Insights into the Microstructural Evolution Occurring during Pyrolysis of Metal-Modified Ceramers Studied through Selective SiO2 Removal
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Structural Characterization of the Ceramer Materials
3.2. Thermal Analysis
3.3. Textural Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaheer, M.; Schmalz, T.; Motz, G.; Kempe, R. Polymer derived non-oxide ceramics modified with late transition metals. Chem. Soc. Rev. 2012, 41, 5102–5116. [Google Scholar] [CrossRef]
- Babonneau, F.; Sorarú, G.D.; Thorne, K.J.; Mackenzie, J.D. Chemical characterization of Si-Al-C-O precursor and its pyrolysis. J. Am. Ceram. Soc. 1991, 74, 1725–1728. [Google Scholar] [CrossRef]
- Suttor, D.; Kleebe, H.J.; Ziegler, G. Formation of mullite from filled siloxanes. J. Am. Ceram. Soc. 1997, 80, 2541–2548. [Google Scholar] [CrossRef]
- Schiavon, M.A.; Gervais, C.; Babonneau, F.; Soraru, G.D. Crystallization Behavior of Novel Silicon Boron Oxycarbide Glasses. J. Am. Ceram. Soc. 2004, 87, 203–208. [Google Scholar] [CrossRef]
- Stabler, C.; Ionescu, E.; Graczyk-Zajac, M.; Gonzalo-Juan, I.; Riedel, R. Silicon oxycarbide glasses and glass-ceramics:“All-Rounder” materials for advanced structural and functional applications. J. Am. Ceram. Soc. 2018, 101, 4817–4856. [Google Scholar] [CrossRef]
- Zeydanli, D.; Akman, S.; Vakifahmetoglu, C. Polymer-derived ceramic adsorbent for pollutant removal from water. J. Am. Ceram. Soc. 2018, 101, 2258–2265. [Google Scholar] [CrossRef]
- Lu, K. Porous and high surface area silicon oxycarbide-based materials—A review. Mater. Sci. Eng. R Rep. 2015, 97, 23–49. [Google Scholar] [CrossRef]
- Colombo, P. Engineering porosity in polymer-derived ceramics. J. Eur. Ceram. Soc. 2008, 28, 1389–1395. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Pippel, E.; Woltersdorf, J.; Colombo, P. Growth of One-Dimensional Nanostructures in Porous Polymer-Derived Ceramics by Catalyst-Assisted Pyrolysis. Part I: Iron Catalyst. J. Am. Ceram. Soc. 2010, 93, 959–968. [Google Scholar] [CrossRef]
- Ionescu, E.; Terzioglu, C.; Linck, C.; Kaspar, J.; Navrotsky, A.; Riedel, R. Thermodynamic control of phase composition and crystallization of metal-modified silicon oxycarbides. J. Am. Ceram. Soc. 2013, 96, 1899–1903. [Google Scholar] [CrossRef]
- Li, N.; Cao, Y.; Zhao, R.; Xu, Y.; An, L. Polymer-derived SiAlOC ceramic pressure sensor with potential for high-temperature application. Sens. Actuators A Phys. 2017, 263, 174–178. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, X.; Zhao, R.; Chen, Y.; Li, N.; An, L. Giant piezoresistivity in polymer-derived amorphous SiAlCO ceramics. J. Mater. Sci. 2016, 51, 5646–5650. [Google Scholar] [CrossRef]
- Ma, Q.S.; Xu, T.H. High-temperature evolution behavior of polymer-derived SiAlOC ceramics under inert atmosphere. J. Alloys Compd. 2017, 723, 17–20. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Z.; Song, Z.; Deng, K.; Ren, Z. Preparation of porous Al2O3 ceramics with in situ formed C-nanowires derived form silicone resin. Mater. Lett. 2018, 212, 271–274. [Google Scholar] [CrossRef]
- Cao, Y.; An, L. Anomalous piezo-dielectricity of a polymer-derived amorphous silicoaluminum oxycarbide (SiAlCO). Ceram. Int. 2018, 44, 1467–1470. [Google Scholar] [CrossRef]
- Ichikawa, H. Polymer-Derived Ceramic Fibers. In Annual Review of Materials Research; Clarke, D.R., Ed.; Annual Reviews: Palo Alto, CA, USA, 2016; Volume 46, pp. 335–356. [Google Scholar]
- Joo, Y.J.; Kim, C.J.; Cho, K.Y. Facile synthesis and morphological study of Si-Zr-C-O fiber felts with high-thermal resistance. Ceram. Int. 2019, 45, 16008–16014. [Google Scholar] [CrossRef]
- Macedo, H.P.; Medeiros, R.L.; Ilsemann, J.; Melo, D.M.; Rezwan, K.; Wilhelm, M. Nickel-containing hybrid ceramics derived from polysiloxanes with hierarchical porosity for CO2 methanation. Microporous Mesoporous Mater. 2019, 278, 156–166. [Google Scholar] [CrossRef]
- Seifollahi Bazarjani, M.; Kleebe, H.-J.; Müller, M.M.; Fasel, C.; Baghaie Yazdi, M.; Gurlo, A.; Riedel, R. Nanoporous Silicon Oxycarbonitride Ceramics Derived from Polysilazanes In situ Modified with Nickel Nanoparticles. Chem. Mater. 2011, 23, 4112–4123. [Google Scholar] [CrossRef]
- Schubert, M.; Wilhelm, M.; Bragulla, S.; Sun, C.; Neumann, S.; Gesing, T.M.; Pfeifer, P.; Rezwan, K.; Bäumer, M. The Influence of the Pyrolysis Temperature on the Material Properties of Cobalt and Nickel Containing Precursor Derived Ceramics and their Catalytic Use for CO2 Methanation and Fischer–Tropsch Synthesis. Catal. Lett. 2017, 147, 472–482. [Google Scholar] [CrossRef]
- Scheffler, M.; Greil, P.; Berger, A.; Pippel, E.; Woltersdorf, J. Nickel-catalyzed in situ formation of carbon nanotubes and turbostratic carbon in polymer-derived ceramics. Mater. Chem. Phys. 2004, 84, 131–139. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Colombo, P.; Carturan, S.M.; Pippel, E.; Woltersdorf, J. Growth of One-Dimensional Nanostructures in Porous Polymer-Derived Ceramics by Catalyst-Assisted Pyrolysis. Part II: Cobalt Catalyst. J. Am. Ceram. Soc. 2010, 93, 3709–3719. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N.; Bissessur, R. Tunable room-temperature soft ferromagnetism in magnetoceramics of organometallic dendrimers. J. Mater. Chem. C 2017, 5, 2268–2281. [Google Scholar] [CrossRef]
- Shao, G.; Liang, J.; Zhao, W.; Zhao, B.; Liu, W.; Wang, H.; Fan, B.; Xu, H.; Lu, H.; Wang, Y.; et al. Co decorated polymer-derived SiCN ceramic aerogel composites with ultrabroad microwave absorption performance. J. Alloys Compd. 2020, 813, 152007. [Google Scholar] [CrossRef]
- Fortuniak, W.; Pospiech, P.; Mizerska, U.; Chojnowski, J.; Slomkowski, S.; Nyczyk-Malinowska, A.; Wojteczko, A.; Wisla-Walsh, E.; Hasik, M. Generation of meso-and microporous structures by pyrolysis of polysiloxane microspheres and by HF etching of SiOC microspheres. Ceram. Int. 2018, 44, 374–383. [Google Scholar] [CrossRef]
- Duan, L.; Ma, Q.; Chen, Z. Preparation and characterization of mesoporous silicon oxycarbide ceramics without free carbon from polysiloxane. J. Eur. Ceram. Soc. 2013, 33, 841–846. [Google Scholar] [CrossRef]
- Tamayo, A.; Peña-Alonso, R.; Rubio, J.; Raj, R.; Sorarù, G.D.; Oteo, J.L. Surface Energy of Sol Gel-Derived Silicon Oxycarbide Glasses. J. Am. Ceram. Soc. 2011, 94, 4523–4533. [Google Scholar] [CrossRef]
- Mazo, M.A.; Tamayo, A.; Rubio, J. Highly micro- and mesoporous oxycarbide derived materials from HF etching of silicon oxycarbide materials. Microporous Mesoporous Mater. 2019, 289, 109614. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; p. 303. [Google Scholar]
- Fidalgo, A.; Ilharco, L.M. Correlation between physical properties and structure of silica xerogels. J. Non-Cryst. Solids 2004, 347, 128–137. [Google Scholar] [CrossRef]
- Bois, L.; Maquet, J.; Babonneau, F.; Mutin, H.; Bahloul, D. Structural characterization of sol-gel derived oxycarbide glasses. 1. Study of the pyrolysis process. Chem. Mater. 1994, 6, 796–802. [Google Scholar] [CrossRef]
- Pantano, C.G.; Singh, A.K.; Zhang, H. Silicon Oxycarbide Glasses. J. Sol.-Gel Sci. Technol. 1999, 14, 7–25. [Google Scholar] [CrossRef]
- Almeida, R.M.; Guiton, T.A.; Pantano, C.G. Detection of LO mode in v-SiO2 by infrared diffuse reflectance spectroscopy. J. Non-Cryst. Solids 1990, 119, 238–241. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. Chemical Tailoring of Porous Silica Xerogels: Local Structure by Vibrational Spectroscopy. Chem. A Eur. J. 2004, 10, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Kamiya, K.; Nasu, H. IR study on the structural evolution of sol-gel derived SiO2 gels in the early stage of conversion to glasses. J. Non-Cryst. Solids 1990, 126, 68–78. [Google Scholar] [CrossRef]
- Awazu, K.; Kawazoe, H. Strained Si–O–Si bonds in amorphous SiO2 materials: A family member of active centers in radio, photo, and chemical responses. J. Appl. Phys. 2003, 94, 6243–6262. [Google Scholar] [CrossRef]
- Koike, C.; Noguchi, R.; Chihara, H.; Suto, H.; Ohtaka, O.; Imai, Y.; Matsumoto, T.; Tsuchiyama, A. Infrared spectra of silica polymorphs and the conditions of their formation. Astrophys. J. 2013, 778, 60. [Google Scholar] [CrossRef]
- Su, D.; Li, Y.-L.; An, H.-J.; Liu, X.; Hou, F.; Li, J.-Y.; Fu, X. Pyrolytic transformation of liquid precursors to shaped bulk ceramics. J. Eur. Ceram. Soc. 2010, 30, 1503–1511. [Google Scholar] [CrossRef]
- Ruan, D.-S.; Li, Y.-L.; Wang, L.; Su, D.; Hou, F. Fabrication of silicon oxycarbide fibers from alkoxide solutions along the sol–gel process. J. Sol. Gel Sci. Technol. 2010, 56, 184–190. [Google Scholar] [CrossRef]
- Gu, C.; Guo, C.; Dong, X.; Hu, Z.; Wu, P.; Su, Z.; Lu, Y.; Xu, B.; Yu, Z.; Liu, A. Core-shell structured iron-containing ceramic nanoparticles: Facile fabrication and excellent electromagnetic absorption properties. J. Am. Ceram. Soc. 2019, 102, 7098–7107. [Google Scholar] [CrossRef]
- Ding, D.; Wang, J.; Xiao, G.; Li, Z.; Bai, B.; Ren, J.; He, G. Enhanced electromagnetic wave absorbing properties of Si-O-C ceramics with in-situ formed 1D nanostructures. Int. J. Appl. Ceram. Technol. 2019, 17, 734–744. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Schmidt, H.; Koch, D.; Grathwohl, G.; Colombo, P. Micro-/Macroporous Ceramics from Preceramic Precursors. J. Am. Ceram. Soc. 2001, 84, 2252–2255. [Google Scholar] [CrossRef]
- Tamayo, A.; Rubio, F.; Teresa Colomer, M.; Arroyo, C.; Rodríguez, M.A. Characterization of polymer-derived ceramers subjected to wet-etching and the evolution of the carbon phase during thermal conversion. J. Non-Cryst. Solids 2020, 547, 120302. [Google Scholar] [CrossRef]
- Erb, D.; Lu, K. Additive and pyrolysis atmosphere effects on polysiloxane-derived porous SiOC ceramics. J. Eur. Ceram. Soc. 2017, 37, 4547–4557. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Yang, J.; Tang, M. Synthesis and characterization of ceramic precursor aluminum-containing polycarbosilane and its pyrolysis. J. Inorg. Organomet. Polym. Mater. 2007, 17, 569–575. [Google Scholar] [CrossRef]
- Duan, Y.; Mo, G.; Huangfu, Z.; Liang, Y.; Cui, Z.; He, L.; Huang, Z.; Chai, Z.; Huang, Q. Effects of aluminium content on the molecular structure and properties of polyaluminocarbosilane for SiC fibre fabrication. Ceram. Int. 2019, 45, 16380–16386. [Google Scholar] [CrossRef]
- Bik, M.; Jeleń, P.; Długoń, E.; Bik, E.; Mroczka, K.; Barańska, M.; Sitarz, M. SiAlOC glasses derived from sol-gel synthesized ladder-like silsesquioxanes. Ceram. Int. 2019, 45, 1683–1690. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, X.; Yu, Y.; Zhang, Y. Quantitative determinations in the molecular structures of polyaluminocarbosilane. Polymer 2011, 52, 3811–3818. [Google Scholar] [CrossRef]
- Padmaja, P.; Anilkumar, G.M.; Mukundan, P.; Aruldhas, G.; Warrier, K.G.K. Characterisation of stoichiometric sol–gel mullite by fourier transform infrared spectroscopy. Int. J. Inorg. Mater. 2001, 3, 693–698. [Google Scholar] [CrossRef]
- Harshe, R.R. Synthesis and Processing of Amorphous Si (Al) OC Bulk Ceramics; Technische Universität Darmstadt: Darmstadt, Germany, 2004. [Google Scholar]
- Li, X.; Edirisinghe, M.J. Evolution of the Ceramic Structure during Thermal Degradation of a Si−Al−C−O Precursor. Chem. Mater. 2004, 16, 1111–1119. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, X.; An, L. Electric conductivity and microstructure evolution of polymer-derived SiAlCO ceramics. Ceram. Int. 2016, 42, 4033–4038. [Google Scholar] [CrossRef]
- Dirè, S.; Ceccato, R.; Gialanella, S.; Babonneau, F. Thermal evolution and crystallisation of polydimethylsiloxane–zirconia nanocomposites prepared by the sol–gel method. J. Eur. Ceram. Soc. 1999, 19, 2849–2858. [Google Scholar] [CrossRef]
- Ionescu, E.; Linck, C.; Fasel, C.; Muller, M.; Kleebe, H.J.; Riedel, R. Polymer-Derived SiOC/ZrO2 Ceramic Nanocomposites with Excellent High-Temperature Stability. J. Am. Ceram. Soc. 2010, 93, 241–250. [Google Scholar] [CrossRef]
- Papet, P.; Le Bars, N.; Baumard, J.; Lecomte, A.; Dauger, A. Transparent monolithic zirconia gels: Effects of acetylacetone content on gelation. J. Mater. Sci. 1989, 24, 3850–3854. [Google Scholar] [CrossRef]
- Ismail, H.M. Characterization of the decomposition products of zirconium acetylacetonate: Nitrogen adsorption and spectrothermal investigation. Powder Technol. 1995, 85, 253–259. [Google Scholar] [CrossRef]
- Dirè, S.; Ceccato, R.; Babonneau, F. Structural and microstructural evolution during pyrolysis of hybrid polydimethylsiloxane-titania nanocomposites. J. Sol. Gel Sci. Technol. 2005, 34, 53–62. [Google Scholar] [CrossRef]
- Liu, C.; Pan, R.; Hong, C.; Zhang, X.; Han, W.; Han, J.; Du, S. Effects of Zr on the precursor architecture and high-temperature nanostructure evolution of SiOC polymer-derived ceramics. J. Eur. Ceram. Soc. 2016, 36, 395–402. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.L.; Godoy, N.V.; Ribeiro, E.S.; Segatelli, M.G. Synthesis and structural characterization of hybrid polymeric networks-derived-SiCxOy in the presence and absence of cobalt acetate. J. Anal. Appl. Pyrolysis 2015, 114, 11–21. [Google Scholar] [CrossRef]
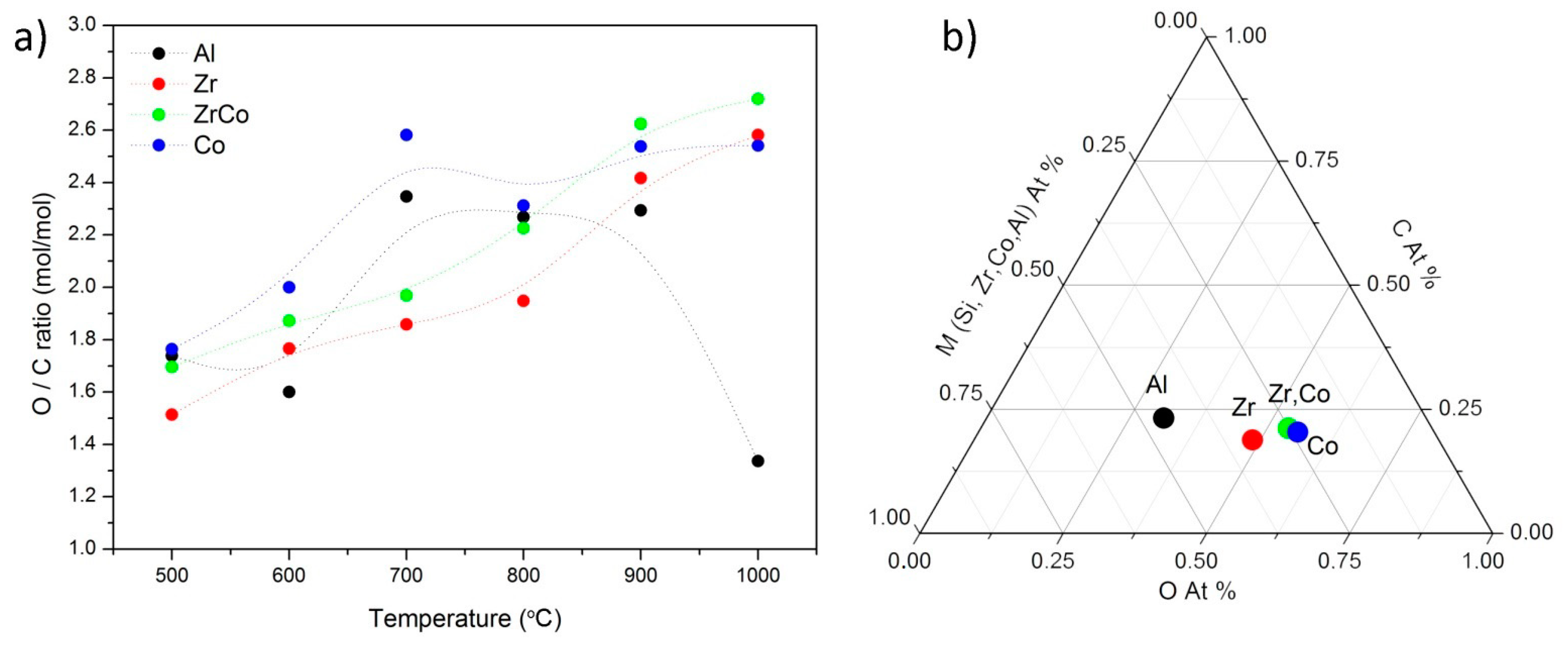

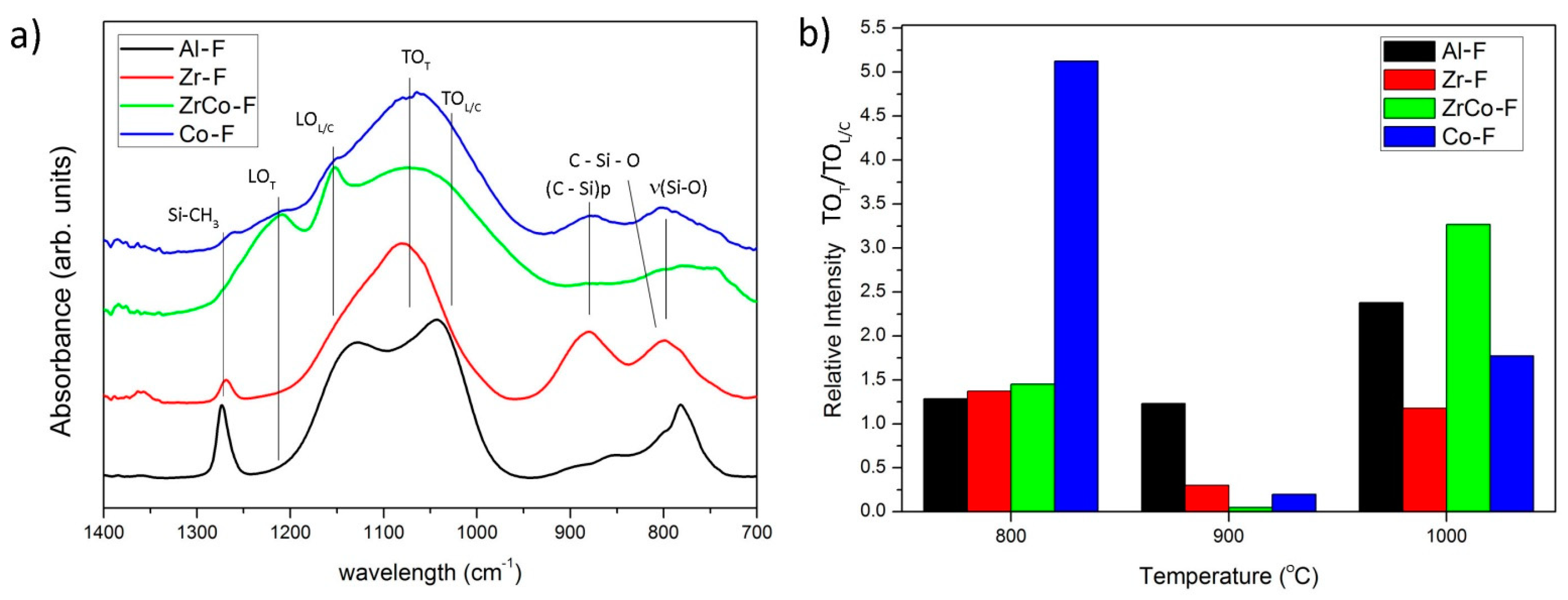

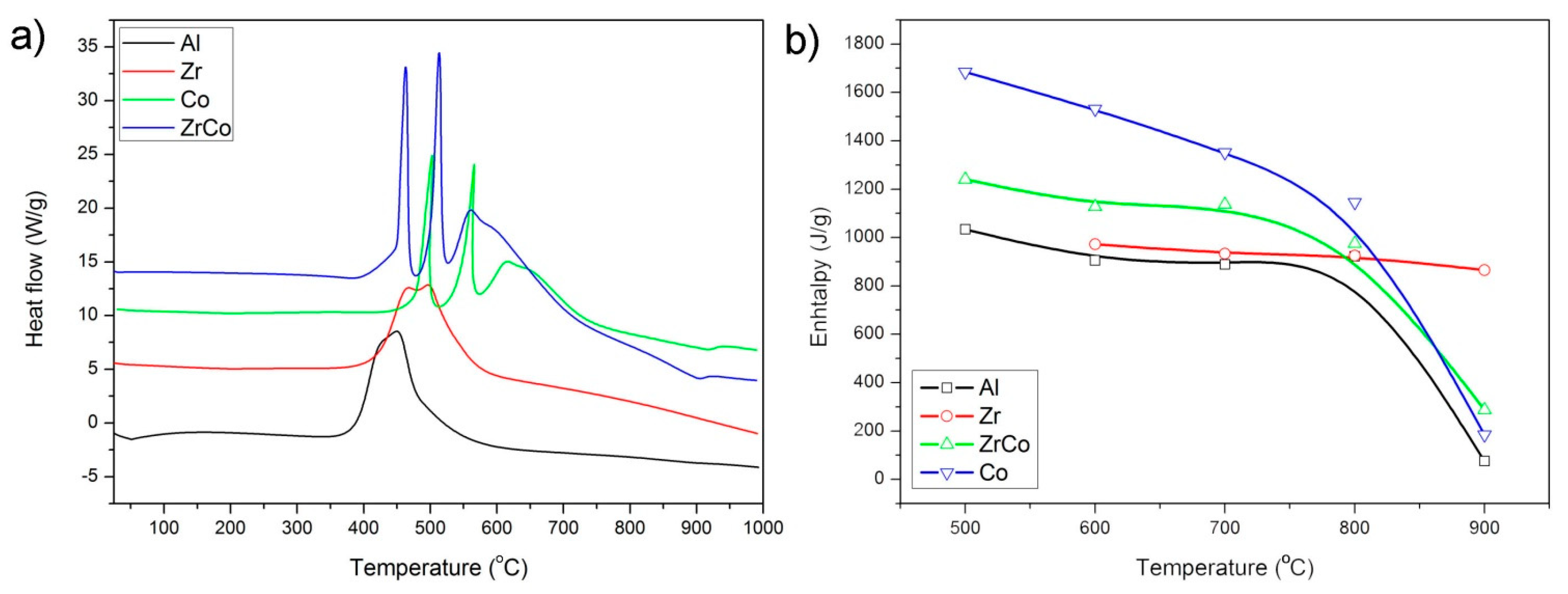

| T (°C) | Al | Zr | Co | Zr/Co |
|---|---|---|---|---|
| 500 | SiAl0.05O1.68C0.97H3.13 | SiZr0.02O1.42C0.94H2.82 | SiCo0.06O1.57C0.89H2.79 | SiZr0.02Co0.03O1.59C0.94H3.02 |
| 600 | SiAl0.05O1.18C0.74H2.31 | SiZr0.02O1.52C0.86H2.41 | SiCo0.07O2.18C1.09H3.75 | SiZr0.02Co0.03O1.93C1.03H2.80 |
| 700 | SiAl0.05O1.39C0.59H1.08 | SiZr0.02O1.08C0.58H1.69 | SiCo0.07O2.53C0.98H2.19 | SiZr0.02Co0.03O1.99C1.01H2.36 |
| 800 | SiAl0.05O1.55C0.68H0.70 | SiZr0.02O1.43C0.73H1.12 | SiCo0.06O1.85C0.80H1.83 | SiZr0.02Co0.03O1.85C0.83H1.32 |
| 900 | SiAl0.05O1.41C0.61H0.23 | SiZr0.02O1.58C0.65H1.01 | SiCo0.06O1.98C0.78H0.11 | SiZr0.02Co0.03O1.94C0.74H1.18 |
| 1000 | SiAl0.05O0.68C0.51H0.04 | SiZr0.02O1.50C0.58H0.03 | SiCo0.07O2.16C0.85H0.07 | SiZr0.02Co0.03O2.35C0.86H0.04 |
| T (°C) | Al | Zr | Co | Zr/Co | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyr | HF Etch | Pyr | HF Etch | Pyr | HF Etch | Pyr | HF Etch | ||||||
| 600 | 450 | n/d | 465 | 495 | n/d | 465 | 515 | n/d | 500 | 565 | n/d | ||
| 700 | 495 | n/d | 500 | n/d | 470 | 600 | 555 | 525 | 575 | n/d | |||
| 800 | 545 | n/d | 550 | n/d | 550 | 585 | 580 | 545 | 600 | 535 | |||
| 900 | 650 | 355 | 550 | 550 | 525 | 475 | 520 | 575 | 480 | 545 | |||
| SSA m2/g | ||||
| T (°C) | Al | Zr | Co * | Zr/Co |
| 500 | 220 | 40 | 190 | 60 |
| 600 | 370 | 390 | 475 | 165 |
| 700 | 320 | 325 | 335 | 360 |
| 800 | 265 | 270 | 255 | 290 |
| 900 | 3.0 | 243 | 2.3 | 2.0 |
| 1000 | 0.5 | 100 | 0.5 | 3.5 |
| SSA m2/g after HF etching | ||||
| 700 | -- | 1.2 | 2.0 | -- |
| 800 | -- | 41 | 43 | -- |
| 900 | 91 | 3.3 | 74 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamayo, A.; Rubio, J.; Rubio, F.; Rodriguez, M.A. Insights into the Microstructural Evolution Occurring during Pyrolysis of Metal-Modified Ceramers Studied through Selective SiO2 Removal. Materials 2021, 14, 3276. https://doi.org/10.3390/ma14123276
Tamayo A, Rubio J, Rubio F, Rodriguez MA. Insights into the Microstructural Evolution Occurring during Pyrolysis of Metal-Modified Ceramers Studied through Selective SiO2 Removal. Materials. 2021; 14(12):3276. https://doi.org/10.3390/ma14123276
Chicago/Turabian StyleTamayo, Aitana, Juan Rubio, Fausto Rubio, and Mᵃ Angeles Rodriguez. 2021. "Insights into the Microstructural Evolution Occurring during Pyrolysis of Metal-Modified Ceramers Studied through Selective SiO2 Removal" Materials 14, no. 12: 3276. https://doi.org/10.3390/ma14123276
APA StyleTamayo, A., Rubio, J., Rubio, F., & Rodriguez, M. A. (2021). Insights into the Microstructural Evolution Occurring during Pyrolysis of Metal-Modified Ceramers Studied through Selective SiO2 Removal. Materials, 14(12), 3276. https://doi.org/10.3390/ma14123276







