Pristine and Poly(Dimethylsiloxane) Modified Multi-Walled Carbon Nanotubes as Supports for Lipase Immobilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of MWCNTs/PDMS Nanocomposites
2.3. Lipase Immobilization
2.4. Analysis of Nanocomposites before Lipase Immobilization
2.4.1. Textural Characterization
2.4.2. Spectral Analysis
2.5. Characterization of Free and Immobilized Lipase
2.6. Stability and Reusability of Free and Immobilized Lipase
3. Results
3.1. Analysis of Nanocomposites before Lipase Immobilization
3.1.1. Parameters of the Porous Structure
3.1.2. Raman Spectroscopy
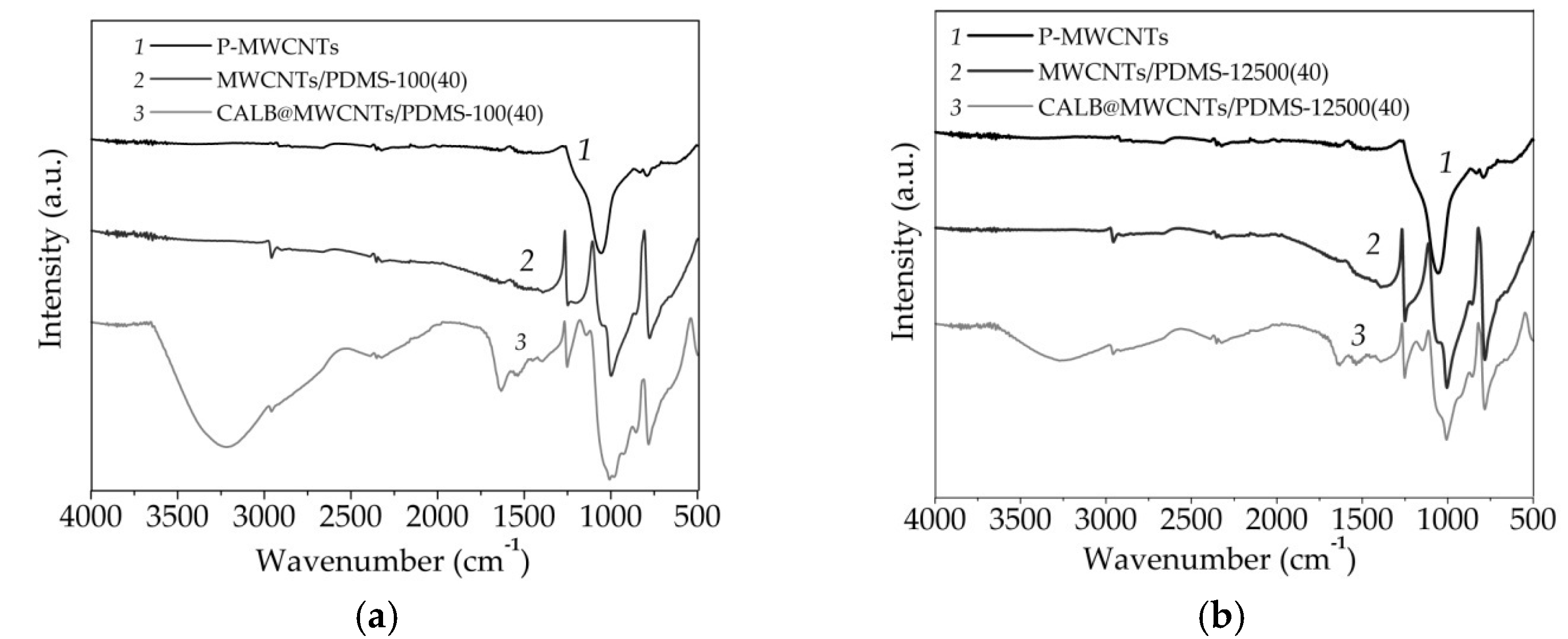
3.1.3. ATR-FTIR Spectroscopy
3.2. Characterization of Free and Immobilized Lipase
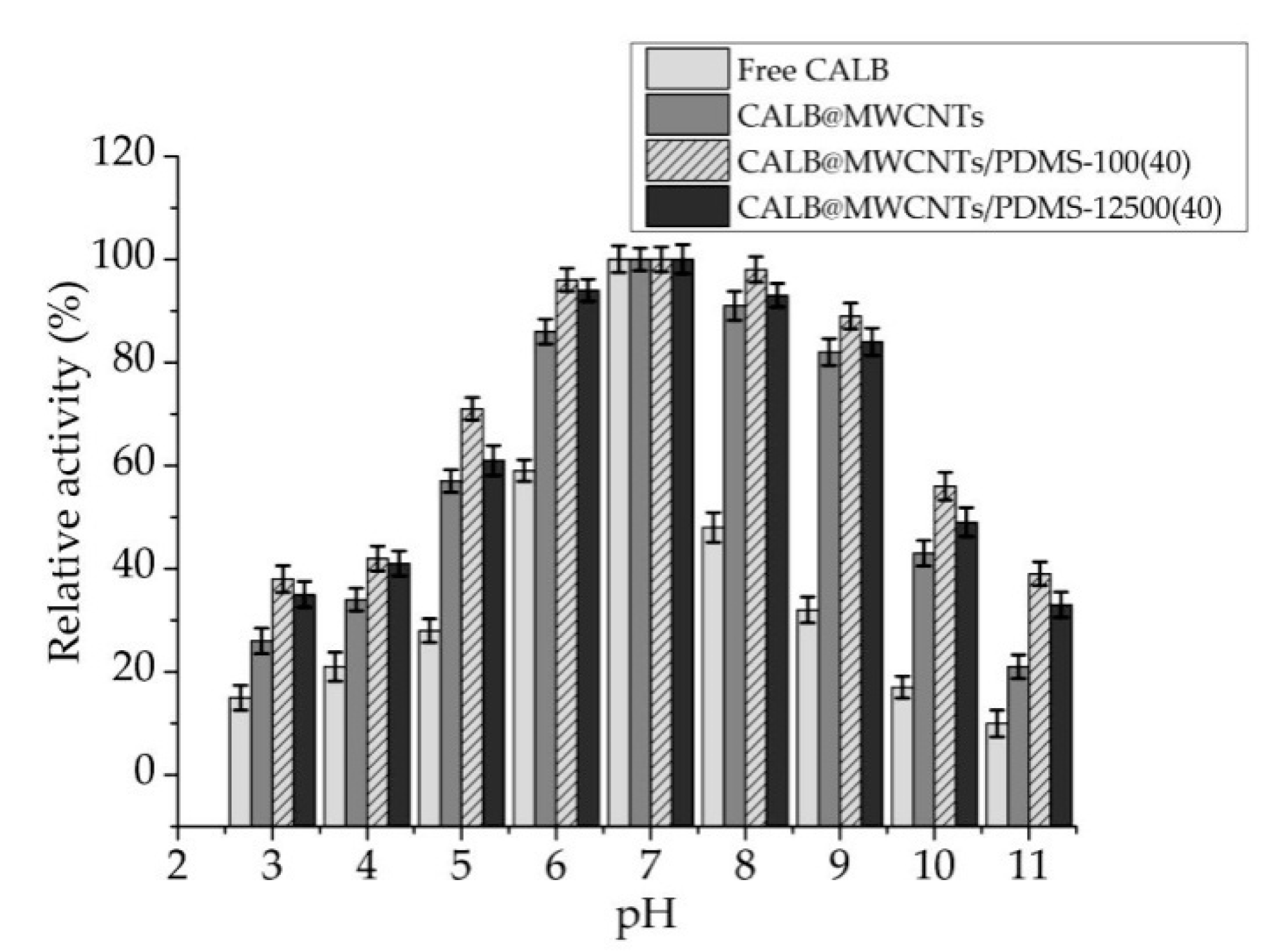
3.2.1. pH Profiles of Free and Immobilized Lipase
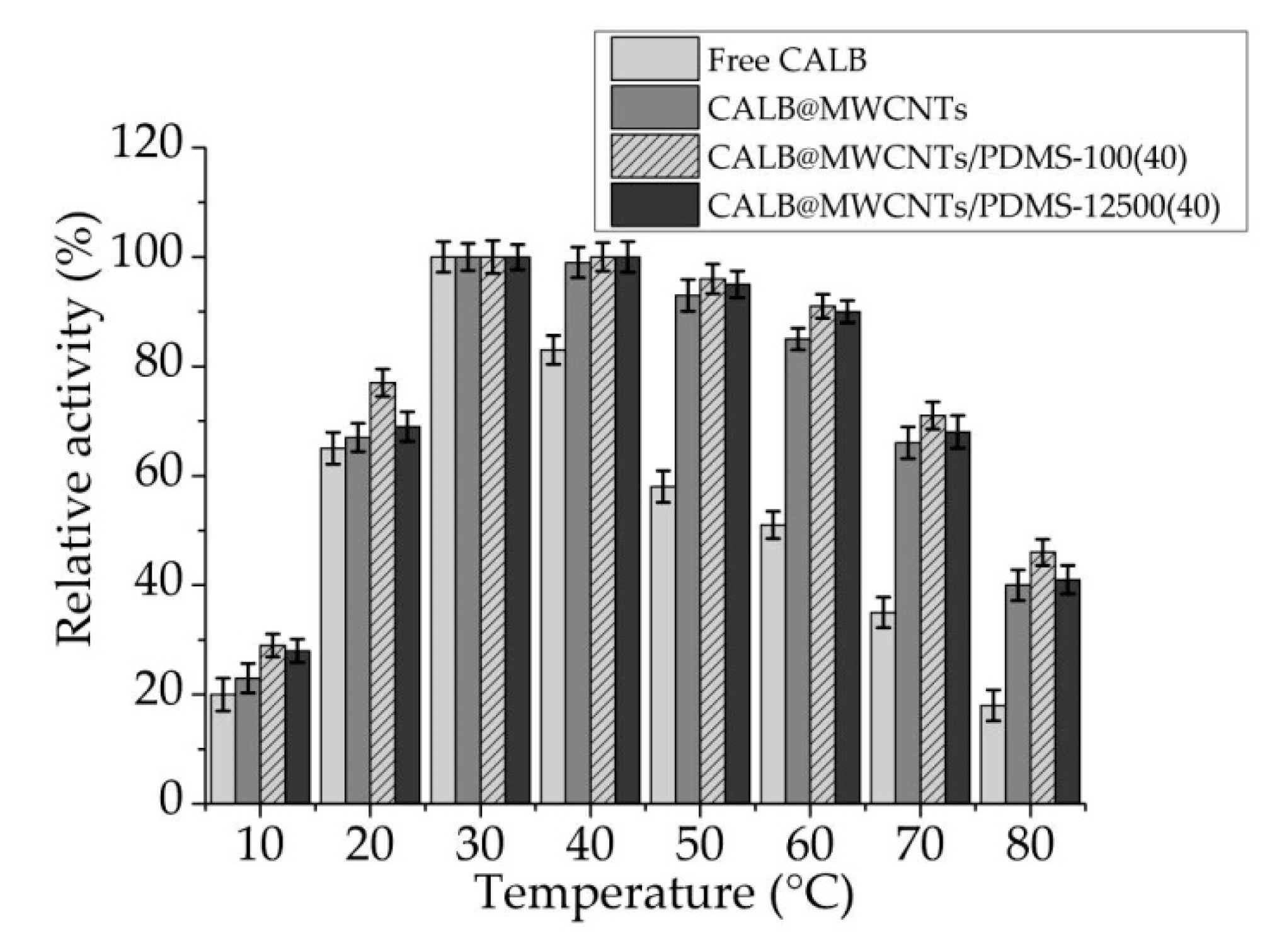
3.2.2. Temperature Profiles of Free and Immobilized Lipase
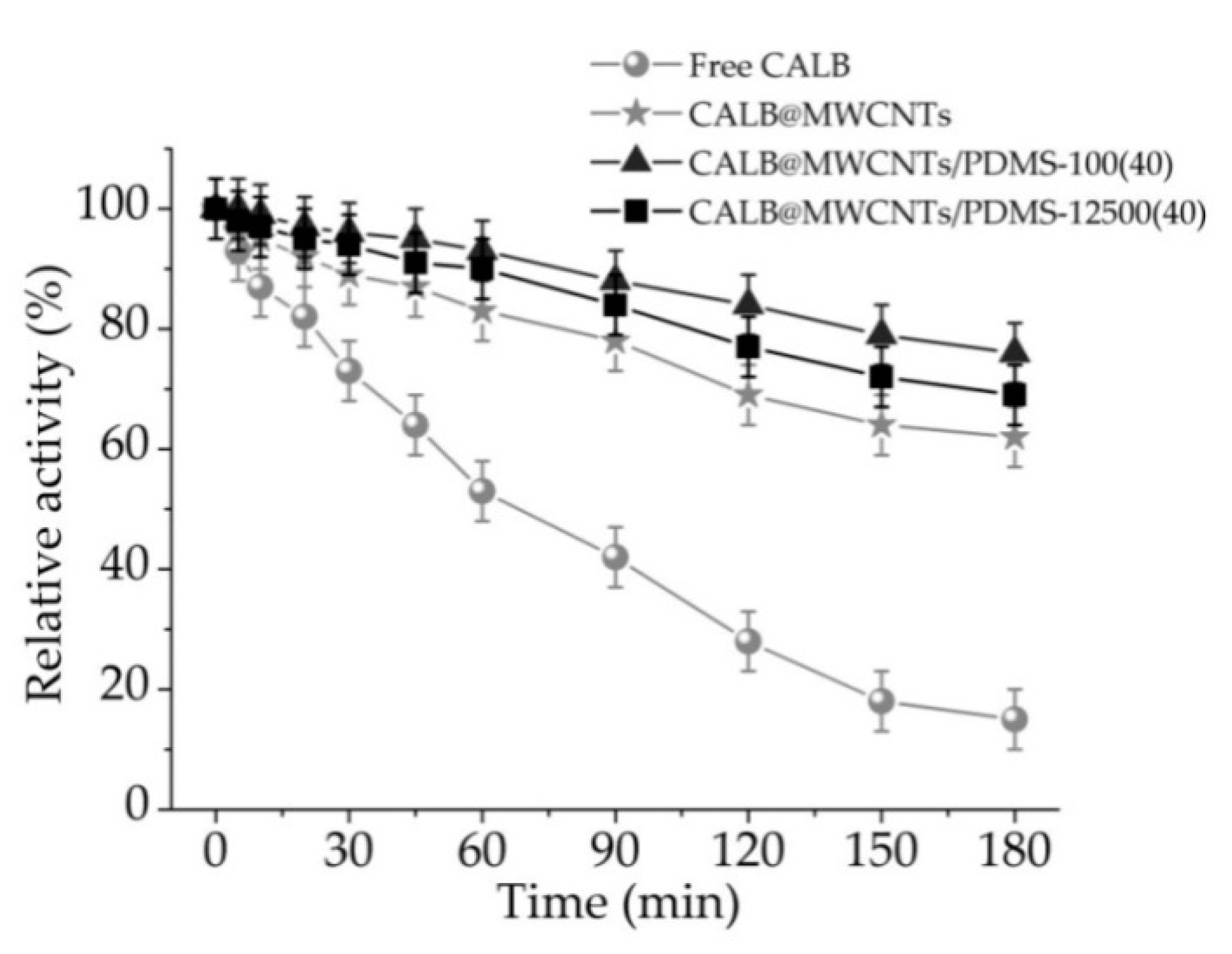
3.2.3. Thermal Stability of Free and Immobilized Lipase
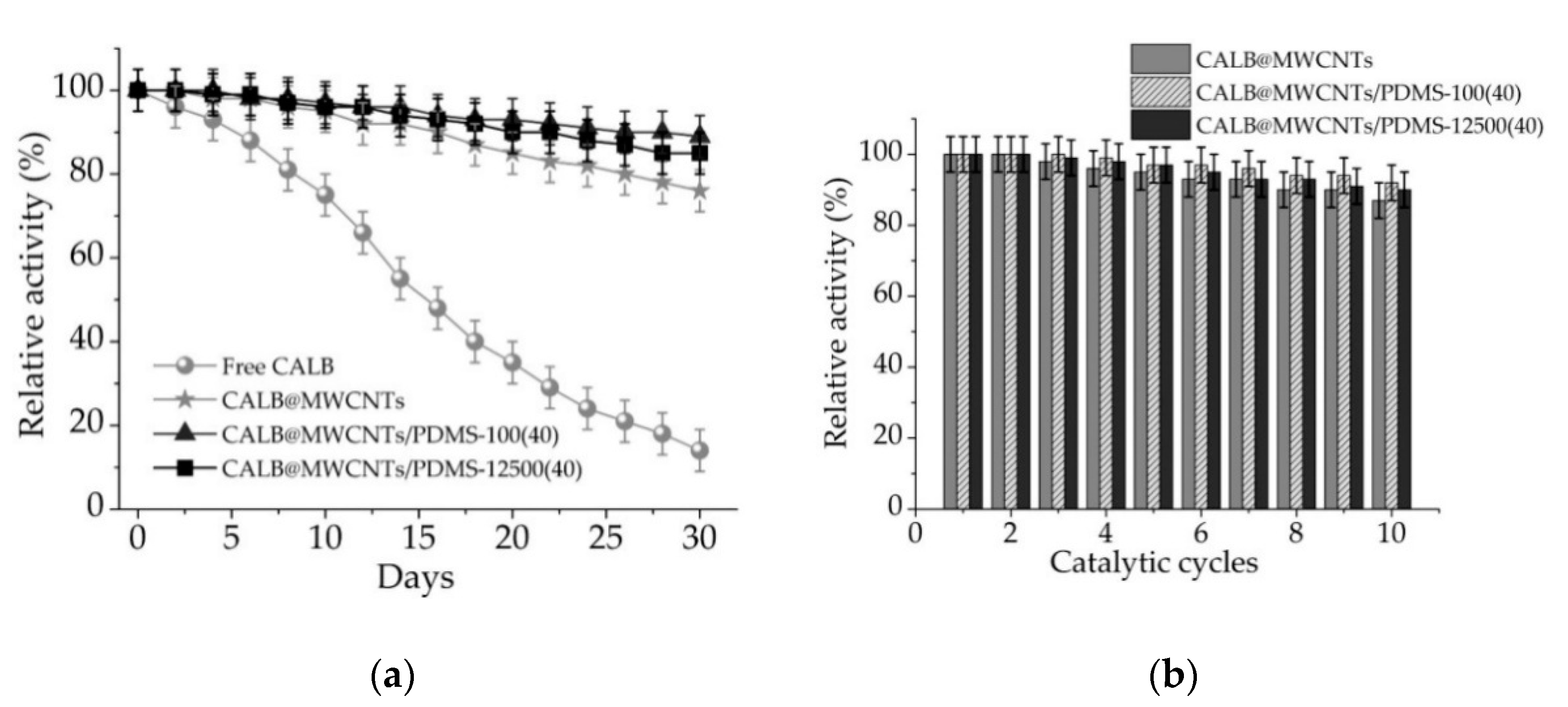
3.2.4. Storage Stability and Reusability of Free and Immobilized Lipase
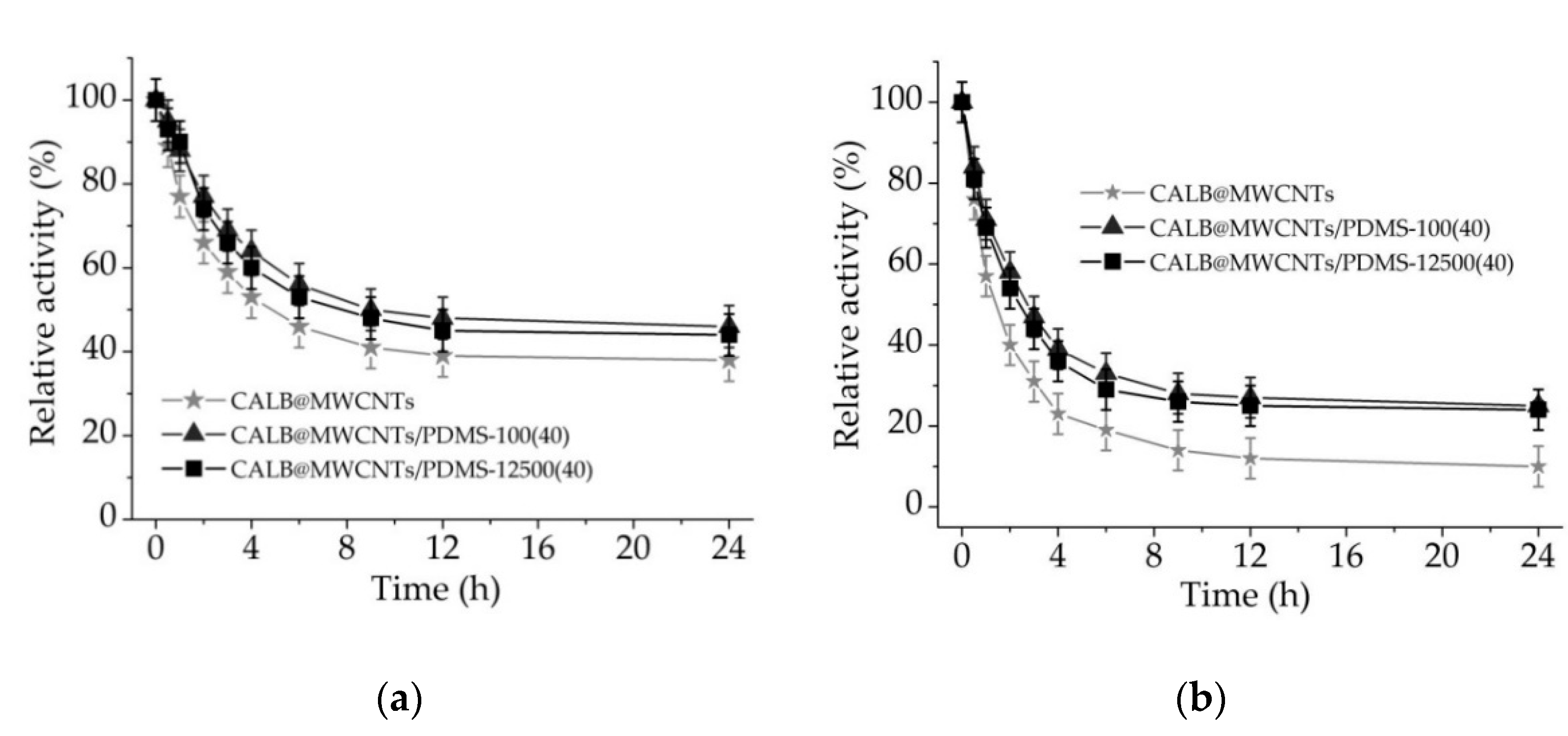
3.2.5. Effect of Solvents on the Immobilized Lipase
4. Discussion
4.1. Analysis of Nanocomposites before Lipase Immobilization
4.2. Immobilized Lipase Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Xu, T.; Yang, J.; Liu, J.; Fu, Q. Surface modification of multi-walled carbon nanotubes by O2 plasma. Appl. Surf. Sci. 2007, 253, 8945–8951. [Google Scholar] [CrossRef]
- Yudianti, R. Analysis of Functional Group Sited on Multi-Wall Carbon Nanotube Surface. Open Mater. Sci. J. 2011, 5, 242–247. [Google Scholar] [CrossRef]
- Karousis, N.; Tsotsou, G.-E.; Evangelista, F.; Rudolf, P.; Ragoussis, N.; Tagmatarchis, N. Carbon Nanotubes Decorated with Palladium Nanoparticles: Synthesis, Characterization, and Catalytic Activity. J. Phys. Chem. C 2008, 112, 13463–13469. [Google Scholar] [CrossRef]
- Ciraci, S.; Dag, S.; Yildirim, T.; Gülseren, O.; Senger, R.T. Functionalized carbon nanotubes and device applications. J. Phys. Condens. Matter 2004, 16, R901–R960. [Google Scholar] [CrossRef]
- Holger, F.; Bettinger, D. Carbon Nanotubes: Basic Concepts and Physical Properties; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Ibrahim, K.S. Carbon nanotubes-properties and applications: A review. Carbon Lett. 2013, 14, 131–144. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010, 396, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kitipornchai, S.; Wang, C.; Liew, K. Modeling of van der Waals force for infinitesimal deformation of multi-walled carbon nanotubes treated as cylindrical shells. Int. J. Solids Struct. 2005, 42, 6032–6047. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltanian, S. Surface functionalization of carbon nanotubes: Fabrication and applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer Nanocomposites Containing Carbon Nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Fujigaya, T.; Nakashima, N. Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci. Technol. Adv. Mater. 2015, 16, 024802. [Google Scholar] [CrossRef]
- Jin, M.; Feng, X.; Xi, J.; Zhai, J.; Cho, K.; Feng, L.; Jiang, L. Super-Hydrophobic PDMS Surface with Ultra-Low Adhesive Force. Macromol. Rapid Commun. 2005, 26, 1805–1809. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Gurney, R.S.; Liu, D. Superhydrophobic and photocatalytic PDMS/TiO2 coatings with environmental stability and multifunctionality. Colloids Surf. Physicochem. Eng. Asp. 2019, 561, 101–108. [Google Scholar] [CrossRef]
- Shena, C.; Wang, H.; Zhang, T.; Zeng, Y.J. Silica coating onto graphene for improving thermal conductivity and electrical insulation of graphene/polydimethylsiloxane nanocomposites. Mater. Res. Technol. 2019, 35, 36–43. [Google Scholar] [CrossRef]
- Deng, W.; Lei, Y.; Lin, Y.; Zhou, S.; Zhang, A. Absorptive supramolecular elastomer wound dressing based on polydimethylsiloxane–(polyethylene glycol)–polydimethylsiloxane copolymer: Preparation and characterization. RSC Adv. 2016, 6, 51694–51702. [Google Scholar] [CrossRef]
- Kong, K.; Mariatti, M.; Rashid, A.; Busfield, J. Enhanced conductivity behavior of polydimethylsiloxane (PDMS) hybrid composites containing exfoliated graphite nanoplatelets and carbon nanotubes. Compos. Part B Eng. 2014, 58, 457–462. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, L.; Yang, X.; Ruan, K.; Han, Y.; Kong, J.; Gu, J. Simultaneous improvement of thermal conductivities and electromagnetic interference shielding performances in polystyrene composites via constructing interconnection oriented networks based on electrospinning technology. Compos. Part A Appl. Sci. Manuf. 2019, 124, 840–848. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Park, S.-J. Superhydrophobic carbon-based materials: A review of synthesis, structure, and applications. Carbon Lett. 2014, 15, 89–104. [Google Scholar] [CrossRef]
- Klonos, P.; Sulym, I.; Borysenko, M.; Kripotou, S.; Kyritsis, A.; Pissis, P.; Gun’Ko, V. Interfacial interactions and complex segmental dynamics in systems based on silica-polydimethylsiloxane core–shell nanoparticles: Dielectric and thermal study. Polymers 2015, 58, 9–21. [Google Scholar] [CrossRef]
- Klonos, P.; Sulym, I.; Kyriakos, K.; Vangelidis, I.; Zidropoulos, S.; Sternik, D.; Borysenko, M.; Kyritsis, A.; Deryło-Marczewska, A.; Gun’Ko, V. Interfacial phenomena in core–shell nanocomposites of PDMS adsorbed onto low specific surface area fumed silica nanooxides: Effects of surface modification. Polymers 2015, 68, 158–167. [Google Scholar] [CrossRef]
- Klonos, P.; Dapei, G.; Sulym, I.Y.; Zidropoulos, S.; Sternik, D.; Deryło-Marczewska, A.; Borysenko, M.V.; Gun’Ko, V.M.; Kyritsis, A.; Pissis, P. Morphology and molecular dynamics investigation of PDMS adsorbed on titania nanoparticles: Effects of polymer molecular weight. Eur. Polym. J. 2016, 74, 64–80. [Google Scholar] [CrossRef]
- Klonos, P.; Sulym, I.Y.; Sternik, D.; Konstantinou, P.; Goncharuk, O.V.; Deryło–Marczewska, A.; Gun’Ko, V.M.; Kyritsis, A.; Pissis, P. Morphology, crystallization and rigid amorphous fraction in PDMS adsorbed onto carbon nanotubes and graphite. Polymers 2018, 139, 130–144. [Google Scholar] [CrossRef]
- Sulym, I.; Kubiak, A.; Jankowska, K.; Sternik, D.; Terpilowski, K.; Sementsov, Y.; Borysenko, M.; Derylo-Marczewska, A.; Jesionowski, T. Superhydrophobic MWCNTs/PDMS-nanocomposite materials: Preparation and characterization. Physicochem. Probl. Miner. Process. 2019, 55, 1394–1400. [Google Scholar] [CrossRef]
- Rodgers, L.; Knott, R.; Holden, P.; Pike, K.; Hanna, J.; Foster, L.; Bartlett, J. Structural evolution and stability of sol–gel biocatalysts. Phys. B Condens. Matter. 2006, 385–386, 508–510. [Google Scholar] [CrossRef]
- Kovalenko, G.A.; Perminova, L.V.; Beklemishev, A.B.; Parmon, V.N. Heterogeneous Biocatalysts Prepared by Immuring Enzymatic Active Components inside Silica Xerogel and Nanocarbons-In-Silica Composites. Catalysts 2018, 8, 177. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Rani, K.Y.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Rodríguez, K.; Martinez, R.; Bernal, C. Selective immobilization of Bacillus subtilis lipase A from cell culture supernatant: Improving catalytic performance and thermal resistance. Process. Biochem. 2020, 92, 214–223. [Google Scholar] [CrossRef]
- Zdarta, J.; Norman, M.; Smułek, W.; Moszyński, D.; Kaczorek, E.; Stelling, A.L.; Ehrlich, H.; Jesionowski, T. Spongin-Based Scaffolds from Hippospongia communis Demosponge as an Effective Support for Lipase Immobilization. Catalysts 2017, 7, 147. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Yang, H.; Huang, X.; Liu, H.; Zhang, J.; Guo, S. Graphene Oxide as a Matrix for Enzyme Immobilization. Langmuir 2010, 26, 6083–6085. [Google Scholar] [CrossRef]
- Asmat, S.; Anwer, A.H.; Husain, Q. Immobilization of lipase onto novel constructed polydopamine grafted multiwalled carbon nanotube impregnated with magnetic cobalt and its application in synthesis of fruit flavours. Int. J. Biol. Macromol. 2019, 140, 484–495. [Google Scholar] [CrossRef]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.-X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Jiang, Y.; Zhou, L.; Ma, L.; He, Y.; Gao, J. Biocatalytic Pickering Emulsions Stabilized by Lipase-Immobilized Carbon Nanotubes for Biodiesel Production. Catalysts 2018, 8, 587. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, S.; Chen, W. Activity of catalase adsorbed to carbon nanotubes: Effects of carbon nanotube surface properties. Talanta 2013, 113, 142–147. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Tsoufis, T.; Enotiadis, A.; Gournis, D.; Stamatis, H. Functionalized Multi-Wall Carbon Nanotubes for Lipase Immobilization. Adv. Eng. Mater. 2010, 12, B179–B183. [Google Scholar] [CrossRef]
- Wang, L.; Xu, R.; Chen, Y.; Jiang, R. Activity and stability comparison of immobilized NADH oxidase on multi-walled carbon nanotubes, carbon nanospheres, and single-walled carbon nanotubes. J. Mol. Catal. B Enzym. 2011, 69, 120–126. [Google Scholar] [CrossRef]
- Feng, W.; Ji, P. Enzymes immobilized on carbon nanotubes. Biotechnol. Adv. 2011, 29, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250. [Google Scholar] [CrossRef]
- Kartel, M.; Sementsov, Y.; Mahno, S.; Trachevskiy, V.; Bo, W. Polymer Composites Filled with Multiwall Carbon Nanotubes. Univ. J. Mater. Sci. 2016, 4, 23–31. [Google Scholar] [CrossRef]
- Melezhyk, A.V.; Sementsov, Y.I.; Yanchenko, V.V. Synthesis of thin carbon nanotubes on co-precipitated metaloxide catalysts. Russ. J. Appl. Chem. 2005, 78, 938–946. [Google Scholar]
- Gun’ko, V.M. Composite materials: Textural characteristics. Appl. Surf. Sci. 2014, 307, 444–454. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Mikhalovsky, S.V. Evaluation of slitlike porosity of carbon adsorbents. Carbon 2004, 42, 843–849. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Kruk, M.; Jaroniec, M. Gas Adsorption Characterization of Ordered Organic−Inorganic Nanocomposite Materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Jorio, A.; Filho, A.S.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Johnson, L.M.; Gao, L.; Iv, C.W.S.; Smith, M.; Efimenko, K.; Cushing, K.; Genzer, J.; López, G.P. Elastomeric microparticles for acoustic mediated bioseparations. J. Nanobiotechnol. 2013, 11, 22. [Google Scholar] [CrossRef]
- Sulym, I.; Goncharuk, O.; Sternik, D.; Terpilowski, K.; Derylo-Marczewska, A.; Borysenko, M.V.; Gun’Ko, V.M. Nanooxide/Polymer Composites with Silica@PDMS and Ceria–Zirconia–Silica@PDMS: Textural, Morphological, and Hydrophilic/Hydrophobic Features. Nanoscale Res. Lett. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Wu, L.; Zhang, L.; Dong, L.; Hu, X.; Li, C.-Z. Second-order Raman spectroscopy of char during gasification. Fuel Process. Technol. 2015, 135, 105–111. [Google Scholar] [CrossRef]
- Wong, P.T.; Wong, R.K.; Caputo, T.A.; Godwin, T.A.; Rigas, B. Infrared spectroscopy of exfoliated human cervical cells: Evidence of extensive structural changes during carcinogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 10988–10992. [Google Scholar] [CrossRef]
- Portaccio, M.B.E.; Della Ventura, B.; Mita, D.G.; Manolova, N.; Stoilova, O.; Rashkov, I.; Lepore, M. FT-IR microscopy characterization of sol–gel layers prior and after glucose oxidase immobilization for biosensing applications. J. SolGel Sci. Technol. 2011, 57, 204–211. [Google Scholar] [CrossRef]
- Colla, L.M.; Ficanha, A.M.M.; Rizzardi, J.; Bertolin, T.E.; Reinehr, C.O.; Costa, J.A.V. Production and Characterization of Lipases by Two New Isolates of Aspergillus through Solid-State and Submerged Fermentation. BioMed Res. Int. 2015, 2015, 725959. [Google Scholar] [CrossRef]
- Zhu, Y.-T.; Ren, X.-Y.; Liu, Y.-M.; Wei, Y.; Qing, L.-S.; Liao, X. Covalent Immobilization of Porcine Pancreatic Lipase on Carboxyl-Activated Magnetic Nanoparticles: Characterization and Application For Enzymatic Inhibition Assays. Mater. Sci. Eng. C 2014, 38, 278–285. [Google Scholar] [CrossRef]
- Narwal, S.K.; Saun, N.K.; Gupta, R. Characterization and Catalytic Properties of Free and Silica-Bound Lipase: A Comparative Study. J. Oleo Sci. 2014, 63, 599–605. [Google Scholar] [CrossRef]
- Peña, S.A.; Rios, N.S.; Carballares, D.; Gonçalves, L.R.; Fernandez-Lafuente, R. Immobilization of lipases via interfacial activation on hydrophobic supports: Production of biocatalysts libraries by altering the immobilization conditions. Catal. Today 2021, 362, 130–140. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shen, H.; Tao, Y.; Chen, B.; Tan, T. Amino silicones finished fabrics for lipase immobilization: Fabrics finishing and catalytic performance of immobilized lipase. Process. Biochem. 2014, 49, 1488–1496. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Liu, D.; Du, W. Hydrophobic pore space constituted in macroporous ZIF-8 for lipase immobilization greatly improving lipase catalytic performance in biodiesel preparation. Biotechnol. Biofuels 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Dong, L.; Ge, C.; Qin, P.; Chen, Y.; Xu, Q. Immobilization and catalytic properties of candida lipolytic lipase on surface of organic intercalated and modified MgAl-LDHs. Solid State Sci. 2014, 31, 8–15. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Park, C.; Kim, S.W. Effect of a buffer mixture system on the activity of lipases during immobilization process. Bioresour. Technol. 2010, 101, S66–S70. [Google Scholar] [CrossRef]
- Jamie, A.; Alshami, A.S.; Maliabari, Z.O.; Ateih, M.A.; Al Hamouz, O.C.S. Immobilization and enhanced catalytic activity of lipase on modified MWCNT for oily wastewater treatment. Environ. Prog. Sustain. Energy 2016, 35, 1441–1449. [Google Scholar] [CrossRef]
- Khan, A.K.; Mubarak, N.M.; Abdullah, E.; Khalid, M.; Nizamuddin, S.; Baloch, H.A.; Siddiqui, M. Immobilization of Lipase Enzyme Carbon Nanotubes via Adsorption. IOP Conf. Ser. Mater. Sci. Eng. 2019, 495, 012055. [Google Scholar] [CrossRef]
- Macario, A.; Giordano, G.; Frontera, P.; Crea, F.; Setti, L. Hydrolysis of Alkyl Ester on Lipase/Silicalite-1 Catalyst. Catal. Lett. 2007, 122, 43–52. [Google Scholar] [CrossRef]
- Gonqalves, A.P.V.; Lopes, J.M.; Lemos, F.; Ram, F.; Ramoa Ribeiro, F.; Prazeres, D.M.F.; Cabral, J.M.S.; Aires-Barros, M. Effect of the immobilization support on the hydrolytic activity of a cutinase from Fusarium solanipisi. J. Mol. Catal. B Enzym. 1996, 1, 53–60. [Google Scholar]
- Soumanou, M.M.; Bornscheuer, U.T. Improvement in lipase-catalyzed synthesis of fatty acid methyl esters from sunflower oil. Enzym. Microb. Technol. 2003, 33, 97–103. [Google Scholar] [CrossRef]
- Reetz, M.T.; Zonta, A.; Simpelkamp, J. Efficient immobilization of lipases by entrapment in hydrophobic sol-gel materials. Biotechnol. Bioeng. 1996, 49, 527–534. [Google Scholar] [CrossRef]
- Zdarta, J.; Wysokowski, M.; Norman, M.; Kołodziejczak-Radzimska, A.; Moszyński, D.; Maciejewski, H.; Ehrlich, H.; Jesionowski, T. Candida antarctica Lipase B Immobilized onto Chitin Conjugated with POSS® Compounds: Useful Tool for Rapeseed Oil Conversion. Int. J. Mol. Sci. 2016, 17, 1581. [Google Scholar] [CrossRef] [PubMed]


| Sample | SBET (m2/g) | Smicro (m2/g) | Smeso (m2/g) | Smacro (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Vmacro (cm3/g) | Vp (cm3/g) | Rp,V (nm) |
|---|---|---|---|---|---|---|---|---|---|
| P-MWCNTs | 222 | 74 | 134 | 14 | 0.039 | 0.418 | 0.357 | 0.814 | 23 |
| MWCNTs/PDMS-100(40) | 76 | 0 | 56 | 20 | 0 | 0.056 | 0.203 | 0.259 | 62 |
| MWCNTs/PDMS-12500(40) | 77 | 0 | 51 | 26 | 0 | 0.054 | 0.283 | 0.337 | 65 |
| Parameter | Free CALB | CALB@P-MWCNTs | CALB@MWCNTs/ PDMS-100(40) | CALB@MWCNTs/ PDMS-12500(40) |
|---|---|---|---|---|
| kD (min−1) | 0.01075 | 0.00268 | 0.00156 | 0.00208 |
| t1/2 (min) | 64.74 | 259.70 | 446.15 | 334.61 |
| Enzyme | Support | Type of Immobilization | Reusability | Storage Stability | Activity Retention | Process Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Lipase from Rhizomucor miehei | Pure silica zeolites | Adsorption | 60% after 4 catalytic cycles | n.a. | 68% | 93% of methyl myristate conversion | [64] |
| Fusarium solanipisi recombinant cutinase with high lipolytic activity | Zeolite | Adsorption | n.a. | 89% after 45 days | 74% | 91% of trycaprylin transformation | [65] |
| Commercial lipases from Rhizomucor miehei | Polypropylene | Adsorption | 85% after 8 catalytic cycles | n.a. | over 70% | 90% of sunflower oil methanolysis | [66] |
| Lipase from Rhizornucor rniehei | Sol–gel silica | Entrapment | n.a. | 75% after 20 days | 86% | n.a. | [67] |
| Lipase B from Candida antarctica | Hippospongiacommunis spongin scaffolds | Adsorption | 82% after 20 catalytic cycles | 85% after 20 days | 91% | 100% of rapeseed oil methanolysis | [30] |
| Lipase B from Candida antarctica | Chitin modified by POSS * compounds | Adsorption | 87% after 15 catalytic cycles | 90% after 20 days | 87% | 100% of rapeseed oil methanolysis | [68] |
| Lipase B from Candida antarctica | MWCNTs modified by PDMS | Adsorption | 91% after 10 catalytic cycles | 90% after 20 days | 94% | n.a. | this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulym, I.; Zdarta, J.; Ciesielczyk, F.; Sternik, D.; Derylo-Marczewska, A.; Jesionowski, T. Pristine and Poly(Dimethylsiloxane) Modified Multi-Walled Carbon Nanotubes as Supports for Lipase Immobilization. Materials 2021, 14, 2874. https://doi.org/10.3390/ma14112874
Sulym I, Zdarta J, Ciesielczyk F, Sternik D, Derylo-Marczewska A, Jesionowski T. Pristine and Poly(Dimethylsiloxane) Modified Multi-Walled Carbon Nanotubes as Supports for Lipase Immobilization. Materials. 2021; 14(11):2874. https://doi.org/10.3390/ma14112874
Chicago/Turabian StyleSulym, Iryna, Jakub Zdarta, Filip Ciesielczyk, Dariusz Sternik, Anna Derylo-Marczewska, and Teofil Jesionowski. 2021. "Pristine and Poly(Dimethylsiloxane) Modified Multi-Walled Carbon Nanotubes as Supports for Lipase Immobilization" Materials 14, no. 11: 2874. https://doi.org/10.3390/ma14112874
APA StyleSulym, I., Zdarta, J., Ciesielczyk, F., Sternik, D., Derylo-Marczewska, A., & Jesionowski, T. (2021). Pristine and Poly(Dimethylsiloxane) Modified Multi-Walled Carbon Nanotubes as Supports for Lipase Immobilization. Materials, 14(11), 2874. https://doi.org/10.3390/ma14112874








