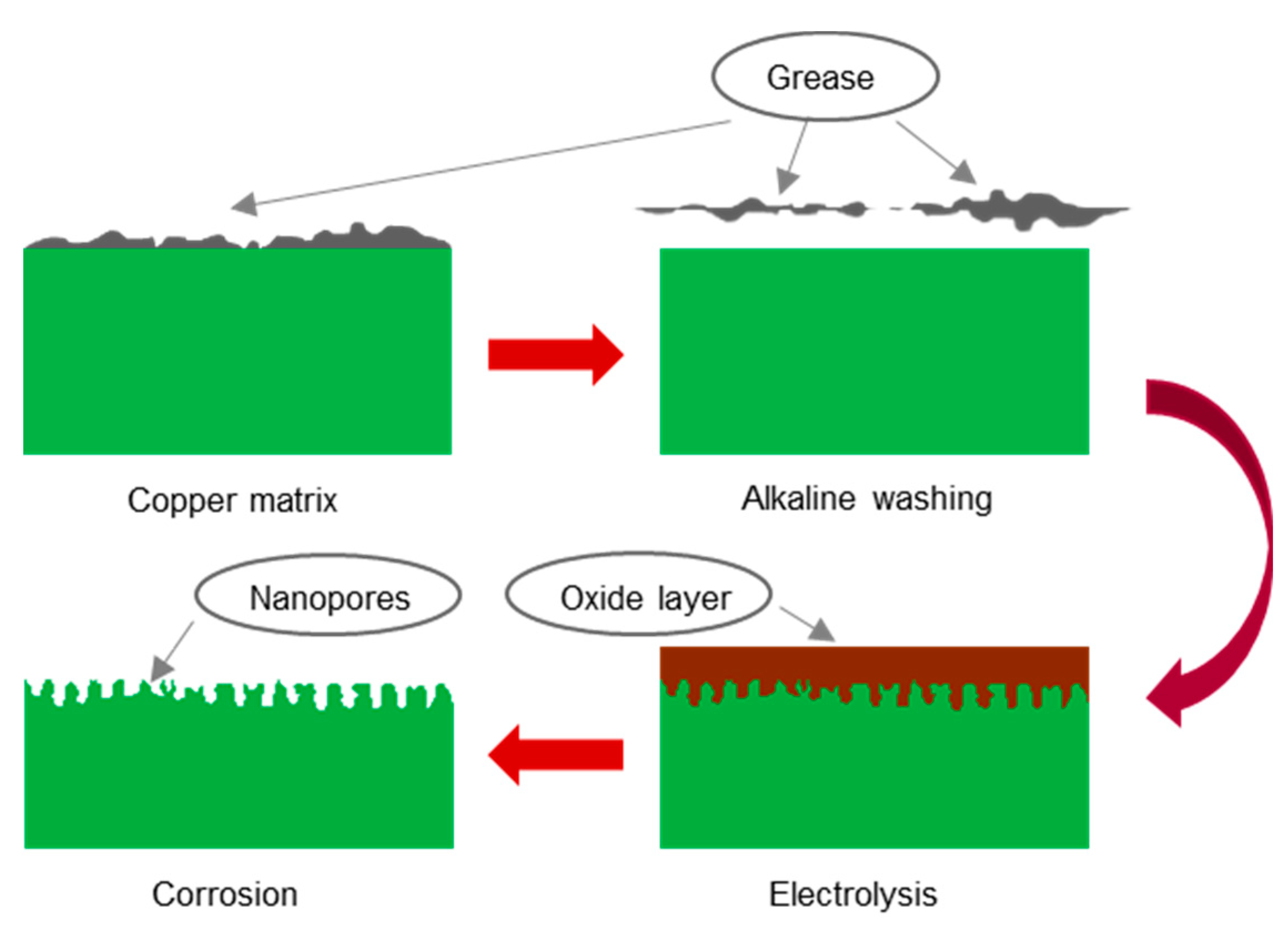
2.2. Experimental Plan for Surface Treatment
The copper surface treatment process was similar to that of aluminum and stainless steel, except that an electrolysis process was applied prior to chemical corrosion.
Figure 1 displays the entire experimental process.
(a) Pretreatment: Before anodic oxidation, the copper surface needed to be cleaned to remove oil stains, rust stains, and dust. First, the copper was rinsed with tap water to remove dust. Second, the copper was rinsed with alcohol and acetone for 5 min to remove oil stains. Third, the copper was rinsed with a 1% sodium hydroxide solution for 3 min for further oil stain removal. Finally, the copper sheet was washed with deionized water and dried.
(b) Preparation of the electrolyte and etching solution: After consulting the relevant literature [
19,
20], Two different electrolyte solutions were prepared: one was a mixture of sodium hydroxide and sodium molybdate, and the other was a mixture of sodium carbonate and sodium molybdate. Three etching solutions were prepared: one was a mixture of hydrochloric acid and sodium chloride, another was a mixture of phosphoric acid and sodium dihydrogen phosphate, and another was a mixture of nitric acid and sodium nitrate.
(c) Anodic oxidation: The copper matrix was used as the anode, and a graphite carbon rod was used as the cathode. Both the anode and the cathode were partially immersed in the electrolyte, and the distance between them was approximately 10 cm. Electrolysis was performed at 15 V for 5 min.
(d) Chemical corrosion: First, the anodized copper sample was immersed in the etching solution. The solution was then stirred gently. Finally, the corroded copper sample was removed, washed with deionized water, and dried.
2.3. Characterization of the Surface Treatment
A JEOL JSM-7800F Prime super resolution field-emission scanning electron microscope (SEM) (JEOL, Tokyo, Japan) and a Thermo Scientific NORAN™ System 7 energy-dispersive X-ray spectrometer (EDS) (Thermo Scientific, Waltham, MA, USA) were used to evaluate the surface morphology of copper after surface treatment at a high acceleration voltage of 5 kV. EDS was performed at an acceleration voltage of 15 kV to determine the composition and content of the copper surface microstructure.
As shown in
Figure 2, Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, Maryland, USA) was used to calculate the processed copper surface porosity, and the porosity was used to judge the quality of the copper surface morphology.
2.4. Phenomena of Surface Treatment in Copper
During the anodic oxidation, the energized copper sheet began to react violently, the area around the anode copper sheet gradually turned light blue, and a large amount of gas was generated near the cathode carbon rod. This occurred because the anode copper sheet lost electrons and was oxidized into copper ions, whereas the cathode water molecules gained electrons and decomposed into
H2 and
OH−. Gradually, the anode copper sheet changed color from purplish red with a metallic luster to gray because as the reaction progressed, the
Cu2+ concentration in the electrolyte continued to increase and
Cu2+ combined with
OH− to precipitate
Cu(OH)2. As the electrolysis reaction was exothermic, when the electrolyte temperature reached 60–80 °C, the
Cu(OH)2 precipitate decomposed into black
CuO and water. The current density remained basically stable throughout the process; specifically, it was proportional to the electrolysis voltage and was independent of other parameters such as electrolyte concentration and type.
Figure 3a,b show the color change of the copper surface.
During chemical corrosion, the anodized copper sheet was immersed in the etching solution. The reaction between the copper sheet and the etching solution was relatively gentle. The color of the copper surface gradually changed from grayish black to the original purplish red, and the etching solution near the copper surface turned light blue. If the solution is uniformly stirred, then no significant changes will occur because a small amount of
CuO attached to the copper surface should react with the etching solution to generate a small amount of
Cu2+.
Figure 3c shows the color of the copper surface after chemical etching. The following chemical reactions occurred during the aforementioned processes.
Figure 4 shows the EDS analysis of the treated copper surface, and
Table 1 shows the elemental composition of the copper surface. These results indicate that compared with the pure copper before surface treatment, the metal content basically remained unchanged, whereas the oxygen and carbon contents increased slightly. The increase in oxygen content was due to a small amount of copper oxide residue on the copper surface, and the increase in carbon content was due to copper oxide reacting with carbon dioxide in the air to form a basic copper carbonate. The EDS analysis results can also explain the experimental phenomenon of the copper surface turning grayish black during electrolysis. The aforementioned analysis demonstrated that after anodic oxidation and chemical corrosion, no residue remained on the copper surface.
2.5. Selection of the Electrolyte and Etching Solution for Surface Treatment
To determine the formulation of electrolytes and etching solutions, this study de-signed six experimental schemes to investigate two types of electrolytes and three types of etching solutions.
Table 2 shows the electrolyte and etching solution ratios in each experimental scheme.
Surface treatment experiments were performed according to the six experimental schemes listed in
Table 2, and the corresponding microstructure morphology of the copper surface was ascertained. These six schemes were intuitively compared using SEM images, as shown in
Figure 5.
Dividing
Figure 5 into two groups—(a,b,c) and (d,e,f)—reveals that when the same electrolyte was used, the corrosion effect of the etching solution containing phosphoric acid was better than that of the etching solution containing hydrochloric acid and nitric acid. Dividing
Figure 5 into three groups—(a,d), (b,e), and (c,f)—reveals that when the same etching solution was used, the oxidation effect of the electrolyte containing sodium carbonate was better than that of the electrolyte containing sodium hydroxide. At the same time, the corrosion effect in
Figure 5e is better than others, where the electrolyte containing sodium carbonate and the etching solution containing phosphoric acid were used. Therefore, in this study, the electrolyte containing sodium carbonate and the etching solution containing phosphoric acid were selected for evaluating the microstructure formation process on the copper surface.
2.6. Relationship between Surface Treatment Process and Morphology of Copper Surface
Based on comparisons of the above six experimental schemes, the electrolyte containing sodium carbonate and the etching solution containing phosphoric acid were finally selected for use. However, the specific concentration ratio remains uncertain; this issue requires further experimental study. In this regard, porosity was selected as the objective function, and the phosphoric acid concentration, sodium dihydrogen phosphate concentration, corrosion time, electrolysis time, sodium carbonate concentration, and electrolysis voltage were selected as six influencing factors. The L
25(5
6) orthogonal table was designed to arrange the experiment. There were 25 groups of experiments. In each group of experiments, one sample was selected, and four photos (with 1000×, 2000×, 5000× and 10,000× magnifications) were selected for observation, and the porosity of the copper surface microstructure obtained through the 25 groups of experiments was determined.
Table 3 lists details of the orthogonal experimental scheme, experimental results, and range analysis.
Where
K1–
K5 are the average values of porosity of the microstructure on the copper surface under different levels. The
K value was calculated as follows.
where
i is the level,
j is the factor,
n is the level number of each factor (in this experiment,
n = 5),
m is the number of experimental groups under the same factor and same level, and
p is the porosity. Taking
K12 as an example, the
K value is the mean of five porosity values corresponding to the second factor (concentration of sodium dihydrogen phosphate) and the first level (1%). The range
R is the difference between the maximum value and the minimum value of
K1–
K5 for each factor.
Figure 6 shows relationships between the factors and porosity. It shows that the factors most influenced the microstructure, in descending order, were phosphoric acid concentration, sodium dihydrogen phosphate concentration, electrolysis voltage, electrolysis time, chemical corrosion time, and sodium carbonate concentration. The phosphoric acid concentration had the largest range and had a much larger influence than the other factors. Although the other factors had different ranges, the difference was small; therefore, this study focused on the influence of only the phosphoric acid concentration.
The most important step in the microstructure preparation process was to corrode the oxide film formed by anodic oxidation. The acidity of the etching solution plays a very important role during the corrosion process. If the acidity of the etching solution is too strong, in addition to reacting with the oxide film, it will also react with copper and destroy the microstructure. However, if the acidity of the etching solution is not strong enough, then it will not fully react with the oxide film and only some pits or large-scale microstructures will form on the copper surface. The phosphoric acid concentration directly determines the strength of its acidity and therefore has a particularly significant influence on the microstructure. By contrast, other factors have less influence on the chemical corrosion process and on the formation of the final microstructure.
A single factor and single target experiment was performed to study the influence of the phosphoric acid concentration in the etching solution on porosity. The phosphoric acid concentration was set to 16%, 18%, 20% and 22%, and other parameters were set according to the optimal combination (B
5, C
4, D
4, E
5, F
4); specifically, the sodium dihydrogen phosphate concentration was 5%, corrosion time was 28 min, electrolysis time was 18 min, sodium carbonate concentration was 25%, and electrolysis voltage was 18 V. After the surface treatment of copper, the micromorphology of the copper surface was evaluated. The result is displayed in
Figure 7.
Figure 7 shows that when the phosphoric acid concentration was 16%, only some uneven structures formed from corrosion on the copper surface; although the surface was much rougher after corrosion, micropores did not form. When the phosphoric acid concentration increased to 18%, many porous structures with an average diameter of 1800–2000 nm formed on the copper surface. At a phosphoric acid concentration of 20%, the micropores that formed on the copper surface were denser and more uniform and the average diameter was smaller, at 800–1500 nm. Finally, when the phosphoric acid concentration increased to 22%, the size of the micropores on the copper surface was less affected, and some gully structures formed. A phosphoric acid concentration of 20% was seen to produce microstructures with the best size and uniformity on the copper surface. Five samples with a phosphoric acid concentration of 20% were selected for analyzing the pore size statistics, and the average pore size was calculated to be 1200 nm, as shown in
Table 4.