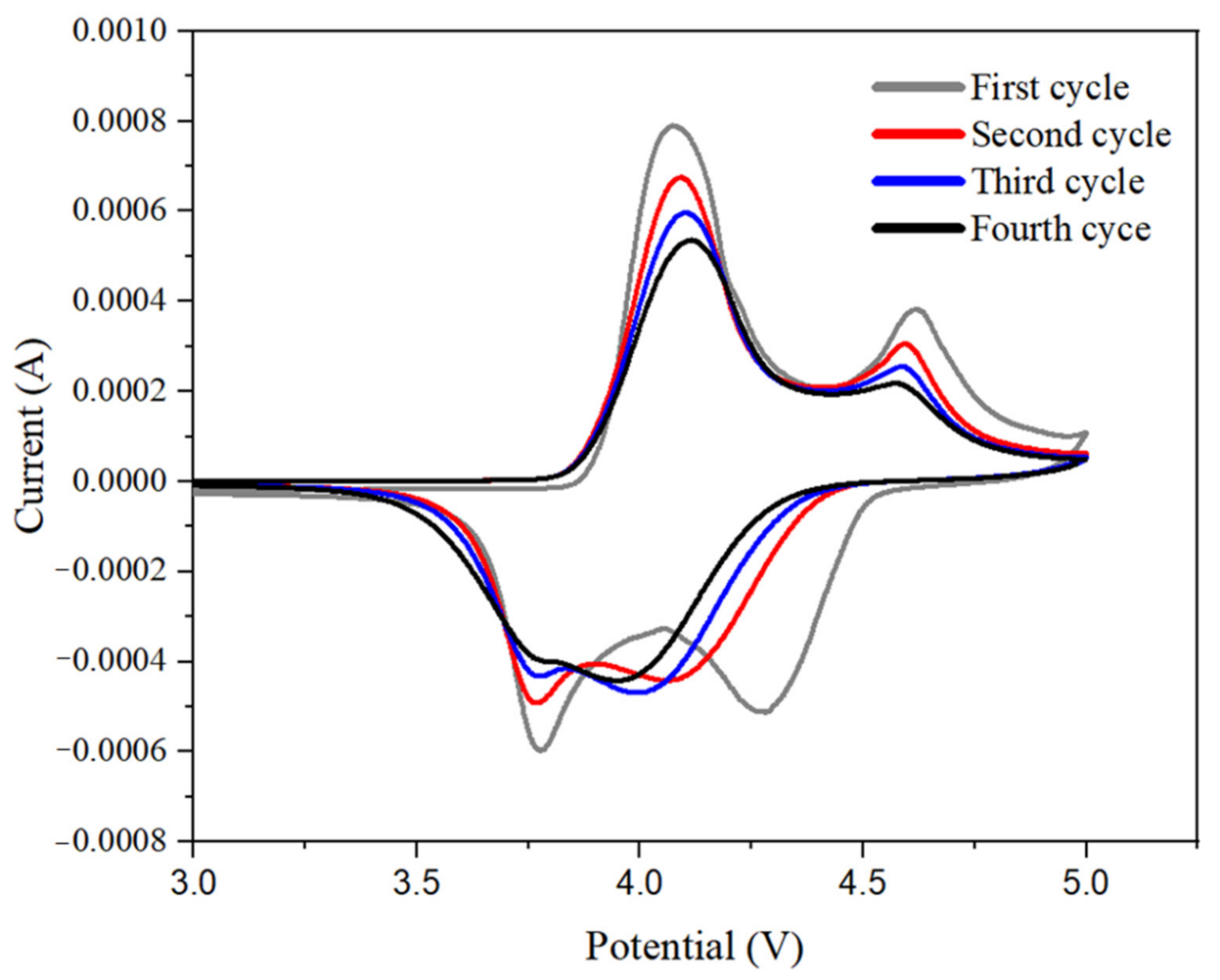
| OVAPOSS Doping Amount | Thickness (μm) | Impedance (Ω) | Room Temperature Conductivity (×10−3 S·cm−1) |
|---|---|---|---|
| P1D5O2%P10% | 14 | 0.835 | 0.855 |
| P1D5O5%P10% | 29 | 0.913 | 1.62 |
| P1D5O7%P10% | 34 | 1.5 | 1.16 |
| P1D5P10% | 189 | 11.03 | 0.874 |
| P1D5 | 41 | 3.064 | 0.683 |
| Temperature (°C) | 20 | 30 | 40 | 50 | 60 | 70 | 80 | |
|---|---|---|---|---|---|---|---|---|
| Ion conductivity (×10−3 S·cm−1) | P1D5P10% | 0.874 | 1.378 | 1.580 | 2.105 | 2.487 | 2.962 | 3.189 |
| P1D5O2%P10% | 0.855 | 0.893 | 1.007 | 1.113 | 1.156 | 1.211 | 1.258 | |
| P1D5O5%P10% | 1.621 | 1.800 | 2.01 | 2.046 | 2.808 | 3.082 | 4.239 | |
| P1D5O7%P10% | 1.156 | 1.255 | 1.527 | 2.01 | 2.574 | 3.349 | 3.755 | |
| Group | P1D5 | P1D5P10% | P1D5O2%P10% | P1D5O5%P10% | P1D5O7%P10% |
|---|---|---|---|---|---|
| Ea/kJ·mol−1 | 7.82 | 7.91 | 2.56 | 5.67 | 8.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Li, S.; Chen, F.; Chu, Y.; Wang, X.; Wan, W.; Zhao, L.; Zhu, Y. Performance Improvement of PVDF–HFP-Based Gel Polymer Electrolyte with the Dopant of Octavinyl-Polyhedral Oligomeric Silsesquioxane. Materials 2021, 14, 2701. https://doi.org/10.3390/ma14112701
Guo X, Li S, Chen F, Chu Y, Wang X, Wan W, Zhao L, Zhu Y. Performance Improvement of PVDF–HFP-Based Gel Polymer Electrolyte with the Dopant of Octavinyl-Polyhedral Oligomeric Silsesquioxane. Materials. 2021; 14(11):2701. https://doi.org/10.3390/ma14112701
Chicago/Turabian StyleGuo, Xin, Shunchang Li, Fuhua Chen, Ying Chu, Xueying Wang, Weihua Wan, Lili Zhao, and Yongping Zhu. 2021. "Performance Improvement of PVDF–HFP-Based Gel Polymer Electrolyte with the Dopant of Octavinyl-Polyhedral Oligomeric Silsesquioxane" Materials 14, no. 11: 2701. https://doi.org/10.3390/ma14112701
APA StyleGuo, X., Li, S., Chen, F., Chu, Y., Wang, X., Wan, W., Zhao, L., & Zhu, Y. (2021). Performance Improvement of PVDF–HFP-Based Gel Polymer Electrolyte with the Dopant of Octavinyl-Polyhedral Oligomeric Silsesquioxane. Materials, 14(11), 2701. https://doi.org/10.3390/ma14112701






