Composite Fe3O4-MXene-Carbon Nanotube Electrodes for Supercapacitors Prepared Using the New Colloidal Method
Abstract
:1. Introduction
2. Materials and Methods
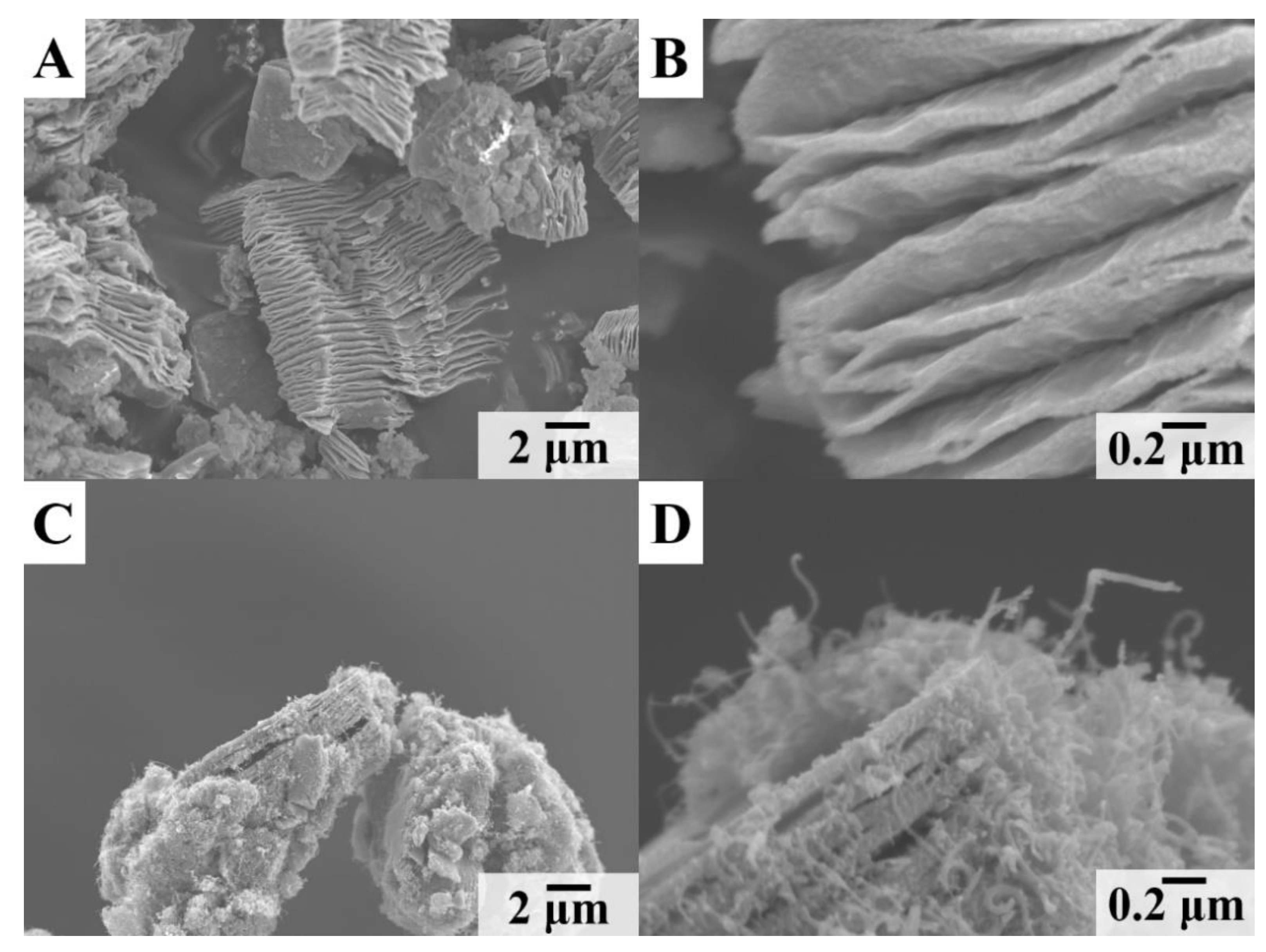
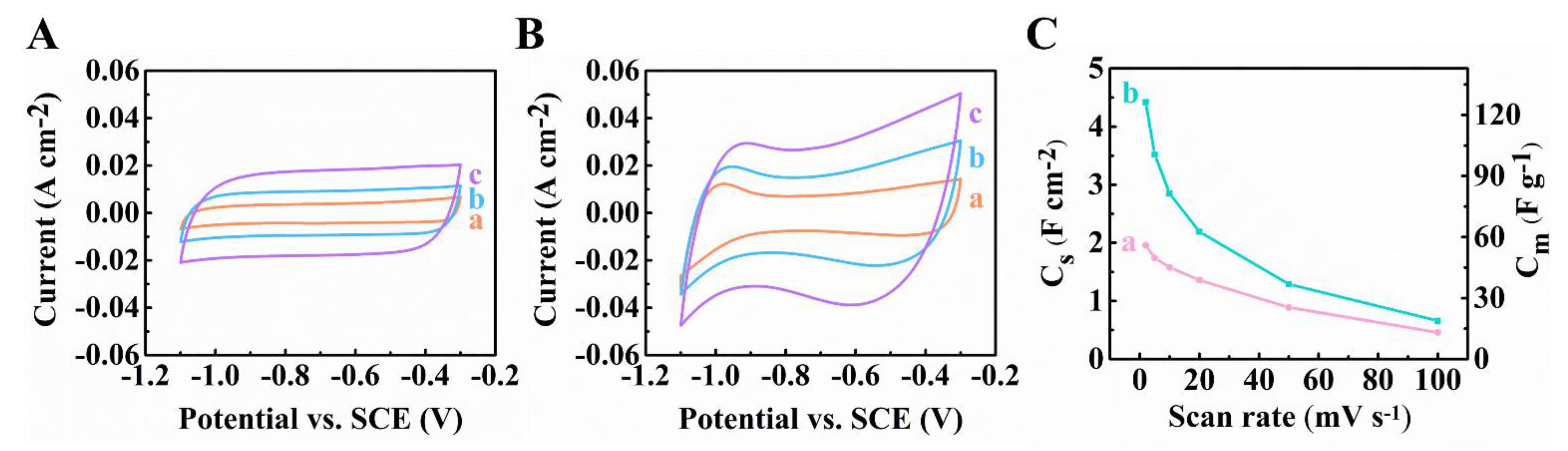
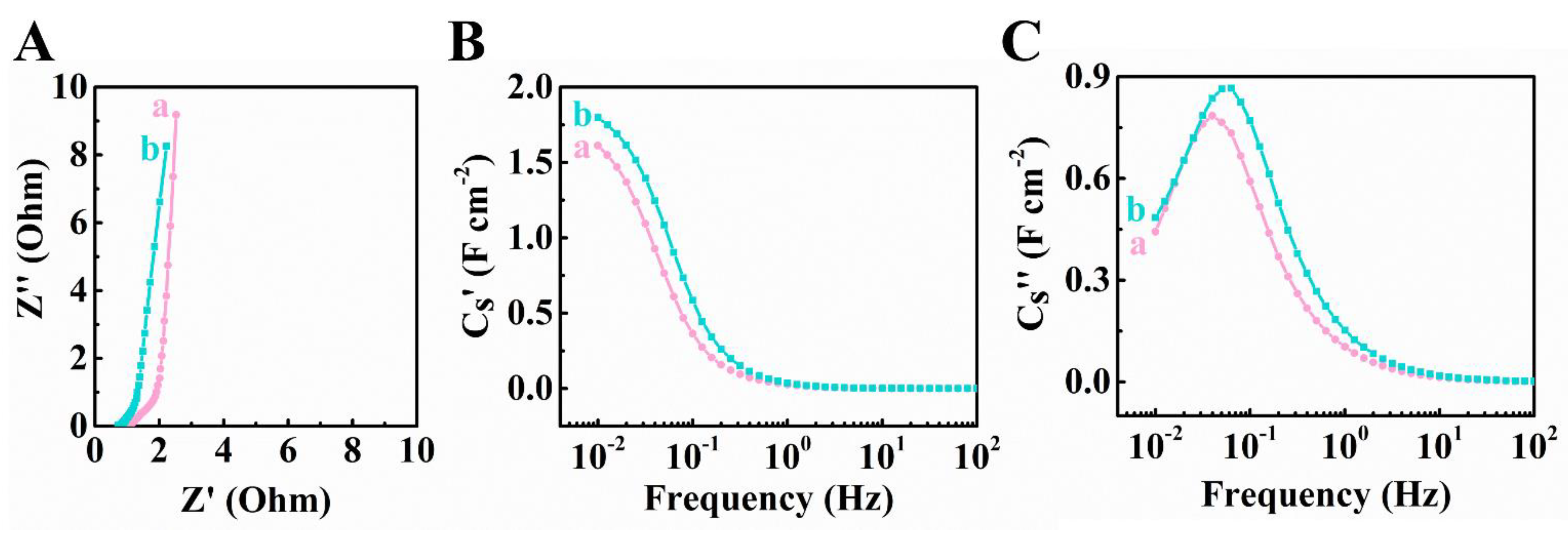
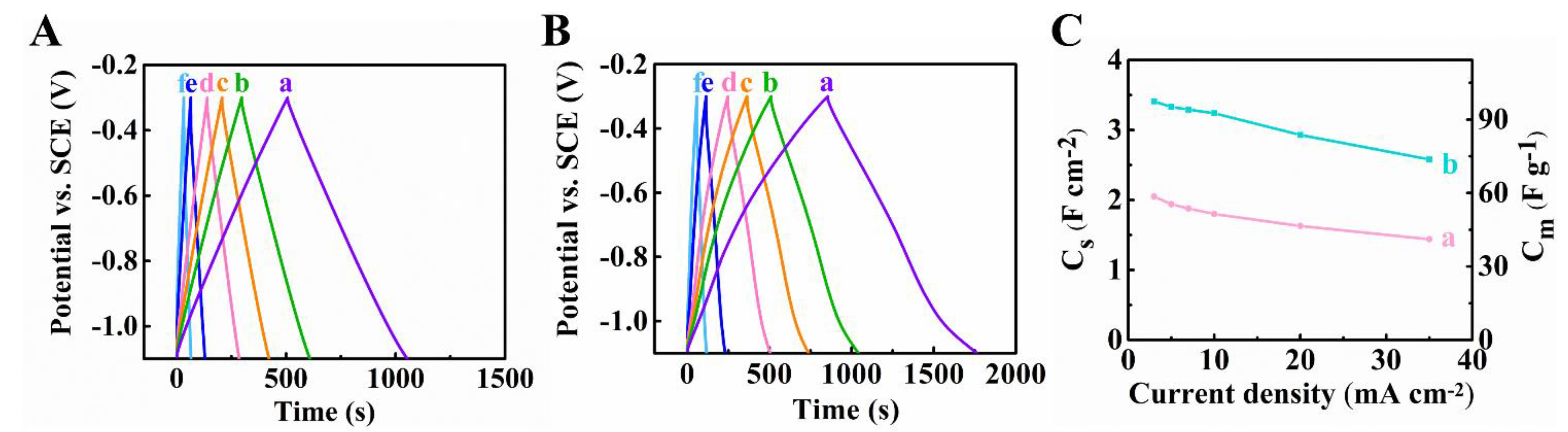
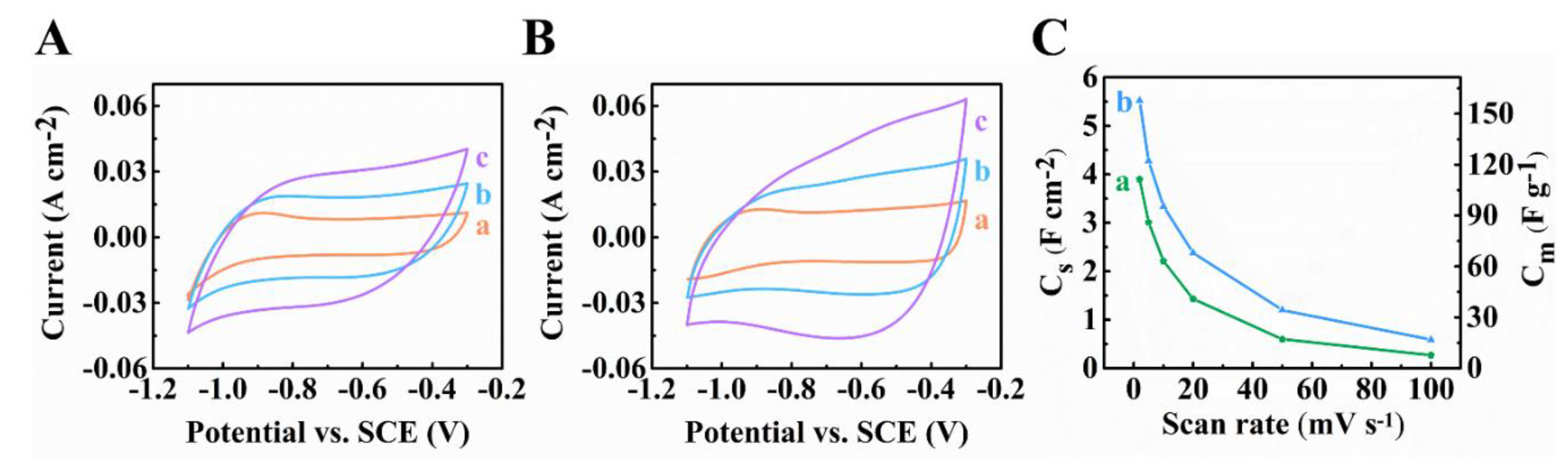
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Xu, S.; Wei, G.; Li, J.; Han, W.; Gogotsi, Y. Flexible MXene–graphene electrodes with high volumetric capacitance for integrated co-cathode energy conversion/storage devices. J. Mater. Chem. A 2017, 5, 17442–17451. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zheng, B.; Mackinder, M.; Baule, N.; Qiao, H.; Jin, H.; Schuelke, T.; Fan, Q.H. Graphene wrapped MXene via plasma exfoliation for all-solid-state flexible supercapacitors. Energy Storage Mater. 2019, 20, 299–306. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, Y.; Jiang, N.; Liu, A.; Gao, L.; Li, Y.; Wang, H.; Ma, T. One-pot synthesis of 2D Ti3C2/Ni2CO3(OH)2 composite as electrode material with superior capacity and high stability for hybrid supercapacitor. Electrochim. Acta 2018, 292, 168–179. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Li, Z.; Zhang, Y.; Xing, B.; Zhang, C.; Zhou, A. Electrochemical performance of Ti3C2 supercapacitors in KOH electrolyte. J. Adv. Ceram. 2015, 4, 130–134. [Google Scholar] [CrossRef]
- Tang, Y.; Zhu, J.; Wu, W.; Yang, C.; Lv, W.; Wang, F. Synthesis of Nitrogen-Doped Two-Dimensional Ti3C2 with Enhanced Electrochemical Performance. J. Electrochem. Soc. 2017, 164, A923–A929. [Google Scholar] [CrossRef]
- Tian, Y.; Que, W.; Luo, Y.; Yang, C.; Yin, X.; Kong, L.B. Surface nitrogen-modified 2D titanium carbide (MXene) with high energy density for aqueous supercapacitor applications. J. Mater. Chem. A 2019, 7, 5416–5425. [Google Scholar] [CrossRef]
- Wen, Y.; Rufford, T.E.; Chen, X.; Li, N.; Lyu, M.; Dai, L.; Wang, L. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Wang, F.; Cao, M.; Qin, Y.; Zhu, J.; Wang, L.; Tang, Y. ZnO nanoparticle-decorated two-dimensional titanium carbide with enhanced supercapacitive performance. RSC Adv. 2016, 6, 88934–88942. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Anjum, D.H.; Alshareef, H.N. Direct Chemical Synthesis of MnO2 Nanowhiskers on Transition-Metal Carbide Surfaces for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2016, 8, 18806–18814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, M.; Wang, F.; Wang, L.; Wu, W.; Lv, W.; Zhu, J. Room Temperature Oxidation of Ti3C2MXene for Supercapacitor Electrodes. J. Electrochem. Soc. 2017, 164, A3933–A3942. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Momodu, D.Y.; Naguib, M.; Mirghni, A.A.; Masikhwa, T.M.; Khaleed, A.A.; Kebede, M.; Manyala, N. Electrochemical performance of two-dimensional Ti3C2-Mn3O4 nanocomposites and carbonized iron cations for hybrid supercapacitor electrodes. Electrochim. Acta 2019, 301, 487–499. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, Y.; Huang, H.; Jiang, Q. Achieving high-rate capacitance of multi-layer titanium carbide (MXene) by liquid-phase exfoliation through Li-intercalation. Electrochem. Commun. 2017, 81, 48–51. [Google Scholar] [CrossRef]
- Wu, W.; Niu, D.; Zhu, J.; Gao, Y.; Wei, D.; Zhao, C.; Wang, C.; Wang, F.; Wang, L.; Yang, L. Hierarchical architecture of Ti3C2@PDA/NiCo2S4 composite electrode as high-performance supercapacitors. Ceram. Int. 2019, 45, 16261–16269. [Google Scholar] [CrossRef]
- Yang, C.; Que, W.; Tang, Y.; Tian, Y.; Yin, X. Nitrogen and Sulfur Co-Doped 2D Titanium Carbides for Enhanced Electrochemical Performance. J. Electrochem. Soc. 2017, 164, A1939–A1945. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, L.; Zhang, B.; Wu, H. 2D-Ti3C2 as hard, conductive substrates to enhance the electrochemical performance of MnO2 for supercapacitor applications. Ceram. Int. 2018, 44, 17539–17543. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Lei, W.; Wu, Y.; Li, C.; Khan, M.A.; Ouyang, Y.; Jiao, X.; Ye, H.; Mutahir, S.; et al. Achieving quick charge/discharge rate of 3.0 V s−1 by 2D titanium carbide (MXene) via N-doped carbon intercalation. Mater. Lett. 2019, 234, 21–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Zhang, B.; Li, J.; Lu, C.; Kong, L.; Liu, M. Self-assembly of secondary-formed multilayer La/e-Ti3C2 as high performance supercapacitive material with excellent cycle stability and high rate capability. J. Alloys Compd. 2020, 835, 155343. [Google Scholar] [CrossRef]
- He, X.; Bi, T.; Zheng, X.; Zhu, W.; Jiang, J. Nickel cobalt sulfide nanoparticles grown on titanium carbide MXenes for high-performance supercapacitor. Electrochim. Acta 2020, 332, 135514. [Google Scholar] [CrossRef]
- Hu, M.; Hu, T.; Li, Z.; Yang, Y.; Cheng, R.; Yang, J.; Cui, C.; Wang, X. Surface functional groups and interlayer water determine the electrochemical capacitance of Ti3C2 T x MXene. ACS Nano 2018, 12, 3578–3586. [Google Scholar] [CrossRef] [PubMed]
- Le, T.A.; Tran, N.Q.; Hong, Y.; Lee, H. Intertwined Titanium Carbide MXene within a 3D Tangled Polypyrrole Nanowires Matrix for Enhanced Supercapacitor Performances. Chem. A Eur. J. 2018, 25, 1037–1043. [Google Scholar] [CrossRef]
- Li, Z.; Ma, C.; Wen, Y.; Wei, Z.; Xing, X.; Chu, J.; Yu, C.; Wang, K.; Wang, Z.-K. Highly conductive dodecaborate/MXene composites for high performance supercapacitors. Nano Res. 2019, 13, 196–202. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Zhang, J.; Han, Y.; Zhang, W.; Yang, X.; Zhang, X.; Jiang, W. Tunable energy storage capacity of two-dimensional Ti3C2Tx modified by a facile two-step pillaring strategy for high performance supercapacitor electrodes. Nanoscale 2019, 11, 21981–21989. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Zhang, X. Two-dimensional titanium carbide electrode with large mass loading for supercapacitor. J. Power Sources 2015, 294, 354–359. [Google Scholar] [CrossRef]
- Ramachandran, R.; Rajavel, K.; Xuan, W.; Lin, D.; Wang, F. Influence of Ti3C2Tx (MXene) intercalation pseudocapacitance on electrochemical performance of Co-MOF binder-free electrode. Ceram. Int. 2018, 44, 14425–14431. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, X.; Zhang, X.; Zhang, Y.; Liu, Y.; Wang, W.; Si, W.; Dong, X. Carbon-intercalated Ti3C2Tx MXene for high-performance electrochemical energy storage. J. Mater. Chem. A 2018, 6, 23513–23520. [Google Scholar] [CrossRef]
- Yang, C.; Que, W.; Yin, X.; Tian, Y.; Yang, Y.; Que, M. Improved capacitance of nitrogen-doped delaminated two-dimensional titanium carbide by urea-assisted synthesis. Electrochim. Acta 2017, 225, 416–424. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, J.; Wu, W.; Zhang, B. Hierarchical architecture of PANI@ TiO2/Ti3C2Tx ternary composite electrode for enhanced electrochemical performance. Electrochim. Acta 2017, 228, 282–289. [Google Scholar] [CrossRef]
- Cao, J.; Han, Y.; Zheng, X.; Wang, Q. Preparation and electrochemical performance of modified Ti3C2Tx/polypyrrole composites. J. Appl. Polym. Sci. 2019, 136, 47003. [Google Scholar] [CrossRef]
- Jian, X.; He, M.; Chen, L.; Zhang, M.-M.; Li, R.; Gao, L.-J.; Fu, F.; Liang, Z.-H. Three-dimensional carambola-like MXene/polypyrrole composite produced by one-step co-electrodeposition method for electrochemical energy storage. Electrochim. Acta 2019, 318, 820–827. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wang, L.; Wu, W.; Fang, Y. In–situ growth of carbon nanotubes on two–dimensional titanium carbide for enhanced electrochemical performance. Electrochim. Acta 2017, 258, 291–301. [Google Scholar] [CrossRef]
- Kim, K.; Okubo, M.; Yamada, A. Interfacial Dissociation of Contact-Ion-Pair on MXene Electrodes in Concentrated Aqueous Electrolytes. J. Electrochem. Soc. 2019, 166, A3739–A3744. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification. Adv. Energy Mater. 2017, 7, 1602725. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yuan, H.; Jin, C.; Zhang, L.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.; et al. Pillared Structure Design of MXene with Ultralarge Interlayer Spacing for High-Performance Lithium-Ion Capacitors. ACS Nano 2017, 11, 2459–2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Narayanasamy, M.; Yang, C.; Zhao, L.; Jiang, J.; Angaiah, S.; Yan, C. 3D interpenetrating assembly of partially oxidized MXene confined Mn–Fe bimetallic oxide for superior energy storage in ionic liquid. Electrochim. Acta 2020, 334, 135546. [Google Scholar] [CrossRef]
- Li, H.; Chen, R.; Ali, M.; Lee, H.; Ko, M.J. In Situ Grown MWCNTs/MXenes Nanocomposites on Carbon Cloth for High-Performance Flexible Supercapacitors. Adv. Funct. Mater. 2020, 30, 2002739. [Google Scholar] [CrossRef]
- Pan, Z.; Ji, X. Facile synthesis of nitrogen and oxygen co-doped C@Ti3C2 MXene for high performance symmetric supercapacitors. J. Power Sources 2019, 439, 227068. [Google Scholar] [CrossRef]
- Wu, W.; Wei, D.; Zhu, J.; Niu, D.; Wang, F.; Wang, L.; Yang, L.; Yang, P.; Wang, C. Enhanced electrochemical performances of organ-like Ti3C2 MXenes/polypyrrole composites as supercapacitors electrode materials. Ceram. Int. 2019, 45, 7328–7337. [Google Scholar] [CrossRef]
- Yang, B.; She, Y.; Zhang, C.; Kang, S.; Zhou, J.; Hu, W. Nitrogen Doped Intercalation TiO2/TiN/Ti3C2Tx Nanocomposite Electrodes with Enhanced Pseudocapacitance. Nanomaterials 2020, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.; Zhitomirsky, I. The Development of Pseudocapacitor Electrodes and Devices with High Active Mass Loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Guo, M.; Liu, C.; Zhang, Z.; Zhou, J.; Tang, Y.; Luo, S. Flexible Ti3C2Tx@Al electrodes with Ultrahigh Areal Capacitance: In Situ Regulation of Interlayer Conductivity and Spacing. Adv. Funct. Mater. 2018, 28, 1803196. [Google Scholar] [CrossRef]
- Nawwar, M.; Poon, R.; Chen, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. High areal capacitance of Fe3O4-decorated carbon nanotubes for supercapacitor electrodes. Carbon Energy 2019, 1, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Reddy, R.N.; Reddy, R.G. Sol—Gel MnO2 as an electrode material for electrochemical capacitors. J. Power Sources 2003, 124, 330–337. [Google Scholar] [CrossRef]
- Jeong, Y.U.; Manthiram, A. Nanocrystalline Manganese Oxides for Electrochemical Capacitors with Neutral Electrolytes. J. Electrochem. Soc. 2002, 149, A1419–A1422. [Google Scholar] [CrossRef]
- Dong, W.; Rolison, D.R.; Dunna, B. Electrochemical Properties of High Surface Area Vanadium Oxide Aerogels. Electrochem. Solid State Lett. 1999, 3, 457–459. [Google Scholar] [CrossRef]
- Dong, W.; Sakamoto, J.S.; Dunn, B. Electrochemical properties of vanadium oxide aerogels. Sci. Technol. Adv. Mater. 2003, 4, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Sinan, N.; Unur, E. Fe3O4/carbon nanocomposite: Investigation of capacitive & magnetic properties for supercapacitor applications. Mater. Chem. Phys. 2016, 183, 571–579. [Google Scholar] [CrossRef]
- Ghaly, H.A.; El-Deen, A.G.; Souaya, E.R.; Allam, N.K. Asymmetric supercapacitors based on 3D graphene-wrapped V2O5 nanospheres and Fe3O4@3D graphene electrodes with high power and energy densities. Electrochim. Acta 2019, 310, 58–69. [Google Scholar] [CrossRef]
- Nithya, V.D.; Arul, N.S. Progress and development of Fe3O4 electrodes for supercapacitors. J. Mater. Chem. A 2016, 4, 10767–10778. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhitomirsky, I. Cathodic electrophoretic deposition of manganese dioxide films. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 248–253. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Chen, J.; Fang, K.; Chen, Q.; Xu, J.; Wong, C.-P. Integrated paper electrodes derived from cotton stalks for high-performance flexible supercapacitors. Nano Energy 2018, 53, 337–344. [Google Scholar] [CrossRef]
- Liu, Y.; Zhitomirsky, I. Electrochemical supercapacitor based on multiferroic BiMn2O5. J. Power Sources 2015, 284, 377–382. [Google Scholar] [CrossRef]
- Liang, W.; Zhitomirsky, I. Zn-Fe Double Hydroxide-Carbon Nanotube Anodes for Asymmetric Supercapacitors. Front. Mater. 2020, 7, 137. [Google Scholar] [CrossRef]
- Liu, Y.; Ata, M.S.; Shi, K.; Zhu, G.-Z.; Botton, G.A.; Zhitomirsky, I. Surface modification and cathodic electrophoretic deposition of ceramic materials and composites using celestine blue dye. RSC Adv. 2014, 4, 29652–29659. [Google Scholar] [CrossRef]
- Ata, M.S.; Liu, Y.; Zhitomirsky, I. A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles. RSC Adv. 2014, 4, 22716–22732. [Google Scholar] [CrossRef]
- Ata, M.S.; Poon, R.; Syed, A.M.; Milne, J.; Zhitomirsky, I. New developments in non-covalent surface modification, dispersion and electrophoretic deposition of carbon nanotubes. Carbon 2018, 130, 584–598. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, K.; Zhitomirsky, I. Polypyrrole coated carbon nanotubes for supercapacitor devices with enhanced electrochemical performance. J. Power Sources 2014, 268, 233–239. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Fabrication of Polypyrrole-Coated Carbon Nanotubes Using Oxidant–Surfactant Nanocrystals for Supercapacitor Electrodes with High Mass Loading and Enhanced Performance. ACS Appl. Mater. Interfaces 2013, 5, 13161–13170. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Graphene/Reduced Graphene Oxide-Carbon Nanotubes Composite Electrodes: From Capacitive to Battery-Type Behaviour. Nanomaterials 2021, 11, 1240. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials—Capacitive, Pseudocapacitive, or Battery-like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rorabeck, K.; Zhitomirsky, I. Application of Octanohydroxamic Acid for Salting out Liquid–Liquid Extraction of Materials for Energy Storage in Supercapacitors. Molecules 2021, 26, 296. [Google Scholar] [CrossRef] [PubMed]
- Rorabeck, K.; Zhitomirsky, I. Salting-out aided dispersive extraction of Mn3O4 nanoparticles and carbon nanotubes for application in supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126451. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Zhitomirsky, I. Composite Fe3O4-MXene-Carbon Nanotube Electrodes for Supercapacitors Prepared Using the New Colloidal Method. Materials 2021, 14, 2930. https://doi.org/10.3390/ma14112930
Liang W, Zhitomirsky I. Composite Fe3O4-MXene-Carbon Nanotube Electrodes for Supercapacitors Prepared Using the New Colloidal Method. Materials. 2021; 14(11):2930. https://doi.org/10.3390/ma14112930
Chicago/Turabian StyleLiang, Wenyu, and Igor Zhitomirsky. 2021. "Composite Fe3O4-MXene-Carbon Nanotube Electrodes for Supercapacitors Prepared Using the New Colloidal Method" Materials 14, no. 11: 2930. https://doi.org/10.3390/ma14112930
APA StyleLiang, W., & Zhitomirsky, I. (2021). Composite Fe3O4-MXene-Carbon Nanotube Electrodes for Supercapacitors Prepared Using the New Colloidal Method. Materials, 14(11), 2930. https://doi.org/10.3390/ma14112930





