Virus Inactivation in Water Using Laser-Induced Graphene Filters
Abstract
1. Introduction
2. Materials and Methods
2.1. LIG Filter Fabrication
2.2. Antiviral and Antimicrobial Activity of LIG Filters
2.2.1. Plaque Assay of Vaccinia Virus-Infected Vero Cells
2.2.2. Negative Staining Transmission Electron Microscopy
2.2.3. H2O2 and Free Chlorine Generation Test
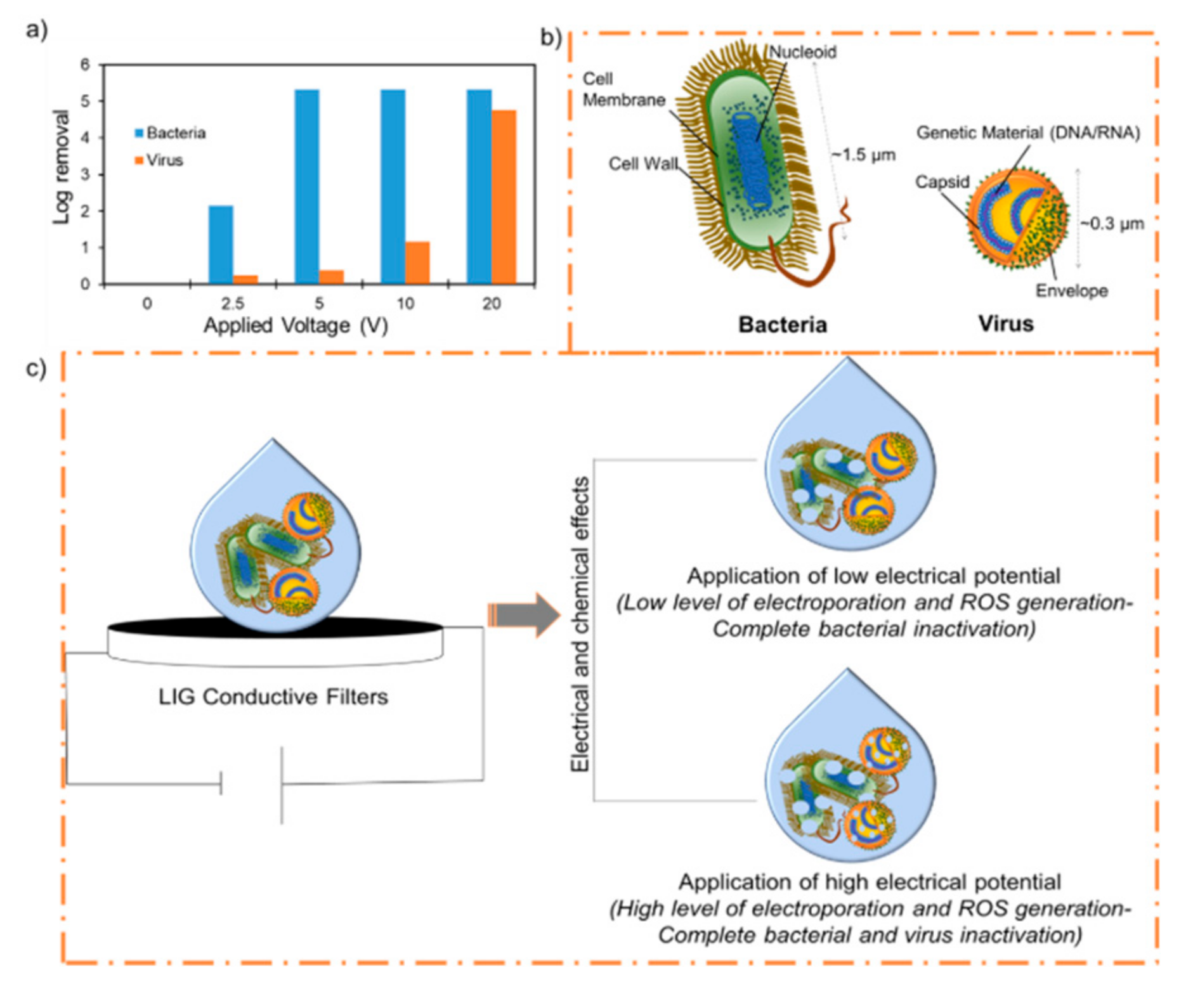
3. Results and Discussion
3.1. Antiviral Activity of the LIG Filters
3.2. H2O2 and Free Chlorine Generation
3.3. Current–Voltage Relationships Present in LIG-PES Filters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Supporting Information File
Conflicts of Interest
References
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef]
- Webby, R.; Hoffmann, E.; Webster, R. Molecular constraints to interspecies transmission of viral pathogens. Nat. Med. 2004, 10, S77–S81. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; 1st Addendum; World Health Organization: Geneva, Switzerland, 2017; pp. 1–4. [Google Scholar]
- Cannon, M.J.; Hyde, T.B.; Schmid, D.S. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev. Med. Virol. 2011, 21, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Gall, A.M.; Mariñas, B.J.; Lu, Y.; Shisler, J.L. Waterborne Viruses: A Barrier to Safe Drinking Water. PLoS Pathog. 2015, 11, e1004867. [Google Scholar] [CrossRef]
- Bogler, A.; Packman, A.; Furman, A.; Gross, A.; Kushmaro, A.; Ronen, A.; Dagot, C.; Hill, C.; Vaizel-Ohayon, D.; Morgenroth, E.; et al. Rethinking wastewater risks and monitoring in light of the COVID-19 pandemic. Nat. Sustain. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Naddeo, V.; Liu, H. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): What is its fate in urban water cycle and how can the water research community respond? Environ. Sci. Water Res. Technol. 2020, 6, 1213–1216. [Google Scholar] [CrossRef]
- Wigginton, K.; Ye, Y.; Ellenberg, R.M. Emerging investigators series: The source and fate of pandemic viruses in the urban water cycle. Environ. Sci. Water Res. Technol. 2015, 1, 735–746. [Google Scholar] [CrossRef]
- Qiu, Y.; E Lee, B.; Neumann, N.F.; Ashbolt, N.; Craik, S.; Maalbared, R.; Pang, X. Assessment of human virus removal during municipal wastewater treatment in Edmonton, Canada. J. Appl. Microbiol. 2015, 119, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Krasner, S.W.; Weinberg, H.S.; Richardson, S.D.; Pastor, S.J.; Chinn, R.; Sclimenti, M.J.; Onstad, G.D.; Thruston, A.D. Occurrence of a New Generation of Disinfection Byproducts. Environ. Sci. Technol. 2006, 40, 7175–7185. [Google Scholar] [CrossRef] [PubMed]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Wang, J.; James, D.K.; Narkhede, P.; Singh, S.P.; Jassby, D.; Tour, J.M.; Arnusch, C.J. Laser-induced graphene and carbon nanotubes as conductive carbon-based materials in environmental technology. Mater. Today 2020, 34, 115–131. [Google Scholar] [CrossRef]
- Barbhuiya, N.H.; Misra, U.; Singh, S.P. Synthesis, fabrication, and mechanism of action of electrically conductive membranes: A review. Environ. Sci. Water Res. Technol. 2021, 7, 671–705. [Google Scholar] [CrossRef]
- Azhar, M.R.; Abid, H.R.; Periasamy, V.; Sun, H.; Tade, M.; Wang, S. Adsorptive removal of antibiotic sulfonamide by UiO-66 and ZIF-67 for wastewater treatment. J. Colloid Interface Sci. 2017, 500, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.R.; Hussain, G.; Tade, M.O.; Silvester, D.S.; Wang, S. Electrodeposited Metal Organic Framework toward Excellent Hydrogen Sensing in an Ionic Liquid. ACS Appl. Nano Mater. 2020, 3, 4376–4385. [Google Scholar] [CrossRef]
- Barbhuiya, N.H.; Kumar, A.; Singh, S.P. A Journey of Laser-Induced Graphene in Water Treatment. Trans. Indian Natl. Acad. Eng. 2021, 6, 159–171. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhi, C.; Wang, X.; Tang, D.; Xu, Y.; Weng, Q.; Jiang, X.; Mitome, M.; Golberg, D.; et al. Three-dimensional strutted graphene grown by substrate-free sugar blowing for high-power-density supercapacitors. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bitounis, D.; Ali-Boucetta, H.; Hong, B.H.; Min, D.-H.; Kostarelos, K. Prospects and Challenges of Graphene in Biomedical Applications. Adv. Mater. 2013, 25, 2258–2268. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; De Faria, A.F.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Singh, S.P.; Li, Y.; Zhang, J.; Tour, J.M.; Arnusch, C.J. Sulfur-Doped Laser-Induced Porous Graphene Derived from Polysulfone-Class Polymers and Membranes. ACS Nano 2018, 12, 289–297. [Google Scholar] [CrossRef]
- Singh, S.P.; Ramanan, S.; Kaufman, Y.; Arnusch, C.J. Laser-Induced Graphene Biofilm Inhibition: Texture Does Matter. ACS Appl. Nano Mater. 2018, 1, 1713–1720. [Google Scholar] [CrossRef]
- Singh, S.P.; Li, Y.; Be’Er, A.; Oren, Y.; Tour, J.M.; Arnusch, C.J. Laser-Induced Graphene Layers and Electrodes Prevents Microbial Fouling and Exerts Antimicrobial Action. ACS Appl. Mater. Interfaces 2017, 9, 18238–18247. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.G.; Li, J.T.; Chen, Y.; McHugh, E.A.; Liopo, A.; Xiao, H.; Tour, J.M. Self-Sterilizing Laser-Induced Graphene Bacterial Air Filter. ACS Nano 2019, 13, 11912–11920. [Google Scholar] [CrossRef]
- Carpio, I.E.M.; Santos, C.M.; Wei, X.; Rodrigues, D.F. Toxicity of a polymer–graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 2012, 4, 4746–4756. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, M.; Calvaresi, M.; Bottoni, A.; Melle-Franco, M.; Zerbetto, F. Graphene Can Wreak Havoc with Cell Membranes. ACS Appl. Mater. Interfaces 2015, 7, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, Y.; Shi, X.; Gao, H. Cellular entry of graphene nanosheets: The role of thickness, oxidation and surface adsorption. RSC Adv. 2013, 3, 15776–15782. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; Bussche, A.V.D.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.; Guo, Y.; Baulin, V.; Al Kobaisi, M.; Crawford, R.; Ivanova, E.P. Graphene Induces Formation of Pores That Kill Spherical and Rod-Shaped Bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral Dimension-Dependent Antibacterial Activity of Graphene Oxide Sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping Bacteria by Graphene Nanosheets for Isolation from Environment, Reactivation by Sonication, and Inactivation by Near-Infrared Irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.J.; Weeks, J.D.; Zhou, R. Dewetting and Hydrophobic Interaction in Physical and Biological Systems. Annu. Rev. Phys. Chem. 2009, 60, 85–103. [Google Scholar] [CrossRef]
- Zhou, R.; Huang, X.; Margulis, C.J.; Berne, B.J. Hydrophobic Collapse in Multidomain Protein Folding. Science 2004, 305, 1605–1609. [Google Scholar] [CrossRef]
- Liu, P.; Huang, X.; Zhou, R.; Berne, B.J. Observation of a dewetting transition in the collapse of the melittin tetramer. Nat. Cell Biol. 2005, 437, 159–162. [Google Scholar] [CrossRef]
- Luan, B.; Huynh, T.; Zhao, L.; Zhou, R. Potential Toxicity of Graphene to Cell Functions via Disrupting Protein–Protein Interactions. ACS Nano 2014, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity Effects of Graphene and Single-Wall Carbon Nanotubes in Neural Phaeochromocytoma-Derived PC12 Cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Daye, A.A.; Eppakayala, V.; Kim, J.-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Musico, Y.L.F.; Santos, C.M.; Dalida, M.L.P.; Rodrigues, D.F. Surface Modification of Membrane Filters Using Graphene and Graphene Oxide-Based Nanomaterials for Bacterial Inactivation and Removal. ACS Sustain. Chem. Eng. 2014, 2, 1559–1565. [Google Scholar] [CrossRef]
- Castrillón, S.R.-V.; Perreault, F.; De Faria, A.F.; Elimelech, M. Interaction of Graphene Oxide with Bacterial Cell Membranes: Insights from Force Spectroscopy. Environ. Sci. Technol. Lett. 2015, 2, 112–117. [Google Scholar] [CrossRef]
- Liu, X.; Sen, S.; Liu, J.; Kulaots, I.; Geohegan, D.; Kane, A.; Puretzky, A.A.; Rouleau, C.M.; More, K.; Palmore, G.T.R.; et al. Antioxidant Deactivation on Graphenic Nanocarbon Surfaces. Small 2011, 7, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Han, H. A new function of graphene oxide emerges: Inactivating phytopathogenic bacterium Xanthomonas oryzae pv. Oryzae. J. Nanoparticle Res. 2013, 15, 1–14. [Google Scholar] [CrossRef]
- An, S.S.A.; Nanda, S.S.; Yi, D.K. Oxidative stress and antibacterial properties of a graphene oxide-cystamine nanohybrid. Int. J. Nanomed. 2015, 10, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Fu, Y.; Wei, T.; Le Guyader, L.; Gao, G.; Liu, R.-S.; Chang, Y.-Z.; Chen, C. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials 2012, 33, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Lalia, B.S.; Kochkodan, V.; Hilal, N.; Hashaikeh, R. Electrically conductive polymeric membranes for fouling prevention and detection: A review. Desalination 2016, 391, 1–15. [Google Scholar] [CrossRef]
- Shim, S.; Hong, S.H.; Tak, Y.; Yoon, J. Prevention of Pseudomonas aeruginosa adhesion by electric currents. Biofouling 2011, 27, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Schnoor, M.H.; Rahaman, S.; Schiffman, J.; Elimelech, M. Electrochemical Multiwalled Carbon Nanotube Filter for Viral and Bacterial Removal and Inactivation. Environ. Sci. Technol. 2011, 45, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Istanbullu, O.; Babauta, J.; Nguyen, H.D.; Beyenal, H. Electrochemical biofilm control: Mechanism of action. Biofouling 2012, 28, 769–778. [Google Scholar] [CrossRef]
- Asadi, M.R.; Torkaman, G. Bacterial Inhibition by Electrical Stimulation. Adv. Wound Care 2014, 3, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Dudchenko, A.; Mende, E.; Flyer, C.; Zhu, X.; Jassby, D. Electrochemical mineral scale prevention and removal on electrically conducting carbon nanotube–polyamide reverse osmosis membranes. Environ. Sci. Process. Impacts 2014, 16, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Iddya, A.; Zhu, X.; Dudchenko, A.V.; Duan, W.; Turchi, C.; Vanneste, J.; Cath, T.Y.; Jassby, D. Enhanced Flux and Electrochemical Cleaning of Silicate Scaling on Carbon Nanotube-Coated Membrane Distillation Membranes Treating Geothermal Brines. ACS Appl. Mater. Interfaces 2017, 9, 38594–38605. [Google Scholar] [CrossRef] [PubMed]
- Tsong, T.Y. Electroporation of Cell Membranes. In Electroporation and Electrofusion in Cell Biology; Springer: New York, NY, USA, 1989; pp. 149–163. [Google Scholar] [CrossRef]
- Wang, Y.; El Deen, A.G.; Li, P.; Oh, B.H.; Guo, Z.; Khin, M.M.; Vikhe, Y.S.; Wang, J.; Hu, R.G.; Boom, R.M.; et al. High-Performance Capacitive Deionization Disinfection of Water with Graphene Oxide-graft-Quaternized Chitosan Nanohybrid Electrode Coating. ACS Nano 2015, 9, 10142–10157. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xie, X.; Zhao, W.; Liu, N.; Maraccini, P.A.; Sassoubre, L.M.; Boehm, A.B.; Cui, Y. Conducting Nanosponge Electroporation for Affordable and High-Efficiency Disinfection of Bacteria and Viruses in Water. Nano Lett. 2013, 13, 4288–4293. [Google Scholar] [CrossRef] [PubMed]
- Shafieian, A.; Khiadani, M.; Azhar, M.R. A solar membrane-based wastewater treatment system for high-quality water production. Energy 2020, 206, 118233. [Google Scholar] [CrossRef]
- Kosaka, K.; Yamada, H.; Matsui, S.; Echigo, A.S.; Shishida, K. Comparison among the Methods for Hydrogen Peroxide Measurements To Evaluate Advanced Oxidation Processes: Application of a Spectrophotometric Method Using Copper(II) Ion and 2,9-Dimethyl-1,10-phenanthroline. Environ. Sci. Technol. 1998, 32, 3821–3824. [Google Scholar] [CrossRef]
- Thakur, A.K.; Singh, S.P.; Kleinberg, M.N.; Gupta, A.; Arnusch, C.J. Laser-Induced Graphene–PVA Composites as Robust Electrically Conductive Water Treatment Membranes. ACS Appl. Mater. Interfaces 2019, 11, 10914–10921. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Singh, S.P.; Thamaraiselvan, C.; Kleinberg, M.N.; Arnusch, C.J. Graphene oxide on laser-induced graphene filters for antifouling, electrically conductive ultrafiltration membranes. J. Membr. Sci. 2019, 591, 117322. [Google Scholar] [CrossRef]
- Drees, K.P.; Abbaszadegan, M.; Maier, R.M. Comparative electrochemical inactivation of bacteria and bacteriophage. Water Res. 2003, 37, 2291–2300. [Google Scholar] [CrossRef]
- Asghari, A. The Inactivation of Bacteria and Viruses by Hydrogen Peroxide. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1993. [Google Scholar]
- Tree, J.A.; Adams, M.R.; Lees, D.N. Chlorination of Indicator Bacteria and Viruses in Primary Sewage Effluent. Appl. Environ. Microbiol. 2003, 69, 2038–2043. [Google Scholar] [CrossRef]
- Wang, X.; Sun, M.; Zhao, Y.; Wang, C.; Ma, W.; Wong, M.S.; Elimelech, M. In Situ Electrochemical Generation of Reactive Chlorine Species for Efficient Ultrafiltration Membrane Self-Cleaning. Environ. Sci. Technol. 2020, 54, 6997–7007. [Google Scholar] [CrossRef]
- Kotnik, T.; Frey, W.; Sack, M.; Meglič, S.H.; Peterka, M.; Miklavčič, D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef]
- El-Hag, A.H.; Jayaram, S.H.; Gonzalez, O.R.; Griffiths, M.W. The Influence of Size and Shape of Microorganism on Pulsed Electric Field Inactivation. IEEE Trans. Nanobioscience 2011, 10, 133–138. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, T.; Xie, X. Locally Enhanced Electric Field Treatment (LEEFT) Promotes the Performance of Ozonation for Bacteria Inactivation by Disrupting the Cell Membrane. Environ. Sci. Technol. 2020, 54, 14017–14025. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, T.; Chen, W.; Lin, B.; Xie, X. Emerging investigator series: Locally enhanced electric field treatment (LEEFT) with nanowire-modified electrodes for water disinfection in pipes. Environ. Sci. Nano 2020, 7, 397–403. [Google Scholar] [CrossRef]
- Guyot, S.; Ferret, E.; Boehm, J.-B.; Gervais, P. Yeast cell inactivation related to local heating induced by low-intensity electric fields with long-duration pulses. Int. J. Food Microbiol. 2007, 113, 180–188. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.Y.; Cho, M.; Choi, W.; Yoon, J. Inactivation of Escherichia coli in the electrochemical disinfection process using a Pt anode. Chemosphere 2007, 67, 652–659. [Google Scholar] [CrossRef]
- Arkhipenko, M.V.; Nikitin, N.A.N.; Baranov, O.A.; Evtushenko, E.A.; Atabekov, J.G.J.G.; Karpova, O.V. Surface Charge Mapping on Virions and Virus-Like Particles of Helical Plant Viruses. Acta Nat. 2019, 11, 73–78. [Google Scholar] [CrossRef]
- Armanious, A.; Aeppli, M.; Jacak, R.; Refardt, D.; Sigstam, T.; Kohn, T.; Sander, M. Viruses at Solid–Water Interfaces: A Systematic Assessment of Interactions Driving Adsorption. Environ. Sci. Technol. 2016, 50, 732–743. [Google Scholar] [CrossRef]
- Koivunen, J.; Heinonen-Tanski, H. Inactivation of enteric microorganisms with chemical disinfectants, UV irradiation and combined chemical/UV treatments. Water Res. 2005, 39, 1519–1526. [Google Scholar] [CrossRef]
- Mamane, H.; Shemer, H.; Linden, K.G. Inactivation of E. coli, B. subtilis spores, and MS2, T4, and T7 phage using UV/H2O2 advanced oxidation. J. Hazard. Mater. 2007, 146, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Yew, P.Y.M.; Owh, C.; Chee, P.L.; Loh, X.J. Sanitizing agents for virus inactivation and disinfection. View 2020, 1, e16. [Google Scholar] [CrossRef]
- Amanna, I.J.; Raué, H.-P.; Slifka, M.K. Development of a new hydrogen peroxide–based vaccine platform. Nat. Med. 2012, 18, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Dembinski, J.L.; Hungnes, O.; Hauge, A.G.; Kristoffersen, A.-C.; Haneberg, B.; Mjaaland, S. Hydrogen peroxide inactivation of influenza virus preserves antigenic structure and immunogenicity. J. Virol. Methods 2014, 207, 232–237. [Google Scholar] [CrossRef]
- Abd-Elghaffar, A.A.; Ali, A.E.; Boseila, A.A.; Amin, M.A. Inactivation of rabies virus by hydrogen peroxide. Vaccine 2016, 34, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Eterpi, M.; McDonnell, G.; Thomas, V. Disinfection efficacy against parvoviruses compared with reference viruses. J. Hosp. Infect. 2009, 73, 64–70. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbhuiya, N.H.; Singh, S.P.; Makovitzki, A.; Narkhede, P.; Oren, Z.; Adar, Y.; Lupu, E.; Cherry, L.; Monash, A.; Arnusch, C.J. Virus Inactivation in Water Using Laser-Induced Graphene Filters. Materials 2021, 14, 3179. https://doi.org/10.3390/ma14123179
Barbhuiya NH, Singh SP, Makovitzki A, Narkhede P, Oren Z, Adar Y, Lupu E, Cherry L, Monash A, Arnusch CJ. Virus Inactivation in Water Using Laser-Induced Graphene Filters. Materials. 2021; 14(12):3179. https://doi.org/10.3390/ma14123179
Chicago/Turabian StyleBarbhuiya, Najmul Haque, Swatantra P. Singh, Arik Makovitzki, Pradnya Narkhede, Ziv Oren, Yaakov Adar, Edith Lupu, Lilach Cherry, Arik Monash, and Christopher J. Arnusch. 2021. "Virus Inactivation in Water Using Laser-Induced Graphene Filters" Materials 14, no. 12: 3179. https://doi.org/10.3390/ma14123179
APA StyleBarbhuiya, N. H., Singh, S. P., Makovitzki, A., Narkhede, P., Oren, Z., Adar, Y., Lupu, E., Cherry, L., Monash, A., & Arnusch, C. J. (2021). Virus Inactivation in Water Using Laser-Induced Graphene Filters. Materials, 14(12), 3179. https://doi.org/10.3390/ma14123179







