Bottom Ash Modification via Sintering Process for Its Use as a Potential Heavy Metal Adsorbent: Sorption Kinetics and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Sintering Process
2.2. Sorbent Property Analysis
2.3. Heavy Metal Sorption Kinetics and Isotherm Experiment
2.4. Statistical Analysis
3. Results and Discussion
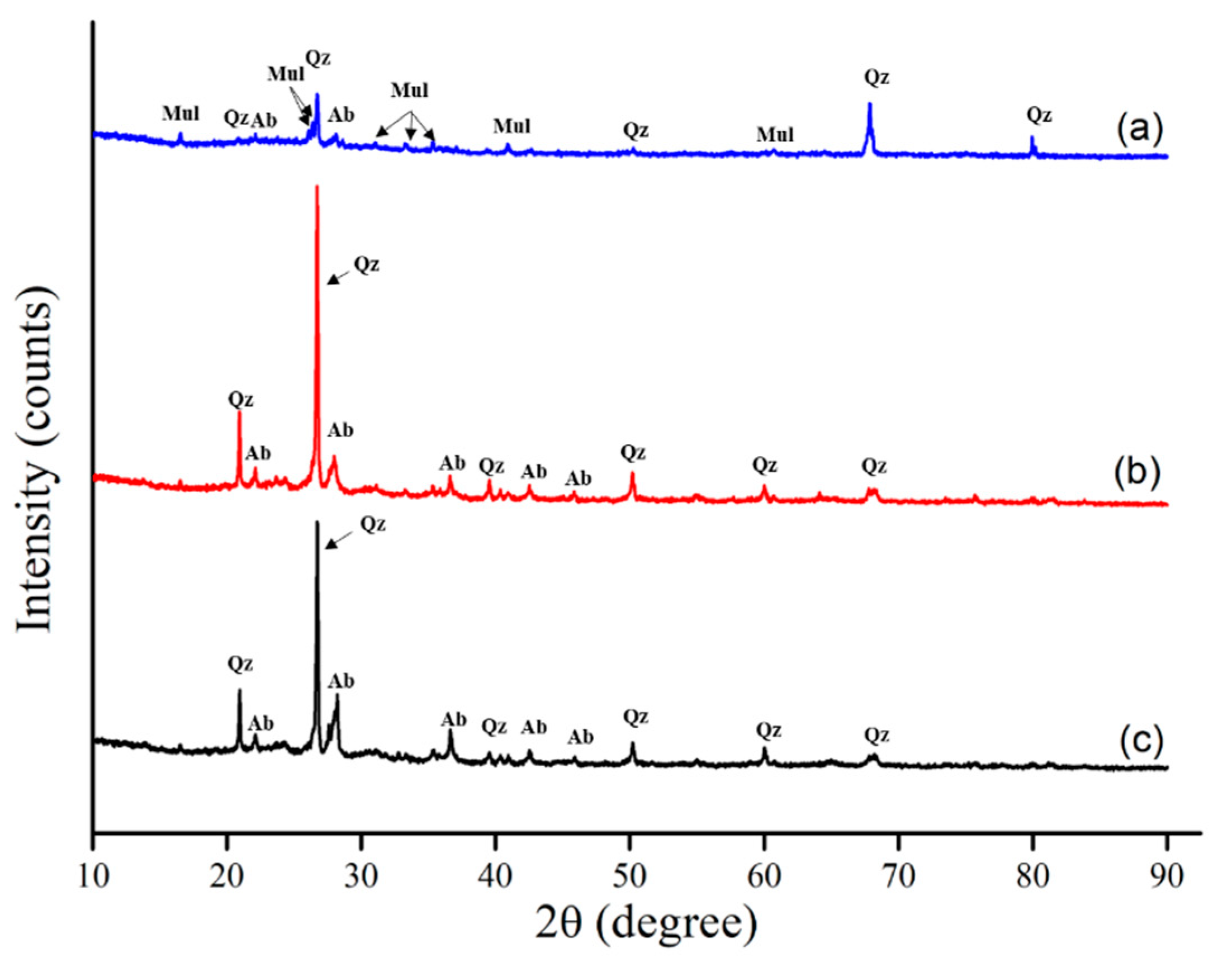
3.1. Physicochemical Properties of Each Sorbent
3.2. Sorption Efficiency of Absorbent for Cd and Pb Removal
3.3. Sorption Isotherms of Cd and Pb
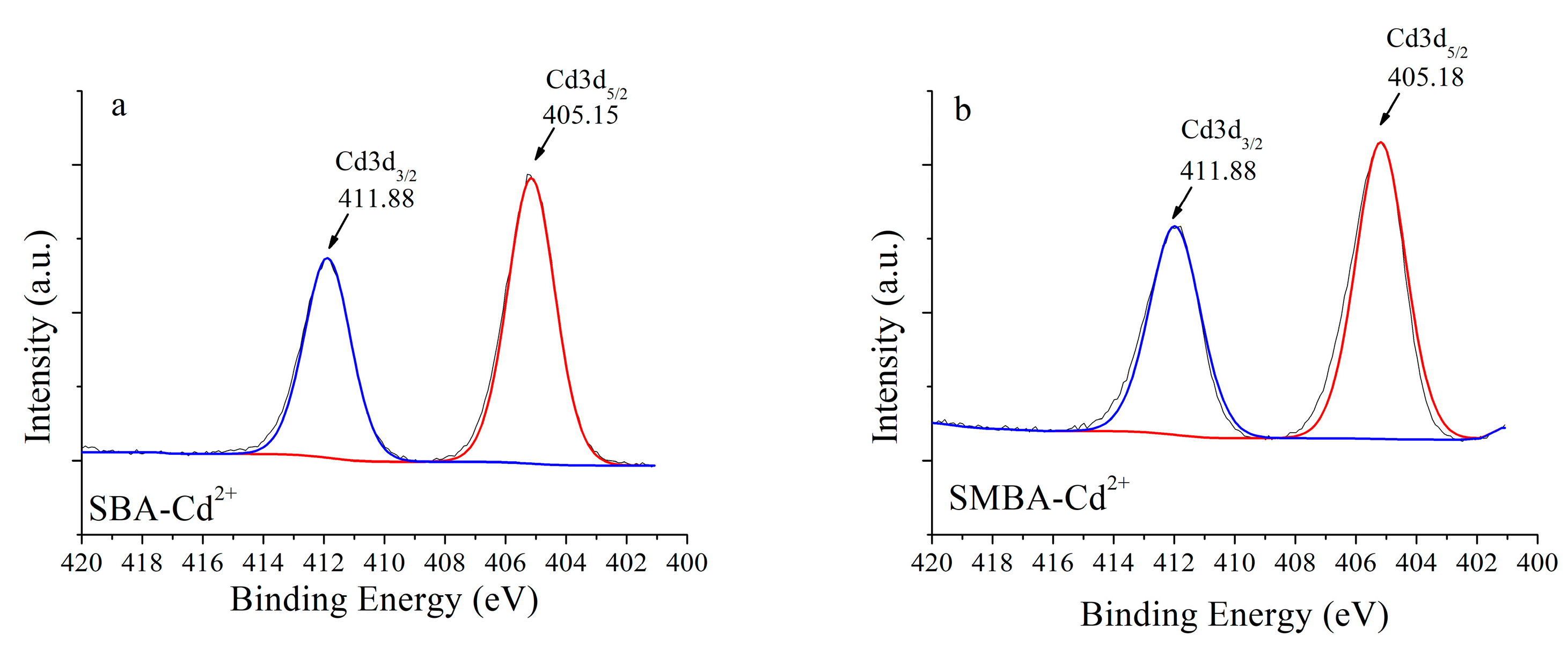
3.4. Sorption Mechanism Evaluation Using XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aljahdali, M.O.; Alhassan, A.B. Ecological risk assessment of heavy metal contamination in mangrove habitats, using biochemical markers and pollution indices: A case study of Avicennia marina L. in the Rabigh lagoon, Red Sea. Saudi J. Biol. Sci. 2020, 27, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, P.; Palma, P.; Goncalves, A.P.; Baiao, N.; Fernandes, R.M.; de Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A.C. Assessment of chemical, biochemical and ecotoxicological aspects in a mine soil amended with sludge of either urban or industrial origin. Chemosphere 2008, 72, 1774–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Critto, A.; Torresan, S.; Semenzin, E.; Giove, S.; Mesman, M.; Schouten, A.J.; Rutgers, M.; Marcomini, A. Development of a site-specific ecological risk assessment for contaminated sites: Part I. A multi-criteria based system for the selection of ecotoxicological tests and ecological observations. Sci. Total Environ. 2007, 379, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Ju, L.; Ling, X.; Liu, C.; Wei, X.; Qiu, H.; Tang, Y.; Morel, J.L.; Qiu, R.; Li, C.; et al. Simultaneous attenuation of phytoaccumulation of Cd and As in soil treated with inorganic and organic amendments. Environ. Pollut. 2019, 250, 464–474. [Google Scholar] [CrossRef]
- Yao, A.; Liu, Y.; Luo, X.; Liu, C.; Tang, Y.; Wang, S.; Huang, X.; Qiu, R. Mediation effects of different sulfur forms on solubility, uptake and accumulation of Cd in soil-paddy rice system induced by organic carbon and liming. Environ. Pollut. 2021, 279, 116862. [Google Scholar] [CrossRef]
- Asgari Lajayer, B.; Najafi, N.; Moghiseh, E.; Mosaferi, M.; Hadian, J. Micronutrient and heavy metal concentrations in basil plant cultivated on irradiated and non-irradiated sewage sludge- treated soil and evaluation of human health risk. Regul. Toxicol. Pharmacol. 2019, 104, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Pan, G.; Li, L.; Bian, R.; Liu, X.; Yan, J.; Quan, G.; Ding, C.; Chen, T.; Liu, Y.; et al. Continuous immobilization of cadmium and lead in biochar amended contaminated paddy soil: A five-year field experiment. Ecol. Eng. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Dedeke, G.A.; Iwuchukwu, P.O.; Aladesida, A.A.; Afolabi, T.A.; Ayanda, I.O. Impact of heavy metal bioaccumulation on antioxidant activities and DNA profile in two earthworm species and freshwater prawn from Ogun River. Sci. Total Environ. 2018, 624, 576–585. [Google Scholar] [CrossRef]
- Kim, D.-M.; Choi, M.-S.; Yun, S.-T.; Yoon, S.; Lee, J.-S. Spatial patterns of Zn, Cd, and Pb isotopic compositions of ground and surface water in mine areas of South Korea reflecting isotopic fractionation during metal attenuation. Sci. Total Environ. 2021, 779, 146453. [Google Scholar] [CrossRef]
- Mignardi, S.; Corami, A.; Ferrini, V. Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 2012, 86, 354–360. [Google Scholar] [CrossRef]
- Cao, D.-Z.; Selic, E.; Herbell, J.-D. Utilization of fly ash from coal-fired power plants in China. J. Zhejiang Univ. Sci. A 2008, 9, 681–687. [Google Scholar] [CrossRef]
- Qin, W.; Han, D.; Song, X.; Liu, S. Sources and migration of heavy metals in a karst water system under the threats of an abandoned Pb-Zn mine, Southwest China. Environ. Pollut. 2021, 277, 116774. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, A.; Lázaro, I.; Razo, I.; Briones-Gallardo, R. Geochemical and mineralogical characterization of stream sediments impacted by mine wastes containing arsenic, cadmium and lead in North-Central Mexico. J. Geochem. Explor. 2021, 221, 106707. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.S.; Choi, Y.J.; Kim, J.G. In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere 2009, 77, 1069–1075. [Google Scholar] [CrossRef]
- Agnello, A.C.; Bagard, M.; van Hullebusch, E.D.; Esposito, G.; Huguenot, D. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Sci. Total Environ. 2016, 563–564, 693–703. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wang, X.; Yang, X.; Cui, Z. Bioaugmentation-assisted phytoremediation of lead and salinity co-contaminated soil by Suaeda salsa and Trichoderma asperellum. Chemosphere 2019, 224, 716–725. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Ling, T.-C.; Lai, S.H. Current development of geopolymer as alternative adsorbent for heavy metal removal. Environ. Technol. Innov. 2020, 18, 100684. [Google Scholar] [CrossRef]
- Zhu, G.; Guo, Q.; Yang, J.; Zhang, H.; Wei, R.; Wang, C.; Peters, M.; Zhou, X.; Yang, J. Washing out heavy metals from contaminated soils from an iron and steel smelting site. Front. Environ. Sci. Eng. 2014, 9, 634–641. [Google Scholar] [CrossRef]
- Alghanmi, S.I.; Al Sulami, A.F.; El-Zayat, T.A.; Alhogbi, B.G.; Abdel Salam, M. Acid leaching of heavy metals from contaminated soil collected from Jeddah, Saudi Arabia: Kinetic and thermodynamics studies. Int. Soil Water Conserv. Res. 2015, 3, 196–208. [Google Scholar] [CrossRef] [Green Version]
- Kaurin, A.; Gluhar, S.; Tilikj, N.; Lestan, D. Soil washing with biodegradable chelating agents and EDTA: Effect on soil properties and plant growth. Chemosphere 2020, 260, 127673. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhang, S.; Zhong, Q.; Wang, G.; Pan, X.; Xu, X.; Zhou, W.; Li, T.; Luo, L.; Zhang, Y. Soil washing remediation of heavy metal from contaminated soil with EDTMP and PAA: Properties, optimization, and risk assessment. J. Hazard. Mater. 2020, 381, 120997. [Google Scholar] [CrossRef] [PubMed]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; Nyambe, I.; et al. Detoxification of lead-bearing zinc plant leach residues from Kabwe, Zambia by coupled extraction-cementation method. J. Environ. Chem. Eng. 2020, 8, 104197. [Google Scholar] [CrossRef]
- Abd Aziz, A.; Lee, B.T.; Han, H.J.; Kim, K.W. Assessment of the stabilization of heavy metal contaminants in soils using chemical leaching and an earthworm bioassay. Environ. Geochem. Health 2019, 41, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Busto, Y.; Cabrera, X.; Tack, F.M.; Verloo, M.G. Potential of thermal treatment for decontamination of mercury containing wastes from chlor-alkali industry. J. Hazard. Mater. 2011, 186, 114–118. [Google Scholar] [CrossRef]
- He, H.; Tam, N.F.Y.; Yao, A.; Qiu, R.; Li, W.C.; Ye, Z. Growth and Cd uptake by rice (Oryza sativa) in acidic and Cd-contaminated paddy soils amended with steel slag. Chemosphere 2017, 189, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.C.; Lee, C.; Lee, J.S.; Baek, K. Simultaneous application of chemical oxidation and extraction processes is effective at remediating soil Co-contaminated with petroleum and heavy metals. J. Environ. Manag. 2017, 186, 314–319. [Google Scholar] [CrossRef]
- Picard, F.; Chaouki, J. NaClO/NaOH soil oxidation for the remediation of two real heavy-metal and petroleum contaminated soils. J. Environ. Chem. Eng. 2017, 5, 2691–2698. [Google Scholar] [CrossRef]
- Bian, R.; Chen, D.; Liu, X.; Cui, L.; Li, L.; Pan, G.; Xie, D.; Zheng, J.; Zhang, X.; Zheng, J.; et al. Biochar soil amendment as a solution to prevent Cd-tainted rice from China: Results from a cross-site field experiment. Ecol. Eng. 2013, 58, 378–383. [Google Scholar] [CrossRef]
- Chaturvedi, P.K.; Seth, C.S.; Misra, V. Sorption kinetics and leachability of heavy metal from the contaminated soil amended with immobilizing agent (humus soil and hydroxyapatite). Chemosphere 2006, 64, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Fan, Y.; Fang, G.; Zhang, H.; Su, B.; Zhou, J. Leachability, availability and bioaccessibility of Cu and Cd in a contaminated soil treated with apatite, lime and charcoal: A five-year field experiment. Ecotoxicol. Environ. Saf. 2016, 134 P1, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Huda, B.N.; Wahyuni, E.T.; Mudasir, M. Eco-friendly immobilization of dithizone on coal bottom ash for the adsorption of lead(II) ion from water. Results Eng. 2021, 10, 100221. [Google Scholar] [CrossRef]
- Xu, D.; Ji, P.; Wang, L.; Zhao, X.; Hu, X.; Huang, X.; Zhao, H.; Liu, F. Effect of modified fly ash on environmental safety of two soils contaminated with cadmium and lead. Ecotoxicol. Environ. Saf. 2021, 215, 112175. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogata, F.; Nakamura, T.; Kawasaki, N. Synthesis of novel zeolites produced from fly ash by hydrothermal treatment in alkaline solution and its evaluation as an adsorbent for heavy metal removal. J. Environ. Chem. Eng. 2020, 8, 103687. [Google Scholar] [CrossRef]
- Guo, R.; Yao, W.; Ma, H.; Yuan, J. Two-step hydrothermal synthesis of nano-kaolinite from fly ash: Thermodynamics and mechanism. J. Clean. Prod. 2020, 271, 122567. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Geopolymers produced from drinking water treatment residue and bottom ash for the immobilization of heavy metals. Chemosphere 2019, 225, 579–587. [Google Scholar] [CrossRef]
- Chiang, Y.W.; Ghyselbrecht, K.; Santos, R.M.; Meesschaert, B.; Martens, J.A. Synthesis of zeolitic-type adsorbent material from municipal solid waste incinerator bottom ash and its application in heavy metal adsorption. Catal. Today 2012, 190, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Giro-Paloma, J.; Mañosa, J.; Maldonado-Alameda, A.; Quina, M.J.; Chimenos, J.M. Rapid sintering of weathered municipal solid waste incinerator bottom ash and rice husk for lightweight aggregate manufacturing and product properties. J. Clean. Prod. 2019, 232, 713–721. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Zhao, X.; Gao, X.; Zheng, Y.; Zuo, H.; Wei, Z. Roles of different humin and heavy-metal resistant bacteria from composting on heavy metal removal. Bioresour. Technol. 2020, 296, 122375. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef]
- Zhou, H.; Bhattarai, R.; Li, Y.; Li, S.; Fan, Y. Utilization of coal fly and bottom ash pellet for phosphorus adsorption: Sustainable management and evaluation. Resour. Conserv. Recycl. 2019, 149, 372–380. [Google Scholar] [CrossRef]
- Luo, H.; He, D.; Zhu, W.; Wu, Y.; Chen, Z.; Yang, E.H. Humic acid-induced formation of tobermorite upon hydrothermal treatment with municipal solid waste incineration bottom ash and its application for efficient removal of Cu(II) ions. Waste Manag. 2019, 84, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wu, Y.; Zhao, A.; Kumar, A.; Liu, Y.; Cao, B.; Yang, E.-H. Hydrothermally synthesized porous materials from municipal solid waste incineration bottom ash and their interfacial interactions with chloroaromatic compounds. J. Clean. Prod. 2017, 162, 411–419. [Google Scholar] [CrossRef]
- Pena, R.; Guerrero, A.; Goni, S. Hydrothermal treatment of bottom ash from the incineration of municipal solid waste: Retention of Cs(I), Cd(II), Pb(II) and Cr(III). J. Hazard. Mater. 2006, 129, 151–157. [Google Scholar] [CrossRef]
- Bethanis, S.; Cheeseman, C.R.; Sollars, C.J. Properties and microstructure of sintered incinerator bottom ash. Ceram. Int. 2002, 28, 881–886. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. J. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Chi, T.; Zuo, J.; Liu, F. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Sharma, A.S.; Biswas, K.; Basu, B. Fine scale characterization of surface/subsurface and nanosized debris particles on worn Cu–10% Pb nanocomposites. J. Nanopart. Res. 2013, 15, 1675. [Google Scholar] [CrossRef]
- Guo, B.; Pan, D.a.; Liu, B.; Volinsky, A.A.; Fincan, M.; Du, J.; Zhang, S. Immobilization mechanism of Pb in fly ash-based geopolymer. Constr. Build. Mater. 2017, 134, 123–130. [Google Scholar] [CrossRef]
- Zheng, X.; Lei, H.; Yang, G.; Ke, W.; Chen, Z.; Chen, C.; Ma, J.; Guo, Q.; Yao, F.; Zhang, Q.; et al. Enhancing efficiency and stability of perovskite solar cells via a high mobility p-type PbS buffer layer. Nano Energy 2017, 38, 1–11. [Google Scholar] [CrossRef]
- Liang, L.; Li, X.; Guo, Y.; Lin, Z.; Su, X.; Liu, B. The removal of heavy metal cations by sulfidated nanoscale zero-valent iron (S-nZVI): The reaction mechanisms and the role of sulfur. J. Hazard. Mater. 2021, 404, 124057. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.; Lin, N.; Ou, C.; Wang, X.; Zhang, L.; Jiang, F. Removal of heavy metals using a novel sulfidogenic AMD treatment system with sulfur reduction: Configuration, performance, critical parameters and economic analysis. Environ. Int. 2020, 136, 105457. [Google Scholar] [CrossRef]




| Heavy Metals | C | N | pH | EC | Surface Area |
|---|---|---|---|---|---|
| % | % | dS/m | m2/g | ||
| BA | 0.30 ± 0.00 c | N.D | 8.02 ± 0.61 ab | 0.19 ± 0.05 c | 0.0002 |
| SBA | 4.51 ± 0.04 a | 0.05 ± 0.00 b | 8.68 ± 0.11 a | 1.29 ± 0.03 b | 7.8477 |
| SMBA | 3.69 ± 0.00 b | 0.28 ± 0.00 a | 6.79 ± 0.02 b | 44.7 ± 1.58 a | 0.3744 |
| Species | Sorbent | 1st Order | 2nd Order | ||||
|---|---|---|---|---|---|---|---|
| qm1 (mg/g) | k1 (1/min) | R2 | qm2 (mg/g) | k2 (1/min) | R2 | ||
| Cd | BA | 0.005 | 0.0009 | 0.71 | 0.009 | 8.549 | 0.99 |
| SBA | 0.010 | 0.0025 | 0.93 | 0.020 | 3.158 | 0.99 | |
| SMBA | 0.002 | 0.0014 | 0.69 | 0.012 | 6.616 | 0.99 | |
| Pb | BA | 0.166 | 0.0025 | 0.79 | 0.319 | 0.116 | 0.99 |
| SBA | 0.646 | 0.0035 | 0.95 | 1.094 | 0.041 | 0.99 | |
| SMBA | 0.219 | 0.0028 | 0.81 | 0.914 | 0.217 | 0.99 | |
| Heavy Metals | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qmax | b | R2 | K | 1/n | R2 | ||
| mg/g | L/mg | mg/g | |||||
| Cd | BA | - | - | - | - | - | - |
| SBA | 0.17 | 0.03 | 0.91 | 0.50 | 0.05 | 0.60 | |
| SMBA | 0.31 | 0.17 | 0.93 | 0.26 | 0.33 | 0.64 | |
| Pb | BA | 0.13 | 0.14 | 0.99 | 0.02 | 0.57 | 0.62 |
| SBA | 1.93 | 0.17 | 0.99 | 0.57 | 0.27 | 0.72 | |
| SMBA | 5.30 | 0.01 | 0.64 | 0.02 | 0.91 | 0.93 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.-K.; Kim, J.-W.; Kim, H.-S.; Lee, S.-P.; Yang, J.-E.; Kim, S.-C. Bottom Ash Modification via Sintering Process for Its Use as a Potential Heavy Metal Adsorbent: Sorption Kinetics and Mechanism. Materials 2021, 14, 3060. https://doi.org/10.3390/ma14113060
Hong Y-K, Kim J-W, Kim H-S, Lee S-P, Yang J-E, Kim S-C. Bottom Ash Modification via Sintering Process for Its Use as a Potential Heavy Metal Adsorbent: Sorption Kinetics and Mechanism. Materials. 2021; 14(11):3060. https://doi.org/10.3390/ma14113060
Chicago/Turabian StyleHong, Young-Kyu, Jin-Wook Kim, Hyuck-Soo Kim, Sang-Phil Lee, Jae-E. Yang, and Sung-Chul Kim. 2021. "Bottom Ash Modification via Sintering Process for Its Use as a Potential Heavy Metal Adsorbent: Sorption Kinetics and Mechanism" Materials 14, no. 11: 3060. https://doi.org/10.3390/ma14113060





