Pyrolysis of Pruning Residues from Various Types of Orchards and Pretreatment for Energetic Use of Biochar
Abstract
1. Introduction
2. Materials and Methods
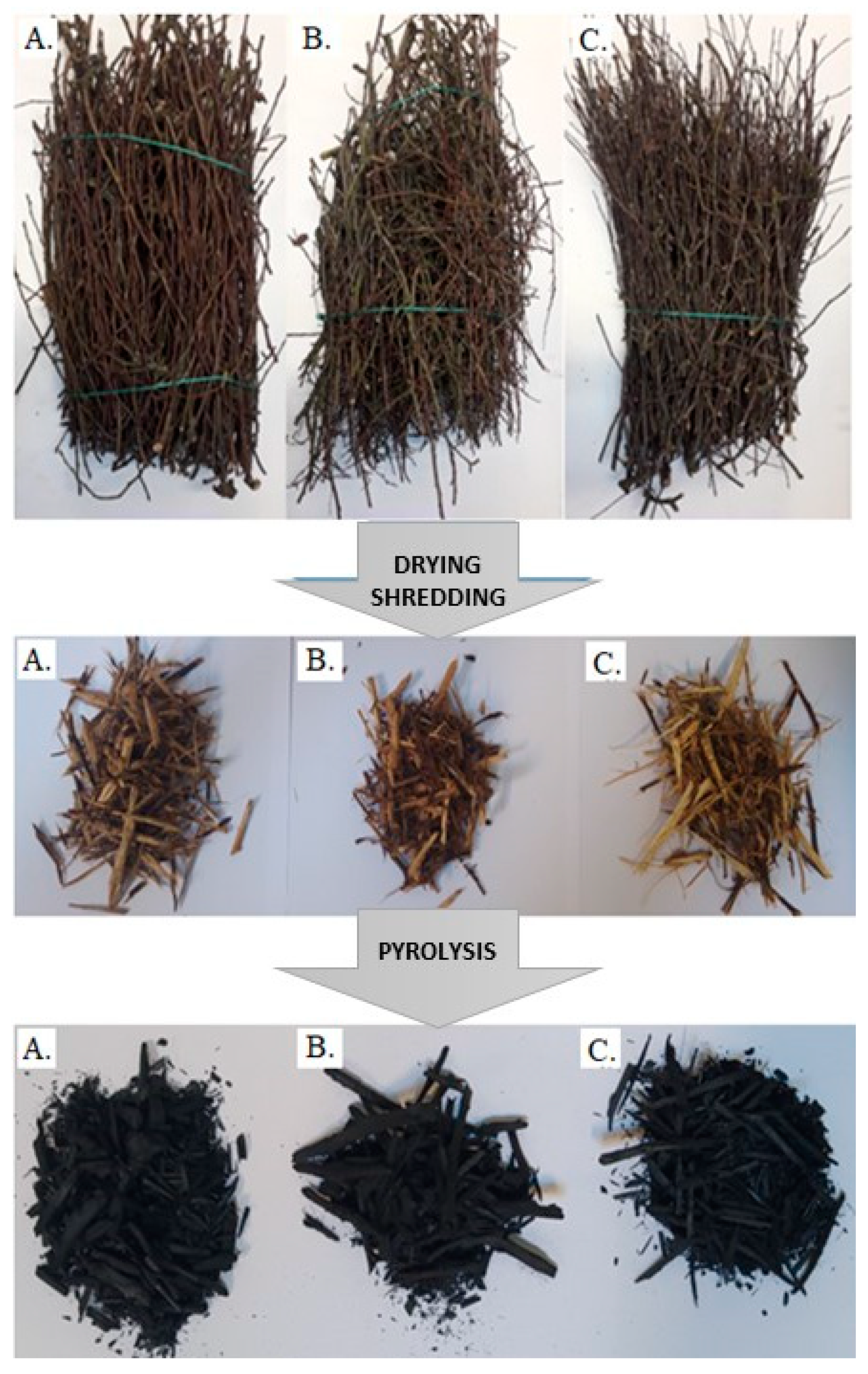
2.1. Materials and Preliminary Preparation of Samples
2.2. Pyrolysis Process
2.3. Proximate and Elemental Analysis
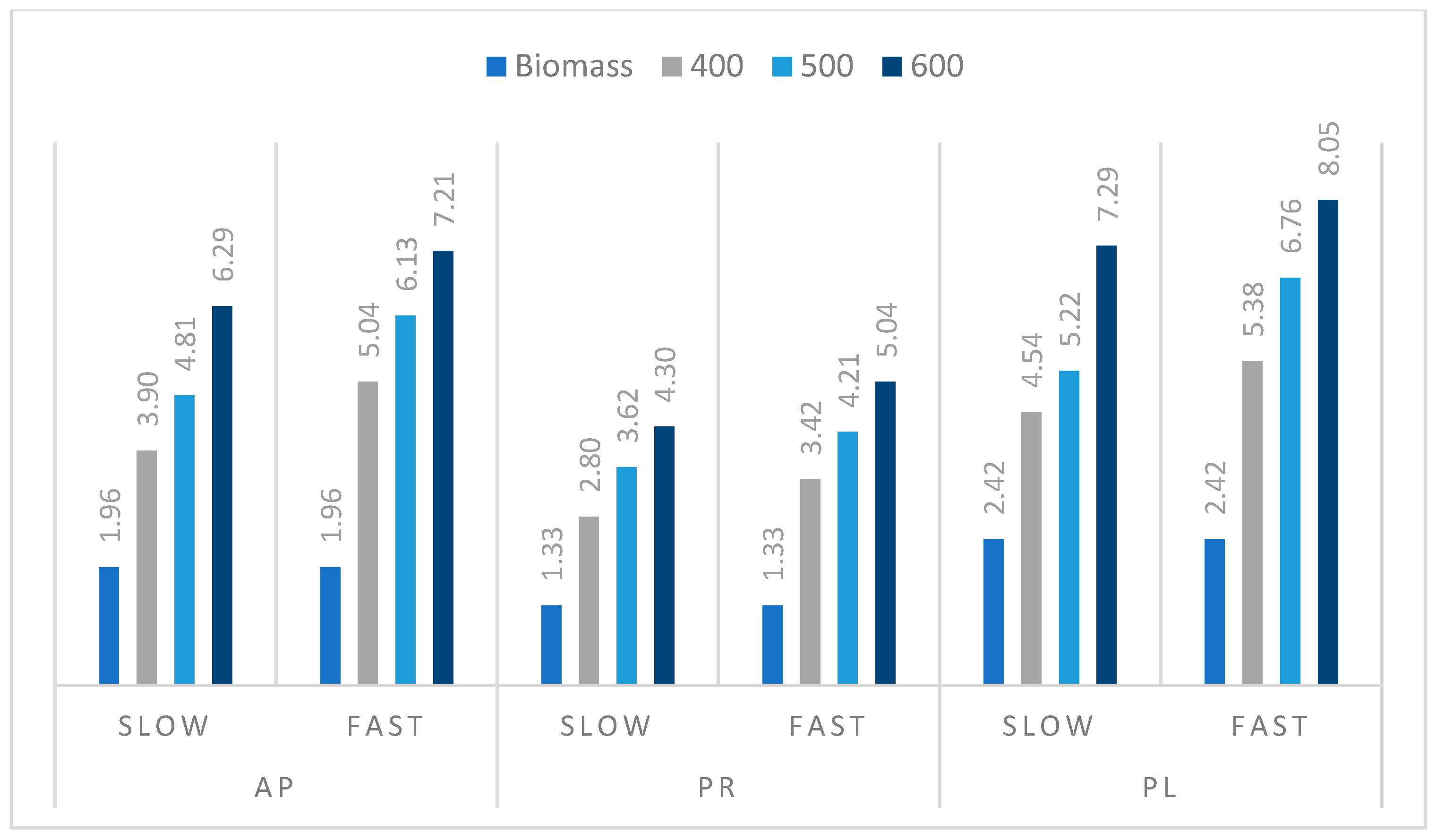
3. Results and Discussion
3.1. Characteristics of the Raw Materials
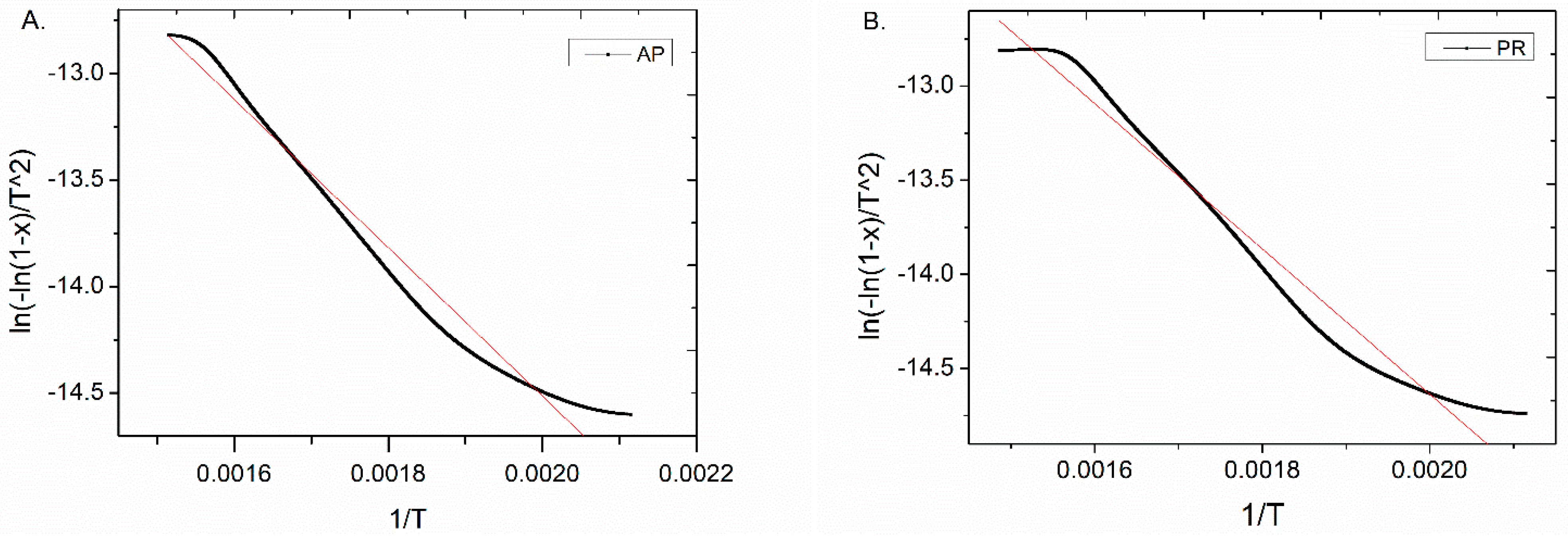
3.2. Pseudo-Activation Energy of the Pyrolysis
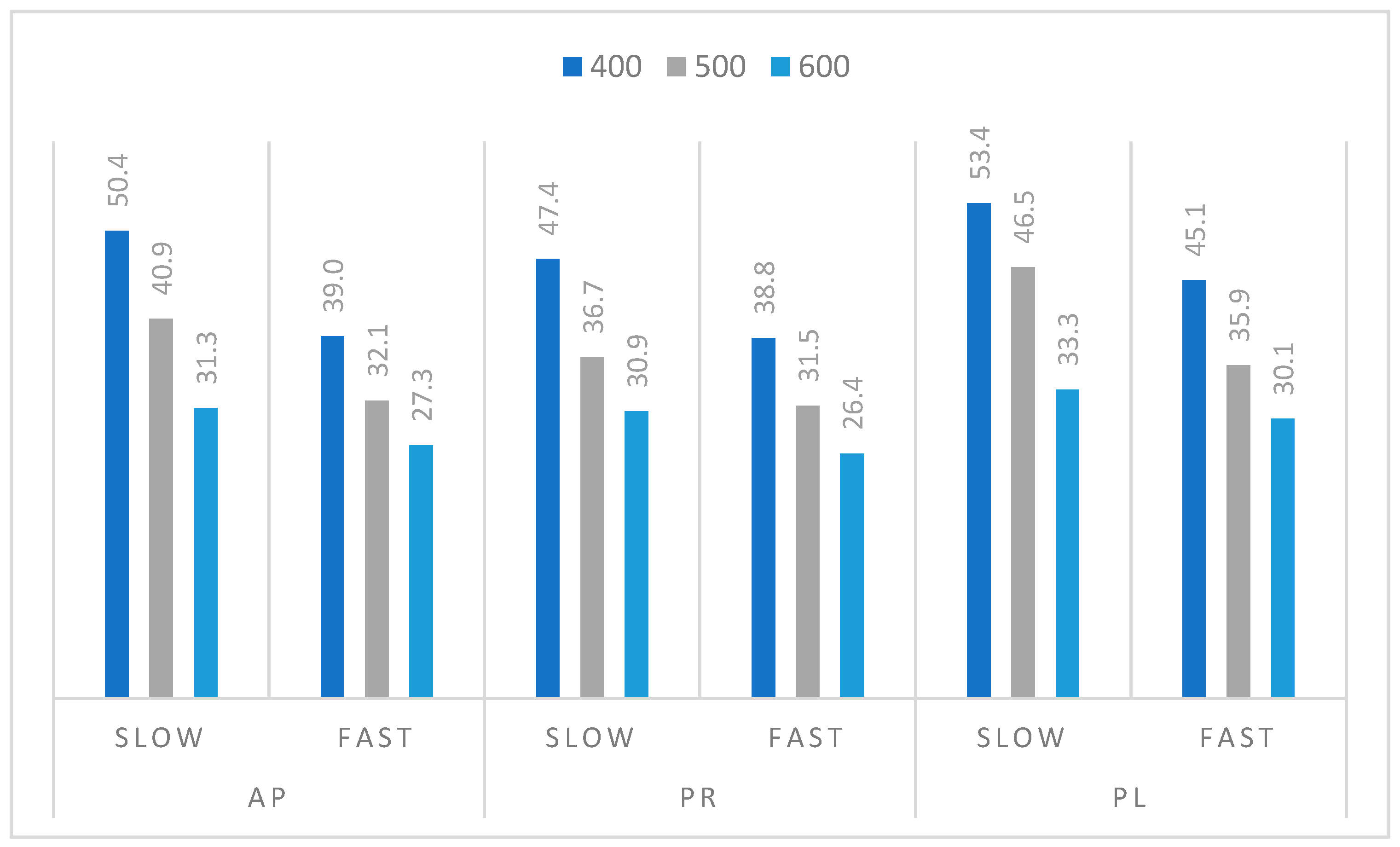
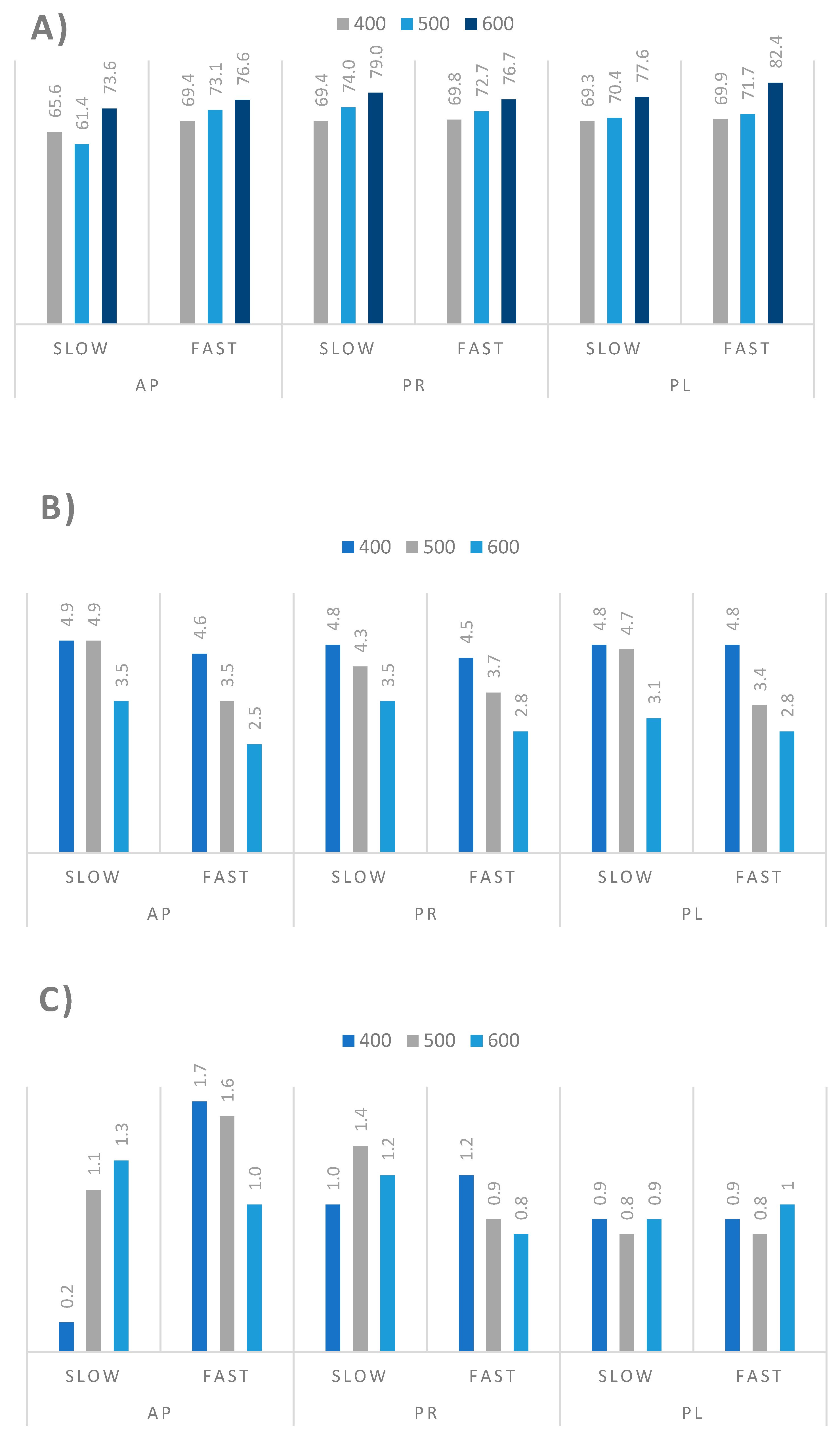
3.3. Pyrolysis Process of Orchard Residues
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Motghare, K.A.; Rathod, A.P.; Wasewar, K.L.; Labhsetwar, N.K. Comparative study of different waste biomass for energy application. Waste Manag. 2016, 47, 40–45. [Google Scholar] [CrossRef]
- Sommer, S.G.; Hamelin, L.; Olesen, J.E.; Montes, F.; Jia, W.; Chen, Q.; Triolo, J.M. Agricultural waste biomass. In Supply Chain Management for Sustainable Food Networks; Wiley: Hoboken, NJ, USA, 2015; pp. 67–106. ISBN 9781118937495. [Google Scholar]
- Ma, L.; Wang, T.; Liu, Q.; Zhang, X.; Ma, W.; Zhang, Q. A review of thermal-chemical conversion of lignocellulosic biomass in China. Biotechnol. Adv. 2012, 30, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Alvarez, J.; Amutio, M.; Hooshdaran, B.; Cortazar, M.; Haghshenasfard, M.; Hosseini, S.H.; Olazar, M. Kinetic modeling and experimental validation of biomass fast pyrolysis in a conical spouted bed reactor. Chem. Eng. J. 2019, 373, 677–686. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Aguado, R.; Artetxe, M.; Bilbao, J.; Olazar, M. Kinetic study of lignocellulosic biomass oxidative pyrolysis. Fuel 2012, 95, 305–311. [Google Scholar] [CrossRef]
- Database—Eurostat. Available online: https://ec.europa.eu/eurostat/web/agriculture/data/database (accessed on 4 August 2020).
- Where Do We Grow Our Fruit and Vegetables?—Product—Eurostat. Available online: https://ec.europa.eu/eurostat/en/web/products-eurostat-news/-/DDN-20191003-1 (accessed on 4 August 2020).
- Dyjakon, A.; Mudryk, K. Energetic Potential of Apple Orchards in Europe in Terms of Mechanized Harvesting of Pruning Residues; Springer: Cham, Germany, 2018; pp. 593–602. [Google Scholar]
- Velázquez-Martí, B.; Fernández-González, E.; López-Cortés, I.; Salazar-Hernández, D.M. Quantification of the residual biomass obtained from pruning of trees in Mediterranean olive groves. Biomass Bioenergy 2011, 35, 3208–3217. [Google Scholar] [CrossRef]
- Velázquez-Martí, B.; Fernández-González, E.; López-Cortés, I.; Salazar-Hernández, D.M. Quantification of the residual biomass obtained from pruning of trees in Mediterranean almond groves. Renew. Energy 2011, 36, 621–626. [Google Scholar] [CrossRef]
- Velázquez-Martí, B.; Fernández-González, E.; López-Cortés, I.; Salazar-Hernández, D.M. Quantification of the residual biomass obtained from pruning of vineyards in Mediterranean area. Biomass Bioenergy 2011, 35, 3453–3464. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Kean, C.; Nichols, D.; Embree, C. Orchard waste as a valuable bio-resource: A chemical composition analysis. Acta Hortic. 2007, 737, 17–23. [Google Scholar] [CrossRef]
- Picchi, G.; Lombardini, C.; Pari, L.; Spinelli, R. Physical and chemical characteristics of renewable fuel obtained from pruning residues. J. Clean. Prod. 2018, 171, 457–463. [Google Scholar] [CrossRef]
- Dyjakon, A. Harvesting and baling of pruned biomass in apple orchards for energy production. Energies 2018, 11, 1680. [Google Scholar] [CrossRef]
- Irawati, D.; Higeta, S.; Wedatama, S.; Ishiguri, F.; Yokota, S. Characterization of Branch Waste of Several Tropical Fruit Tree Species as Considerations for Bioenergy Resources. In Proceedings of the IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2020; Volume 449. [Google Scholar]
- Local Data Bank (Bank Danych Lokalnych, in Polish). Available online: https://bdl.stat.gov.pl/BDLARCH/dane/podgrup/tablica (accessed on 4 August 2020).
- Zhang, N.W.; Zhao, P.; Li, H.X.; Zhao, M.X.; Dong, C.X.; Xu, Y.C. Effects of compost made from pruned pear trees on the fruit, soil nutrients and microorganisms in pear orchards. Acta Hortic. 2018, 1217, 39–43. [Google Scholar] [CrossRef]
- Bonet-Martínez, E.; García-Cobo, P.; Pérez-Villarejo, L.; Castro, E.; Eliche-Quesada, D. Effect of Olive-Pine Bottom Ash on Properties of Geopolymers Based on Metakaolin. Materials 2020, 13, 901. [Google Scholar] [CrossRef]
- Hoffmann, V.; Jung, D.; Zimmermann, J.; Correa, C.R.; Elleuch, A.; Halouani, K.; Kruse, A. Conductive carbon materials from the hydrothermal carbonization of vineyard residues for the application in electrochemical double-layer capacitors (EDLCs) and direct carbon fuel cells (DCFCs). Materials 2019, 12, 1703. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S. Combustion behaviour of biomass fuels and their blends with lignite. Thermochim. Acta 2011, 526, 192–199. [Google Scholar] [CrossRef]
- Brand, M.A.; Jacinto, R.C. Apple pruning residues: Potential for burning in boiler systems and pellet production. Renew. Energy 2020, 152, 458–466. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S.; Kotronakis, M. Fluidized bed combustion of residues from oranges’ plantations and processing. Renew. Energy 2012, 44, 231–237. [Google Scholar] [CrossRef]
- San José, M.J.; Alvarez, S.; Peñas, F.J.; García, I. Thermal exploitation of fruit tree pruning wastes in a novel conical spouted bed combustor. Chem. Eng. J. 2014, 238, 227–233. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Ioannidou, O.; Antonakou, E.; Lappas, A. Experimental study of pyrolysis for potential energy, hydrogen and carbon material production from lignocellulosic biomass. Int. J. Hydrogen Energy 2008, 33, 2433–2444. [Google Scholar] [CrossRef]
- Ayala-Cortés, A.; Arancibia-Bulnes, C.A.; Villafán-Vidales, H.I.; Lobato-Peralta, D.R.; Martínez-Casillas, D.C.; Cuentas-Gallegos, A.K. Solar Pyrolysis of Agave and Tomato Pruning Wastes: Insights of the Effect of Pyrolysis Operation Parameters on the Physicochemical Properties of Biochar. In Proceedings of the AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2019; Volume 2126. [Google Scholar]
- Bartoli, M.; Rosi, L.; Giovannelli, A.; Frediani, P.; Frediani, M. Characterization of bio-oil and bio-char produced by low-temperature microwave-assisted pyrolysis of olive pruning residue using various absorbers. Waste Manag. Res. 2020, 38, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Calahorro, C.V.; Serrano, V.G.; Alvaro, J.H.; García, A.B. The use of waste matter after olive grove pruning for the preparation of charcoal. The influence of the type of matter, particle size and pyrolysis temperature. Bioresour. Technol. 1992, 40, 17–22. [Google Scholar] [CrossRef]
- Pérez, A.; Martín-Lara, M.A.; Gálvez-Pérez, A.; Calero, M.; Ronda, A. Kinetic analysis of pyrolysis and combustion of the olive tree pruning by chemical fractionation. Bioresour. Technol. 2018, 249, 557–566. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Blázquez, G.; Zamora, M.C.; Calero, M. Kinetic modelling of torrefaction of olive tree pruning. Appl. Therm. Eng. 2017, 113, 1410–1418. [Google Scholar] [CrossRef]
- Iáñez-Rodríguez, I.; Martín-Lara, M.Á.; Blázquez, G.; Osegueda, Ó.; Calero, M. Thermal analysis of olive tree pruning and the by-products obtained by its gasification and pyrolysis: The effect of some heavy metals on their devolatilization behavior. J. Energy Chem. 2019, 32, 105–117. [Google Scholar] [CrossRef]
- Dunnigan, L.; Morton, B.J.; Ashman, P.J.; Zhang, X.; Kwong, C.W. Emission characteristics of a pyrolysis-combustion system for the co-production of biochar and bioenergy from agricultural wastes. Waste Manag. 2018, 77, 59–66. [Google Scholar] [CrossRef]
- Rantuch, P.; Ondruška, J.; Wachter, I. A comparison of thermal decomposition of vineyard pruning waste in the flow of air and nitrogen. Int. J. Glob. Warm. 2017, 13, 138–155. [Google Scholar] [CrossRef]
- Uras, Ü.; Carrier, M.; Hardie, A.G.; Knoetze, J.H. Physico-chemical characterization of biochars from vacuum pyrolysis of South African agricultural wastes for application as soil amendments. J. Anal. Appl. Pyrolysis 2012, 98, 207–213. [Google Scholar] [CrossRef]
- Pituello, C.; Francioso, O.; Simonetti, G.; Pisi, A.; Torreggiani, A.; Berti, A.; Morari, F. Characterization of chemical–physical, structural and morphological properties of biochars from biowastes produced at different temperatures. J. Soils Sediments 2015, 15, 792–804. [Google Scholar] [CrossRef]
- PN-139 EN 15403:2011 Solid Recovered Fuels—Determination of Ash Content; Polish Committee for Standardization: Warsaw, Poland, 2011.
- CEN/TS 15414-1:2010 Solid Recovered Fuels—Determination of Moisture Content Using the Oven Dry Method—Part 1: Determination of Total Moisture by a Reference Method; Polish Committee for Standardization: Warsaw, Poland, 2010.
- PN-EN 15414-3:2011 Solid Recovered Fuels—Determination of Moisture Content by Drying Oven Method—Part 3: Moisture in General Analytical Sample; Polish Committee for Standardization: Warsaw, Poland, 2011.
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Polesek-Karczewska, S.; Kardaś, D. Prediction of thermal behavior of pyrolyzed wet biomass by means of model with inner wood structure. J. Therm. Sci. 2015, 24, 82–89. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Kazimierski, P.; Hercel, P.; Januszewicz, K.; Kardaś, D. Pre-Treatment of Furniture Waste for Smokeless Charcoal Production. Materials 2020, 13, 3188. [Google Scholar] [CrossRef]
- Kongkaew, N.; Pruksakit, W.; Patumsawad, S. Thermogravimetric Kinetic Analysis of the Pyrolysis of Rice Straw. In Proceedings of the International Conference on Alternative Energy in Developing Countries and Emerging Economies, Bangkok, Thailand, 28–29 May 2015. [Google Scholar]
- Popescu, F.; Mahu, R.; Ion, I.V.; Rusu, E. A Mathematical Model of Biomass Combustion Physical and Chemical Processes. Energies 2020, 13, 6232. [Google Scholar] [CrossRef]
- Pawel, K.; Dariusz, K. Influence of Temperature on Composition of Wood Pyrolysis Products. Drv. Ind. 2017, 68, 307–313. [Google Scholar] [CrossRef][Green Version]
- Kardaś, D.; Hercel, P.; Polesek-Karczewska, S.; Wardach-Świȩcicka, I. A novel insight into biomass pyrolysis – The process analysis by identifying timescales of heat diffusion, heating rate and reaction rate. Energy 2019, 189, 116159. [Google Scholar] [CrossRef]
- Januszewicz, K.; Kazimierski, P.; Klein, M.; Kardaś, D.; Łuczak, J. Activated carbon produced by pyrolysis of waste wood and straw for potential wastewater adsorption. Materials 2020, 13, 2047. [Google Scholar] [CrossRef]
- Zhao, L.; Giannis, A.; Lam, W.Y.; Lin, S.X.; Yin, K.; Yuan, G.A.; Wang, J.Y. Characterization of Singapore RDF resources and analysis of their heating value. Sustain. Environ. Res. 2016, 26, 51–54. [Google Scholar] [CrossRef]
- Pohl, M.; Gebauer, K.; Beckmann, M. Characterisation of Refuse Derived Fuels in view of the Fuel Technical Properties. In Proceedings of the 8th European Conference on Industrial Furnaces and Boilers (INFUB-8), Vilamoura-Algarve, Portugal, 25–28 March 2008; Volume 8, pp. 1–11. [Google Scholar]
- Islam, M.R.; Islam, M.N.; Mustafi, N.N.; Rahim, M.A.; Haniu, H. Thermal recycling of solid tire wastes for alternative liquid fuel: The first commercial step in Bangladesh. In Proceedings of the 5th BSME International Conference on Thermal Engineering, Dhaka, Bangladesh, 21–23 December 2012. [Google Scholar]
- Colom, X.; Marín-Genescà, M.; Mujal, R.; Formela, K.; Cañavate, J. Structural and physico-mechanical properties of natural rubber/GTR composites devulcanized by microwaves: Influence of GTR source and irradiation time. J. Compos. Mater. 2018, 52, 3099–3108. [Google Scholar] [CrossRef]
- Witanowski, Ł.; Klonowicz, P.; Lampart, P.; Suchocki, T.; Jędrzejewski, Ł.; Zaniewski, D.; Klimaszewski, P. Optimization of an axial turbine for a small scale ORC waste heat recovery system. Energy 2020, 205, 118059. [Google Scholar] [CrossRef]
- Irmak, S. Biomass as Raw Material for Production of High-Value Products. In Biomass Volume Estimation and Valorization for Energy; InTech: London, UK, 2017. [Google Scholar]
- Suchocki, T.; Witanowski, Ł.; Lampart, P.; Kazimierski, P.; Januszewicz, K.; Gawron, B. Experimental investigation of performance and emission characteristics of a miniature gas turbine supplied by blends of kerosene and waste tyre pyrolysis oil. Energy 2021, 215, 119125. [Google Scholar] [CrossRef]
- Claassen, P.A.M.; Lopez Contreras, A.M.; Sijtsma, L.; Weusthuis, R.A.; Van Lier, J.B.; Van Niel, E.W.J.; Stams, A.J.M.; De Vries, S.S. Utilisation of biomass for the supply of energy carriers. Appl. Microbiol. Biotechnol. 1999, 52, 741–755. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]





| AP | PR | PL | |
|---|---|---|---|
| Moisture, (%) | 44.86 | 40.91 | 41.76 |
| HHV 1, (MJ/kg) | 22.39 | 23.60 | 24.90 |
| Ash, (%) | 1.96 | 1.33 | 2.42 |
| Sample | Temp. Range (°C) | E (kJ/mol) | x | R2 |
|---|---|---|---|---|
| AP | 193–387 | 28.97 | 0.097–0.690 | 0.9722 |
| PR | 199–399 | 32.08 | 0.085–0.709 | 0.9738 |
| PL | 209–391 | 37.00 | 0.078–0.713 | 0.9718 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierski, P.; Hercel, P.; Suchocki, T.; Smoliński, J.; Pladzyk, A.; Kardaś, D.; Łuczak, J.; Januszewicz, K. Pyrolysis of Pruning Residues from Various Types of Orchards and Pretreatment for Energetic Use of Biochar. Materials 2021, 14, 2969. https://doi.org/10.3390/ma14112969
Kazimierski P, Hercel P, Suchocki T, Smoliński J, Pladzyk A, Kardaś D, Łuczak J, Januszewicz K. Pyrolysis of Pruning Residues from Various Types of Orchards and Pretreatment for Energetic Use of Biochar. Materials. 2021; 14(11):2969. https://doi.org/10.3390/ma14112969
Chicago/Turabian StyleKazimierski, Paweł, Paulina Hercel, Tomasz Suchocki, Jakub Smoliński, Agnieszka Pladzyk, Dariusz Kardaś, Justyna Łuczak, and Katarzyna Januszewicz. 2021. "Pyrolysis of Pruning Residues from Various Types of Orchards and Pretreatment for Energetic Use of Biochar" Materials 14, no. 11: 2969. https://doi.org/10.3390/ma14112969
APA StyleKazimierski, P., Hercel, P., Suchocki, T., Smoliński, J., Pladzyk, A., Kardaś, D., Łuczak, J., & Januszewicz, K. (2021). Pyrolysis of Pruning Residues from Various Types of Orchards and Pretreatment for Energetic Use of Biochar. Materials, 14(11), 2969. https://doi.org/10.3390/ma14112969










