Testing a Gypsum Composite Based on Raw Gypsum with a Direct Admixture of Paraffin and Polymer to Improve Thermal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Heating and Cooling
2.2. Tests of Hardening Time of Individual Samples—Vicat Apparatus
2.3. Testing the “Ability” of the Material to Accumulate Paraffin—An Uptake Test
2.4. Thermal Conductivity, Volumetric Heat Capacity, and Diffusivity
3. Results
3.1. Heating and Cooling
3.2. Tests of Hardening Time of Individual Samples—Vicat Apparatus
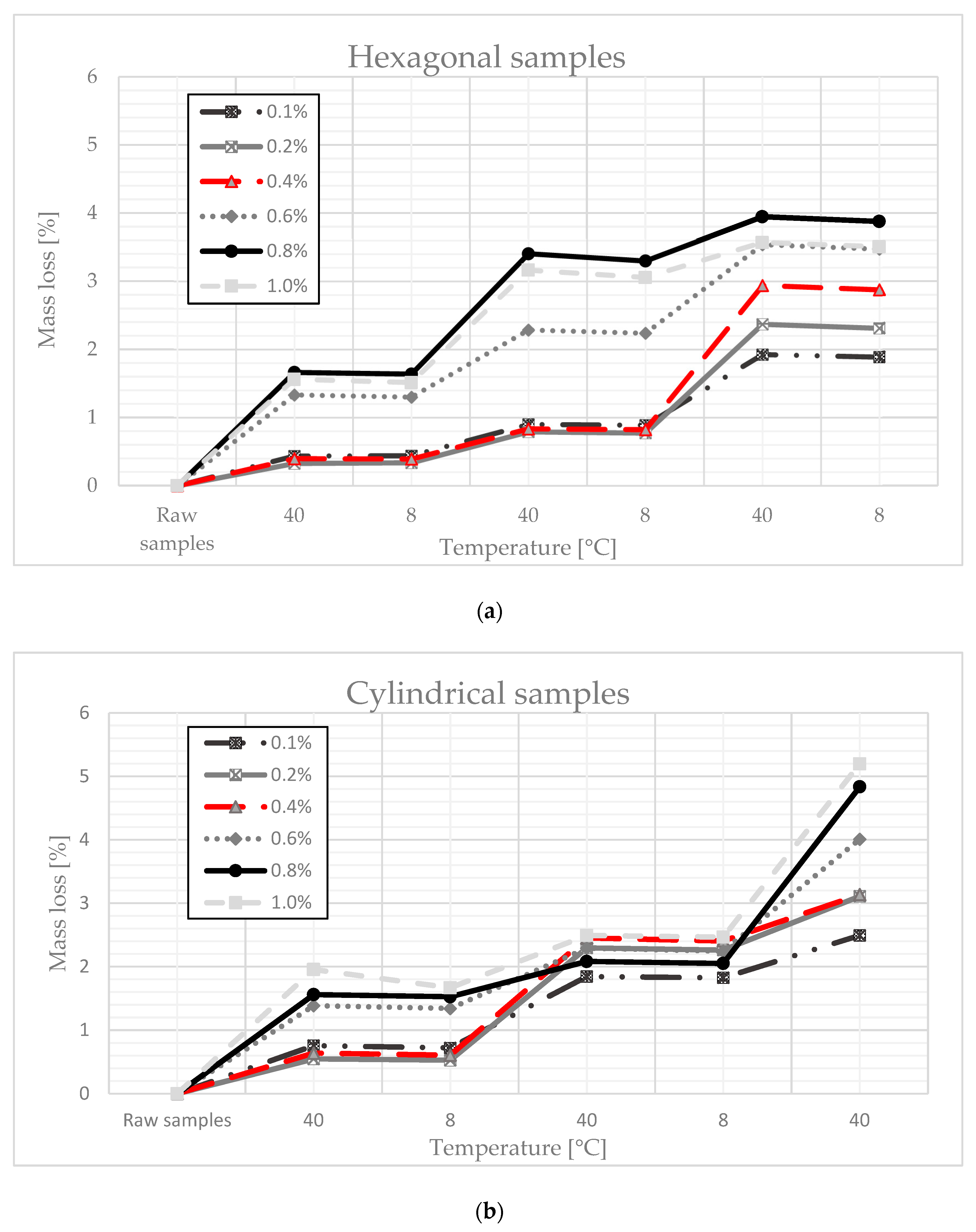
3.3. Testing the “Ability” of the Material to Accumulate Paraffin—An Uptake Test
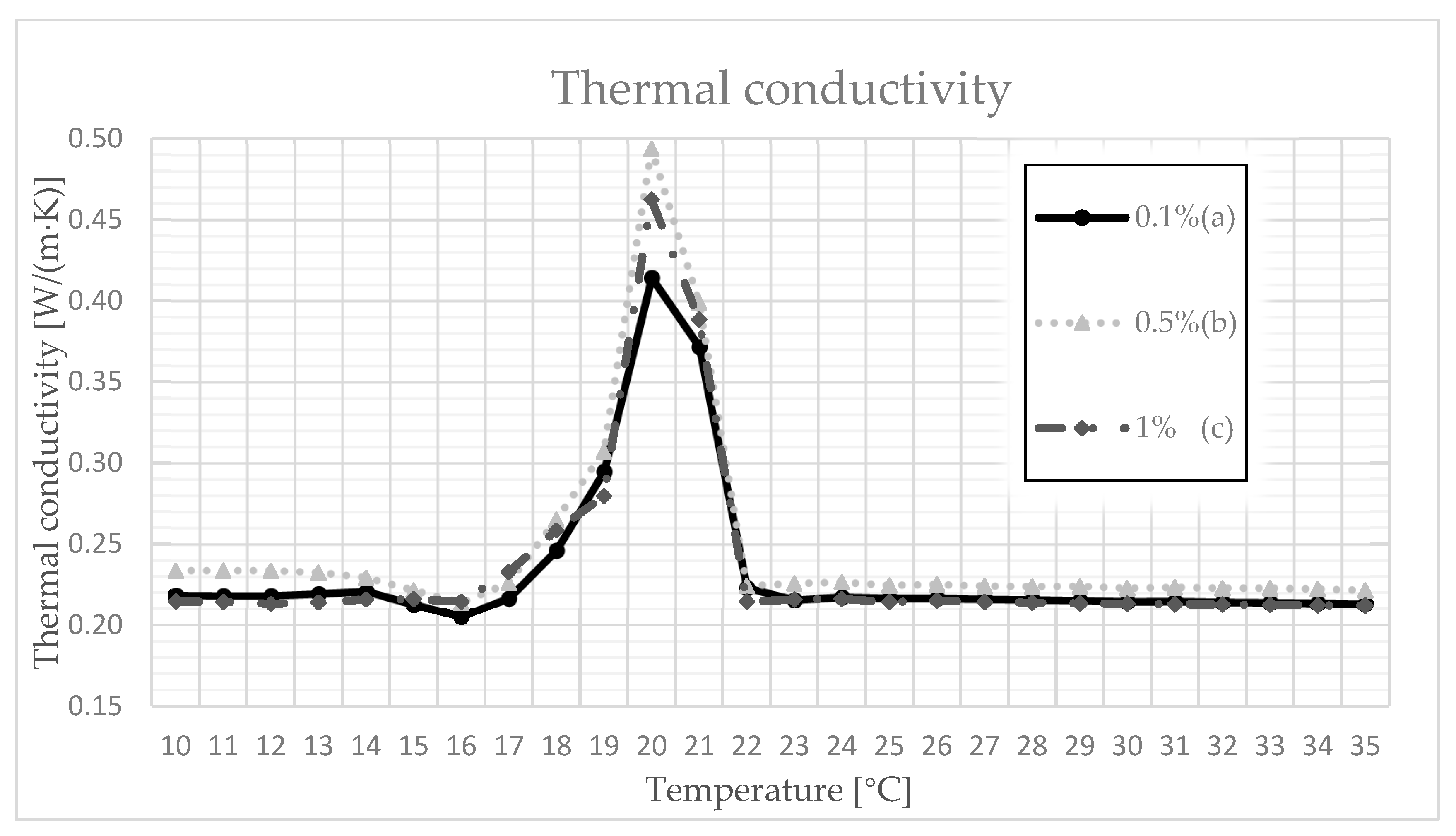
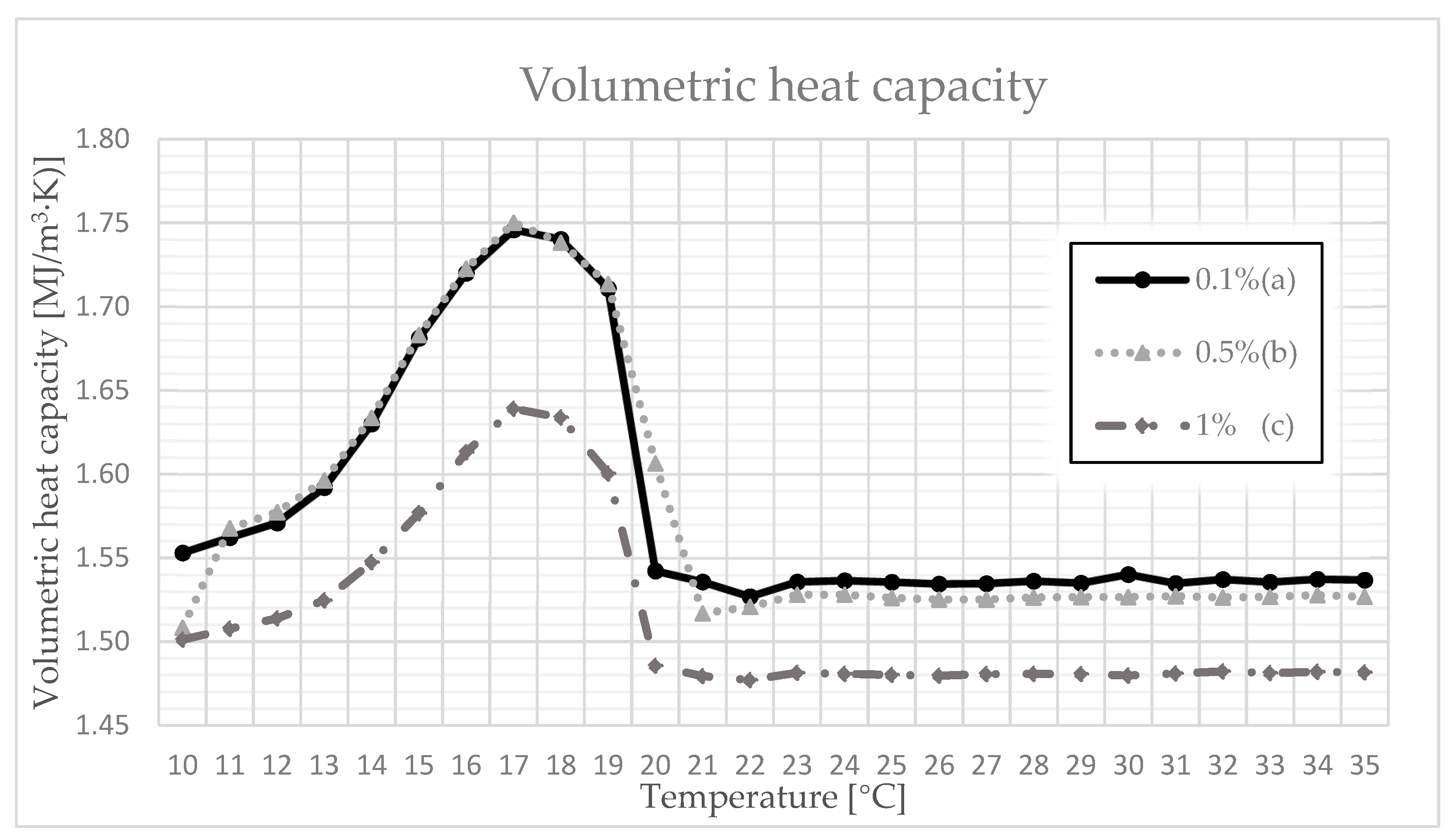
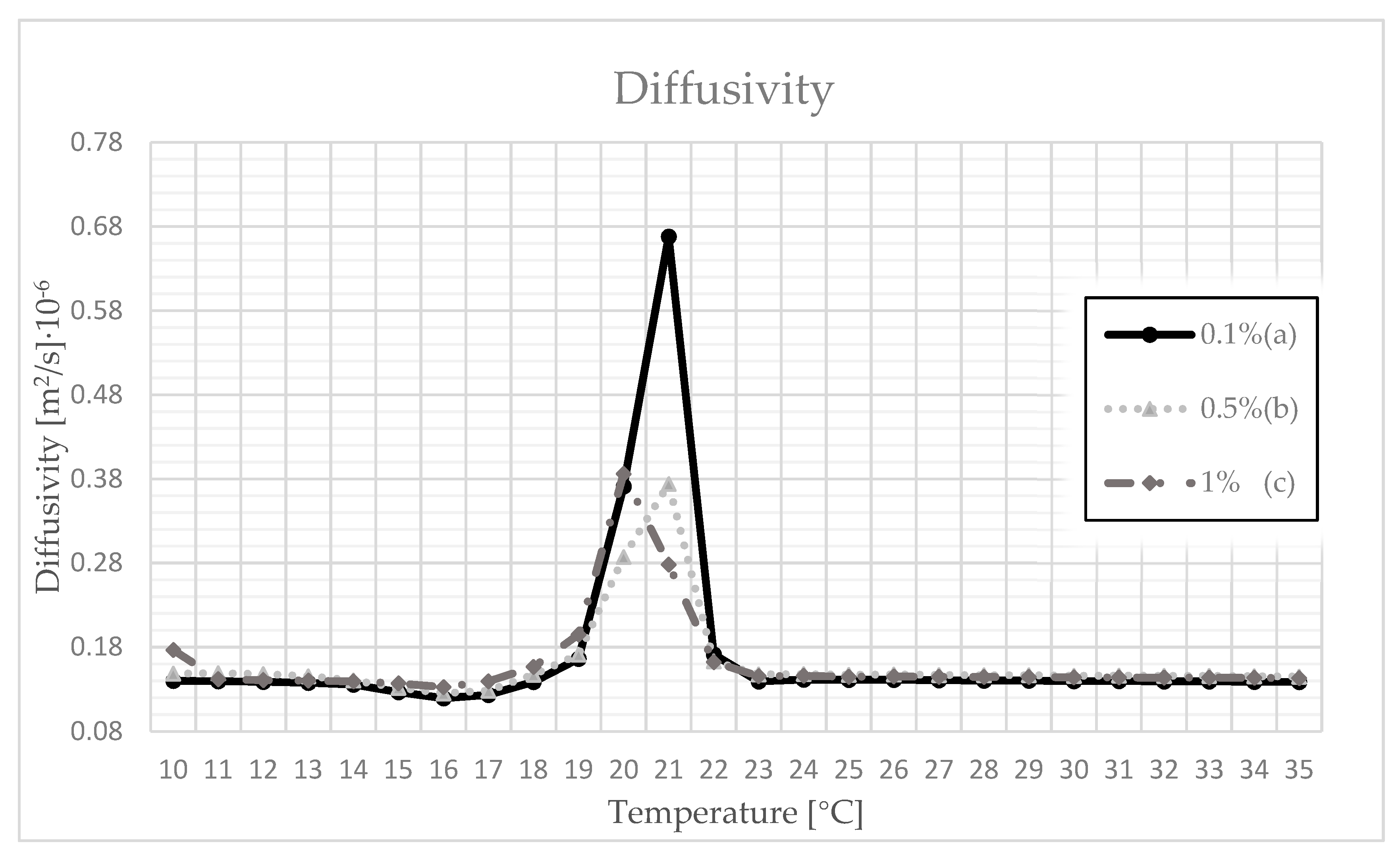
3.4. Thermal Conductivity, Volumetric Heat Capacity, and Diffusivity
4. Conclusions
- When the composite was subjected to a drying and cooling cycle, some mass of paraffin leaked from the composite. The loss of paraffin mass depended on the shape and mass of the sample. For cylindrical samples, the weight loss was 5% and, for cubic samples, the maximum weight loss was 4%, which should be considered a satisfactory result;
- The measurement of the curing time value showed that the polymer additive used in the composite influenced the hardening time of the samples. The PCM sample with the addition of 0.1% polymer achieved a hardening time that was shorter than that of the raw gypsum;
- A modified, proprietary study of paraffin rising proved the benefits of using the polymer in the designed composite. The sample with the addition of polymer soaked slower than the sample made of gypsum alone;
- The thermal conductivity coefficient increased to its maximum value, which was characterized by the phase transition point of the paraffin used, and then adjusted itself to the initial value. It can be assumed that, during the transformation, the designed composite can absorb so much heat that, after another change in aggregate state, it can release the stored heat, e.g., at night, which is a chief idea behind the use of PCM.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ürge-Vorsatz, D.; Cabeza, L.F.; Serrano, S.; Barreneche, C.; Petrichenko, K. Heating and cooling energy trends and drivers in buildings. Renew. Sustain. Energy Rev. 2015, 41, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Munarim, U.; Ghisi, E. Environmental feasibility of heritage buildings rehabilitation. Renew. Sustain. Energy Rev. 2016, 58, 235–249. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y.; Chen, X.; Song, X.; Tang, K. Experimental Study of an Enhanced Phase Change Material of Para ffi n/Expanded Graphite/Nano-Metal Particles for a Personal Cooling System. Materials 2020, 13, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Xiao, X.; Ma, Z.W. A review of the composite phase change materials: Fabrication, characterization, mathematical modeling and application to performance enhancement. Appl. Energy 2016, 165, 472–510. [Google Scholar] [CrossRef]
- Tong, X.; Li, N.; Zeng, M.; Wang, Q. Organic phase change materials confined in carbon-based materials for thermal properties enhancement: Recent advancement and challenges. Renew. Sustain. Energy Rev. 2019, 108, 398–422. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M. Phase Change Materials for Energy E ffi ciency in Buildings and Their Use in Mortars. Materials 2019, 12, 1260. [Google Scholar] [CrossRef] [Green Version]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Jayalath, A.; San Nicolas, R.; Sofi, M.; Shanks, R.; Ngo, T.; Aye, L.; Mendis, P. Properties of cementitious mortar and concrete containing micro-encapsulated phase change materials. Constr. Build. Mater. 2016, 120, 408–417. [Google Scholar] [CrossRef]
- Cunha, S.; Lima, M.; Aguiar, J.B. Influence of adding phase change materials on the physical and mechanical properties of cement mortars. Constr. Build. Mater. 2016, 127, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Guardia, C.; Barluenga, G.; Palomar, I.; Diarce, G. Thermal enhanced cement-lime mortars with phase change materials (PCM), lightweight aggregate and cellulose fibers. Constr. Build. Mater. 2019, 221, 586–594. [Google Scholar] [CrossRef]
- Hadiya, J.P.; Shukla, A.K.N. Thermal energy storage using phase change materials: A way forward. Int. J. Glob. Energy Issues 2018, 41, 108–127. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Zhang, Y. Review of thermal energy storage technologies based on PCM application in buildings. Energy Build. 2013, 67, 56–69. [Google Scholar] [CrossRef]
- Kalnæs, S.E.; Jelle, B.P. Phase change materials and products for building applications: A state-of-the-art review and future research opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Tian, Y.; Jin, X.; Lo, T.Y.; Cui, H. Thermal and Mechanical Properties of Expanded Graphite/Paraffin Gypsum-Based Composite Material Reinforced by Carbon Fiber. Materials 2018, 11, 2205. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Zhong, Y.; Rong, X.; Min, C.; Qi, C. Building Energy Storage Panel Based on Paraffin/Expanded Perlite: Preparation and thermal performance study. Materials 2016, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Wen, R.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X.; Min, X. Preparation and analysis of lightweight wall material with expanded graphite (EG)/paraffin composites for solar energy storage. Appl. Therm. Eng. 2017, 120, 107–114. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storag with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Silakhori, M.; Naghavi, M.S.; Simon, H.; Metselaar, C.; Meurah, T.; Mahlia, I.; Fauzi, H.; Mehrali, M. Accelerated Thermal Cycling Test of Microencapsulated Paraffin Wax/Polyaniline Made by Simple Preparation Method for Solar Thermal Energy Storage. Materials 2013, 6, 1608–1620. [Google Scholar] [CrossRef] [Green Version]
- Xin, C.; Lu, S.F.; Shen, T.W.; Xiao, C.P.; Zhang, Y.S. Preparation and properties of polyurethane microencapsulated phase change materials. Xiandai Huagong/Modern Chem. Ind. 2019, 39. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Tang, G.; Song, G. Preparation and thermal properties of microencapsulated paraffin with polyurea/acrylic resin hybrid shells as phase change energy storage materials. J. Therm. Anal. Calorim. 2020, 143, 3023–3032. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, L.; Fang, G.; Shan, F. Synthesis and characterization of microencapsulated paraffin microcapsules as shape-stabilized thermal energy storage materials. Nanoscale Microscale Thermophys. Eng. 2013, 17, 112–123. [Google Scholar] [CrossRef]
- Schossig, P.; Henning, H.M.; Gschwander, S.; Haussmann, T. Micro-encapsulated phase-change materials integrated into construction materials. Sol. Energy Mater. Sol. Cells 2005, 89, 297–306. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Liu, Z. Novel hybrid microencapsulated phase change materials incorporated wallboard for year-long year energy storage in buildings. Energy Convers. Manag. 2019, 183, 791–802. [Google Scholar] [CrossRef]
- Lucas, S.S.; Ferreira, V.M.; De Aguiar, J.L.B. Latent heat storage in PCM containing mortars—Study of microstructural modifications. Energy Build. 2013, 66, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Cunha, S.; Aguiar, J.B.; Ferreira, V.M.; Tadeu, A. Influence of adding encapsulated phase change materials in aerial lime based mortars. Adv. Mater. Res. 2013, 687, 255–261. [Google Scholar] [CrossRef]
- Cunha, S.; Aguiar, J.; Ferreira, V.; Tadeu, A.; Garbacz, A. Mortars with phase change materials-Part I: Physical and mechanical characterization. Key Eng. Mater. 2015, 634, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Pavlík, Z.; Trník, A.; Keppert, M.; Pavlíková, M.; Žumár, J.; Černý, R. Experimental investigation of the properties of lime-based plaster-containing PCM for enhancing the heat-storage capacity of building envelopes. Int. J. Thermophys. 2014, 35, 767–782. [Google Scholar] [CrossRef]
- Drissi, S.; Ling, T.C. Thermal and durability performances of mortar and concrete containing phase change materials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 431, 062001. [Google Scholar] [CrossRef]
- Cunha, S.; Aguiar, J.; Tadeu, A. Ranking procedure based on mechanical, durability and thermal behavior of mortars with incorporation of phase change materials. Mater. Constr. 2015, 65, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Prałat, K.; Łukasiewicz, M.; Miczko, P. Experimental study of the basic mechanical properties of hardened gypsum paste modified with addition of polyoxymethylene micrograins. Arch. Civ. Eng. 2020, 66, 385–397. [Google Scholar]
- Powała, K.; Heim, D. Paraffin Permeability of Synthetic Gypsum Binders Modified by Individual Polymers. Latv. J. Phys. Tech. Sci. 2019, 56, 47–56. [Google Scholar] [CrossRef]
- Mróz, P.; Mucha, M. Hydroxyethyl methyl cellulose as a modifier of gypsum properties. J. Therm. Anal. Calorim. 2018, 134, 1083–1089. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Unit | Result |
|---|---|---|
| Calcium sulfate hemihydrate content | % | >95 |
| (β-CaSO4·0.5H2O) Crystallization water | % | 5.6–6.0 |
| Mechanical strength after drying to constant weight - for bending - for compression | MPa | >5.0 >12.0 |
| Parameter | Unit | Result |
|---|---|---|
| Melting area | °C | 20–23 |
| Congealing area | °C | 23–20 |
| Heat storage capacity | kJ/kg | 190 |
| Specific heat capacity | kJ/kg·K | 2 |
| Density solid | kg/L | 0.76 |
| Density liquid | kg/L | 0.7 |
| Type of Equipment | Parameter | Model and Company | Country, City |
|---|---|---|---|
| Dryer | Temperature | Model ST3 company Pol-eko | Poland, Wodzisław Śląski |
| Device for testing the thermal conductivity | Thermal conductivity coefficient, volumetric heat capacity, thermal diffusivity | Isomet 2114 company Applied Precision Ltd. | Slovakia, Bratislava |
| Vicat apparatus | Drying time | manual according to PN-EN 196-3 MMC-0051/E by Multi-serv | Poland, Marcyporęba |
| Percentage of Polymer | Drying Time |
|---|---|
| 0% | 21 min 28 s |
| 0.1% | 19 min |
| 0.5% | 38 min 42 s |
| 1% | 55 min 30 s |
| Percentage of Polymer | Temperature | Thermal Conductivity | Volumetric Heat Capacity | Diffusivity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| °C | W/m·K | MJ/(m3·K) | (m2/s) 10−6 | |||||||
| % | Min | Max | Average | Min | Max | Average | Min | Max | Average | |
| 0.1 | 17 | 0.210 | 0.219 | 0.217 | 1.727 | 1.755 | 1.746 | 0.118 | 0.125 | 0.123 |
| 18 | 0.215 | 0.249 | 0.246 | 1.723 | 1.755 | 1.740 | 0.123 | 0.143 | 0.139 | |
| 19 | 0.246 | 0.302 | 0.295 | 1.703 | 1.722 | 1.711 | 0.143 | 0.178 | 0.166 | |
| 20 | 0.304 | 0.546 | 0.414 | 1.518 | 1.695 | 1.542 | 0.256 | 0.514 | 0.371 | |
| 21 | 0.345 | 0.403 | 0.372 | 1.521 | 1.544 | 1.536 | 0.443 | 0.828 | 0.668 | |
| 22 | 0.209 | 0.250 | 0.223 | 1.520 | 1.534 | 1.527 | 0.137 | 0.222 | 0.171 | |
| 0.5 | 17 | 0.214 | 0.227 | 0.226 | 1.727 | 1.767 | 1.750 | 0.124 | 0.130 | 0.128 |
| 18 | 0.225 | 0.269 | 0.265 | 1.721 | 1.751 | 1.738 | 0.129 | 0.156 | 0.147 | |
| 19 | 0.267 | 0.315 | 0.307 | 1.703 | 1.732 | 1.713 | 0.154 | 0.185 | 0.171 | |
| 20 | 0.454 | 0.548 | 0.494 | 1.551 | 1.657 | 1.606 | 0.204 | 0.353 | 0.287 | |
| 21 | 0.334 | 0.470 | 0.399 | 1.510 | 1.528 | 1.517 | 0.221 | 0.489 | 0.374 | |
| 22 | 0.218 | 0.241 | 0.224 | 1.515 | 1.528 | 1.521 | 0.143 | 0.215 | 0.163 | |
| 1 | 17 | 0.221 | 0.250 | 0.233 | 1.629 | 1.655 | 1.639 | 0.135 | 0.152 | 0.139 |
| 18 | 0.247 | 0.272 | 0.258 | 1.626 | 1.647 | 1.634 | 0.151 | 0.166 | 0.157 | |
| 19 | 0.269 | 0.298 | 0.280 | 1.572 | 1.621 | 1.600 | 0.166 | 0.229 | 0.195 | |
| 20 | 0.431 | 0.510 | 0.462 | 1.470 | 1.499 | 1.486 | 0.292 | 0.489 | 0.386 | |
| 21 | 0.339 | 0.480 | 0.388 | 1.469 | 1.480 | 1.479 | 0.229 | 0.325 | 0.278 | |
| 22 | 0.208 | 0.224 | 0.214 | 1.473 | 1.482 | 1.477 | 0.141 | 0.181 | 0.162 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powała, K.; Obraniak, A.; Heim, D. Testing a Gypsum Composite Based on Raw Gypsum with a Direct Admixture of Paraffin and Polymer to Improve Thermal Properties. Materials 2021, 14, 3241. https://doi.org/10.3390/ma14123241
Powała K, Obraniak A, Heim D. Testing a Gypsum Composite Based on Raw Gypsum with a Direct Admixture of Paraffin and Polymer to Improve Thermal Properties. Materials. 2021; 14(12):3241. https://doi.org/10.3390/ma14123241
Chicago/Turabian StylePowała, Krzysztof, Andrzej Obraniak, and Dariusz Heim. 2021. "Testing a Gypsum Composite Based on Raw Gypsum with a Direct Admixture of Paraffin and Polymer to Improve Thermal Properties" Materials 14, no. 12: 3241. https://doi.org/10.3390/ma14123241






