Preparation of Poly(acrylic acid-acrylamide/starch) Composite and Its Adsorption Properties for Mercury (II)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
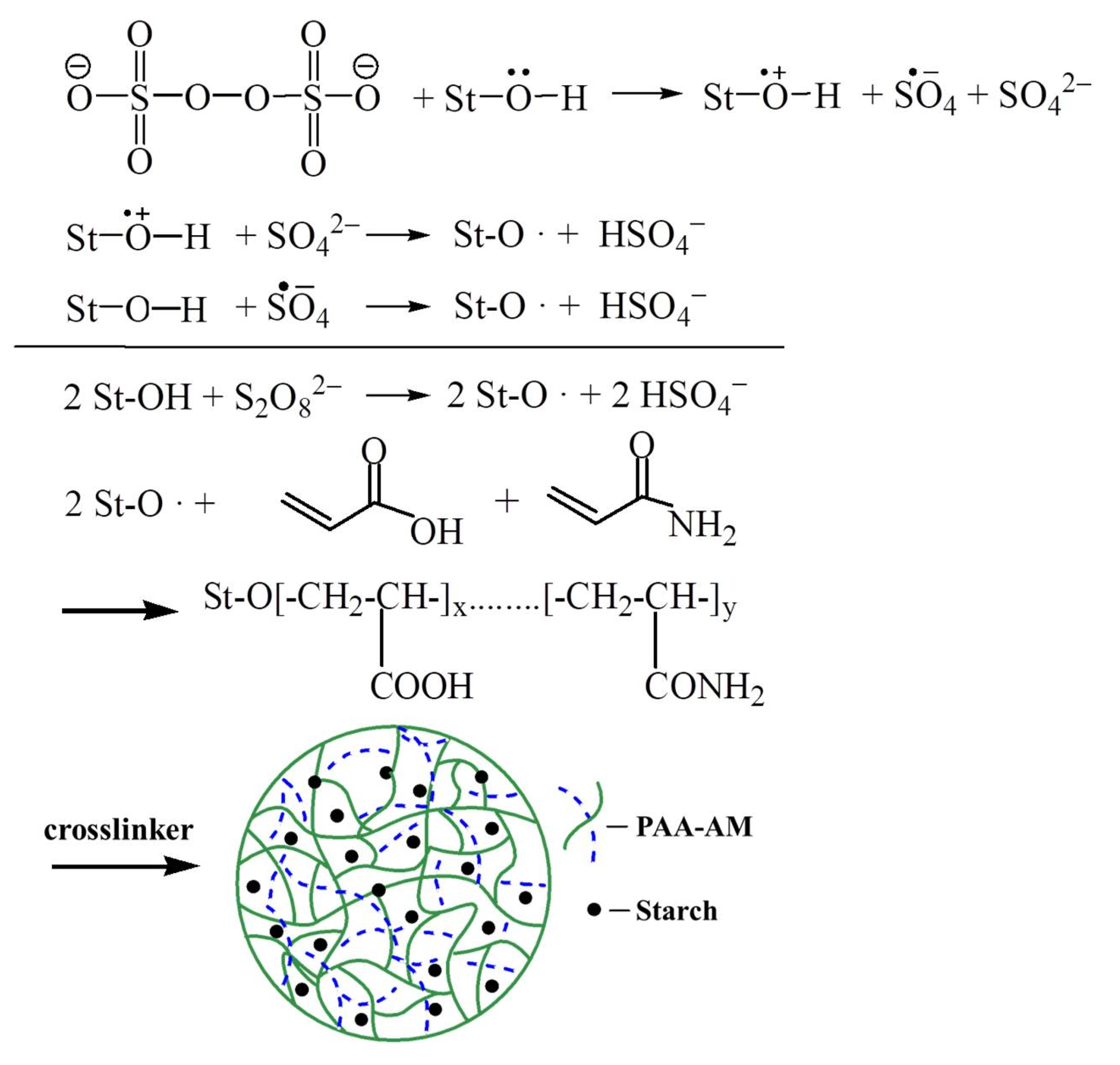
2.2. Synthesis of PAA-AM/St Superabsorbent Composite
2.3. Characterization
2.4. Adsorption of Hg(II) Ions
2.5. Regeneration of the PAA-AM/St Composite
3. Results and Discussion
3.1. Measurement of Molecular Weight
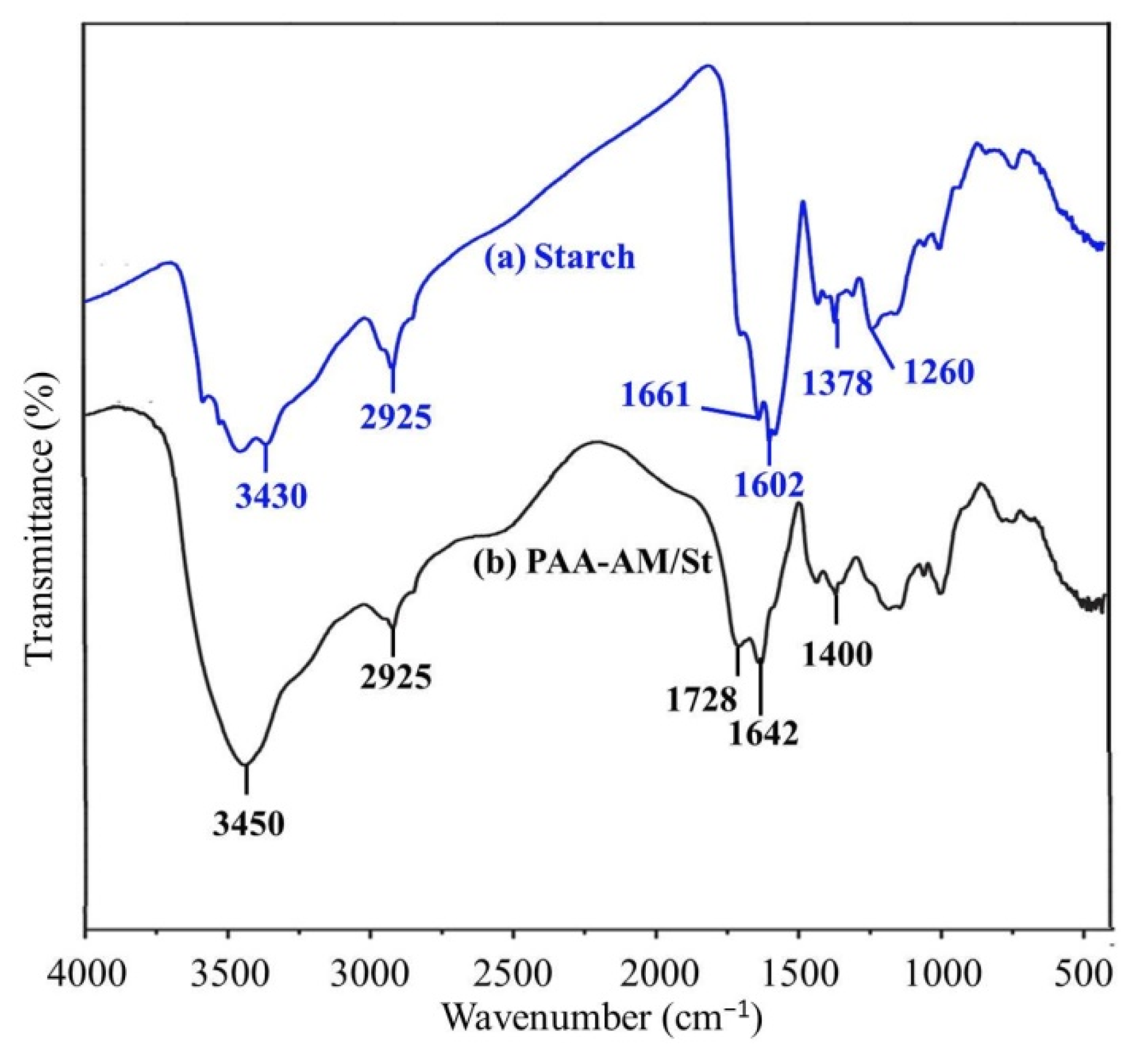
3.2. FT-IR Spectra
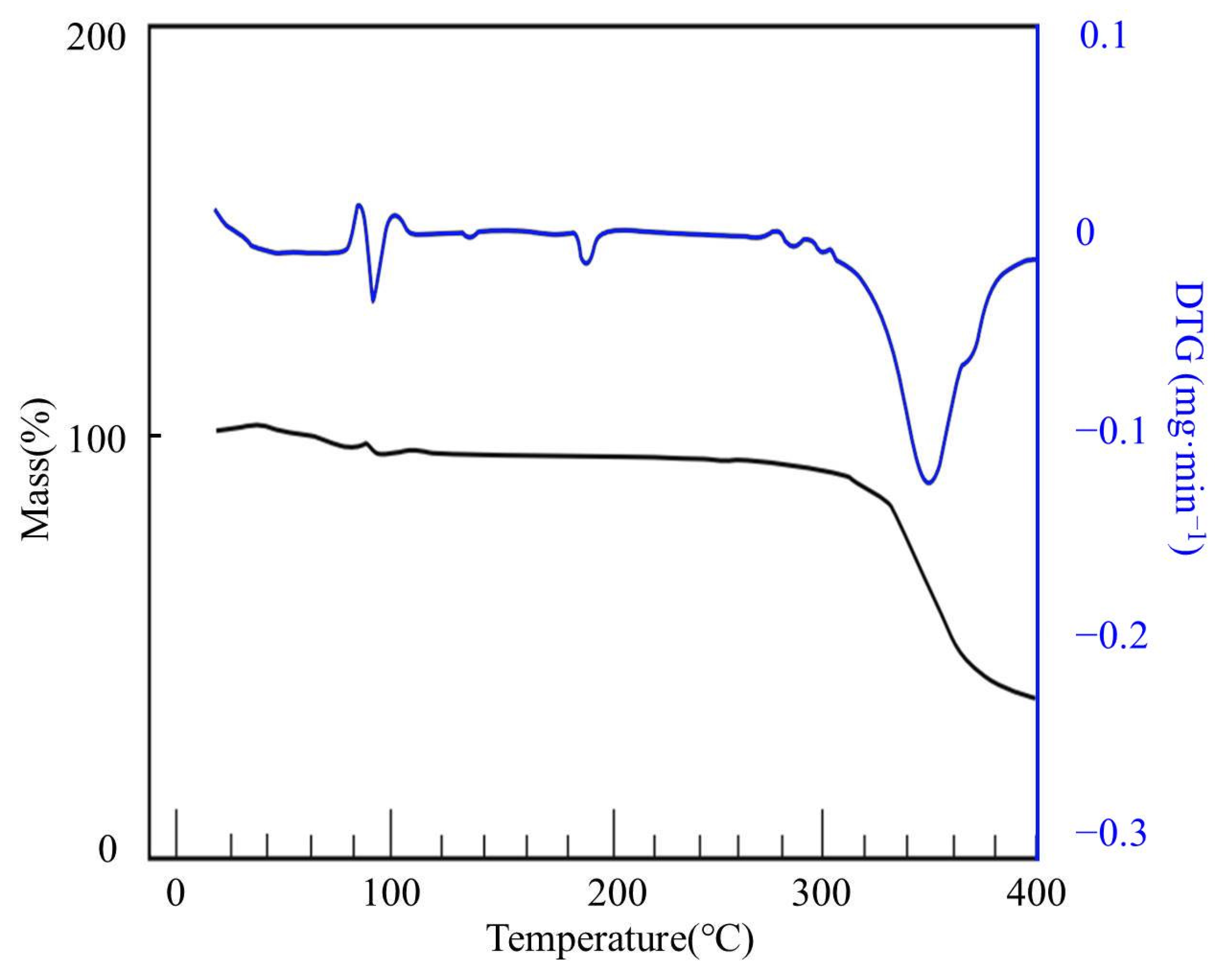
3.3. Thermal Stability
3.4. Morphology Analysis
3.5. DLS Study
3.6. Optimization of Hg(II) Ions Adsorption Conditions
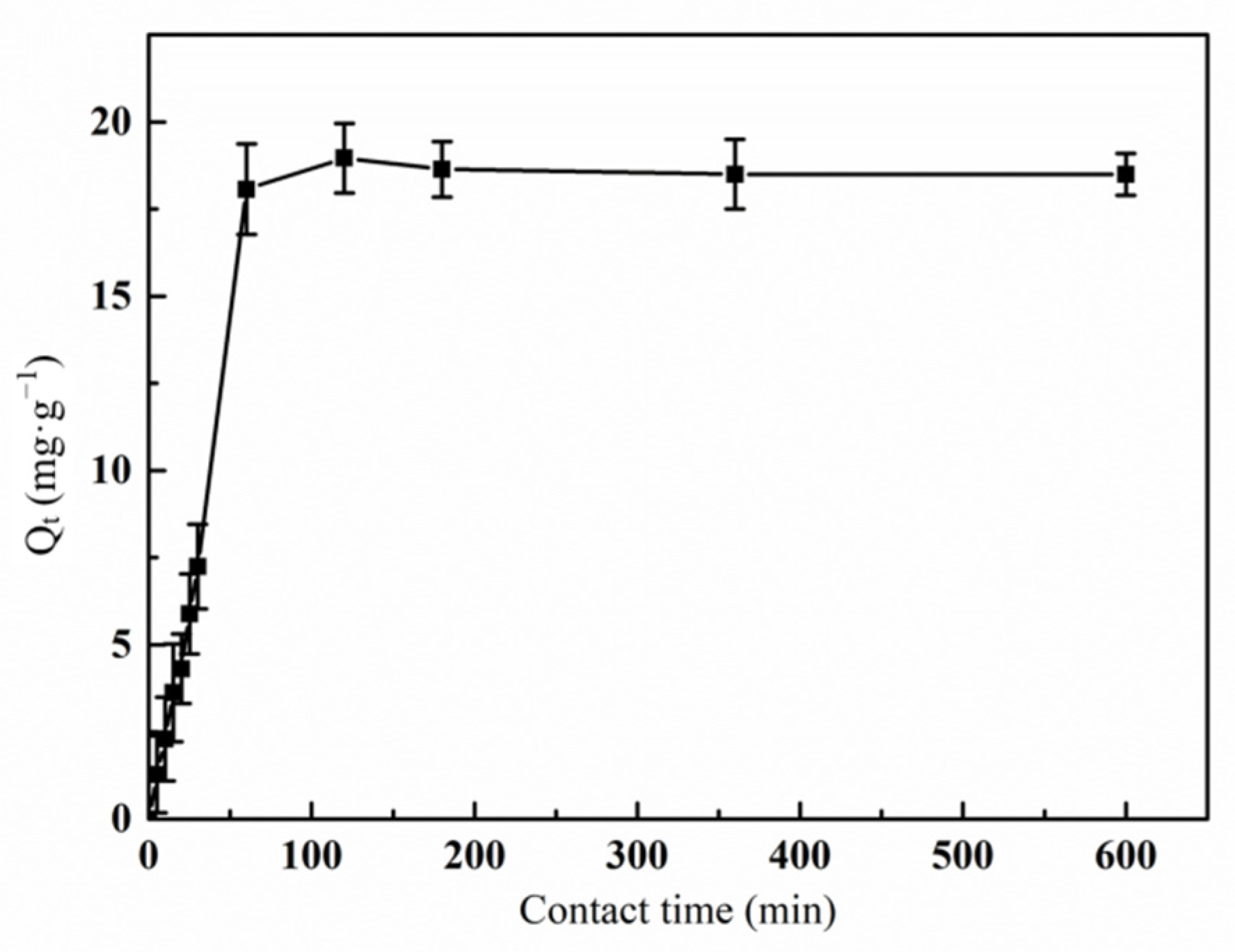
3.6.1. Effect of Contact Time on the Adsorption of Hg(II) Ions by the Graft Polymer
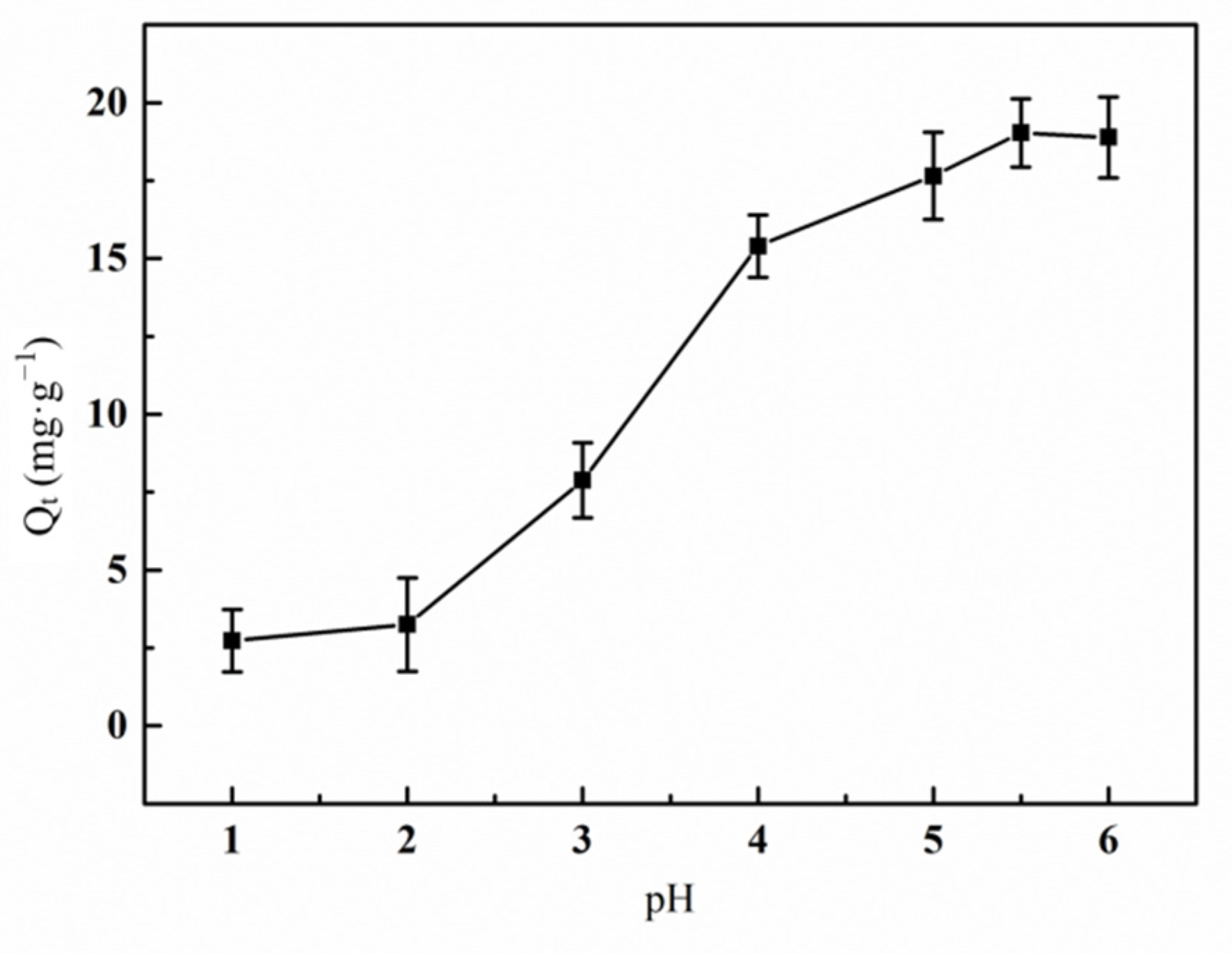
3.6.2. Effect of pH Value on the Adsorption of Hg(II) Ions by the Graft Polymer
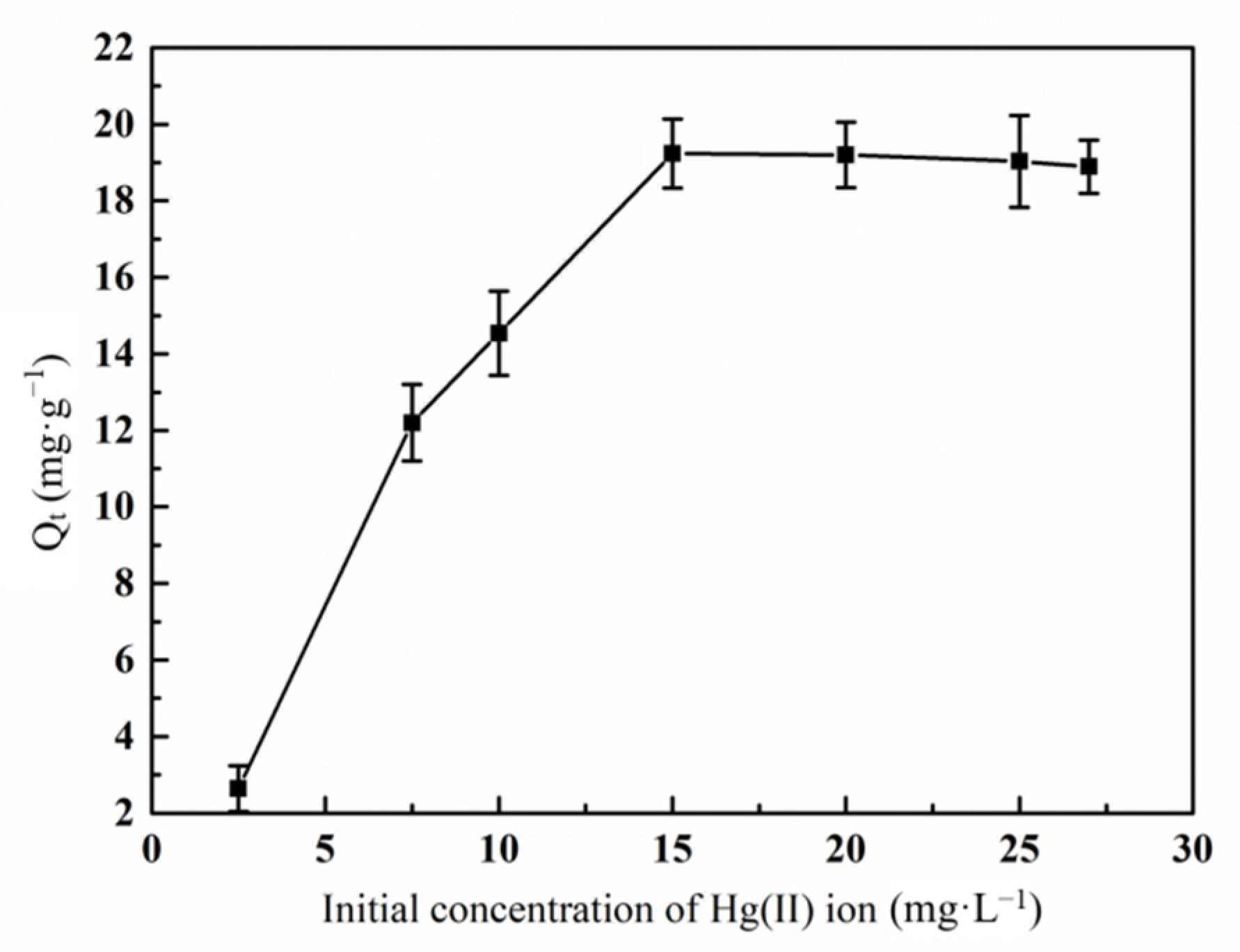
3.6.3. Effect of Initial Hg(II) Ions Concentration on the Adsorption Property by Graft Polymer
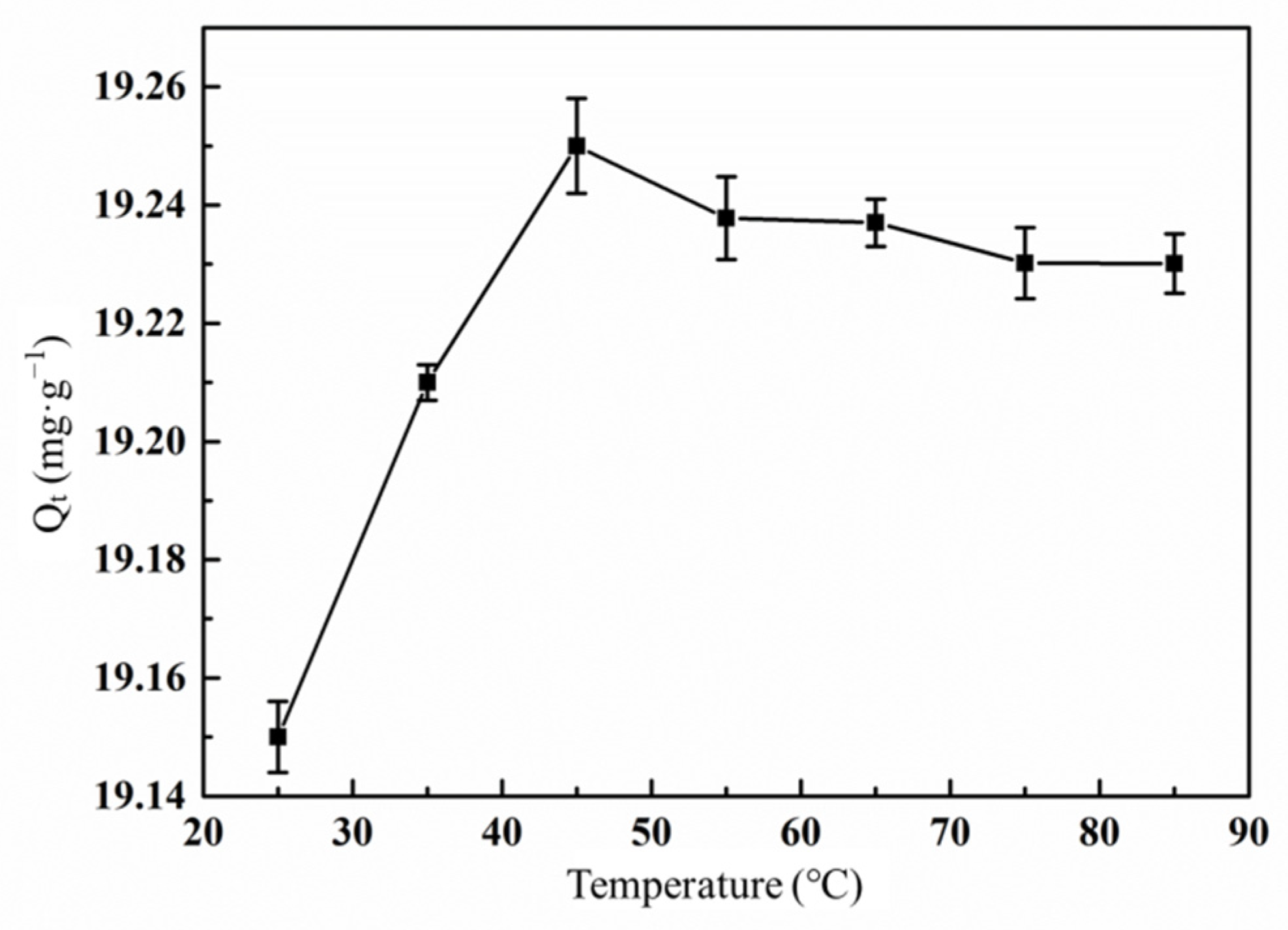
3.6.4. Effect of Temperature on the Adsorption of Hg(II) Ions by the Graft Polymer
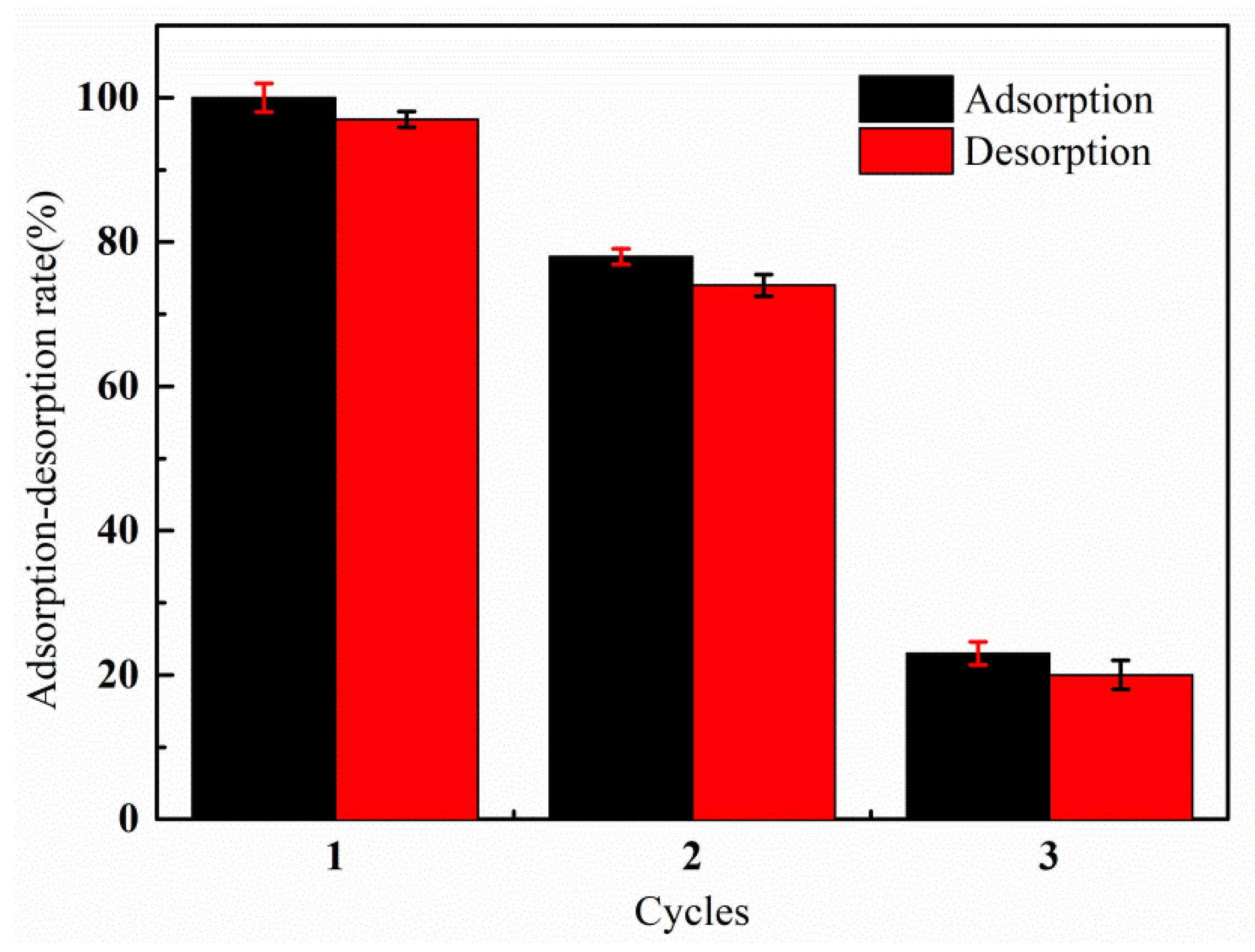
3.7. Recyclability Study
3.8. Comparison Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Zahhar, A.A.; Idris, A.M. Mercury(II) decontamination using a newly synthesized poly(acrylonitrile-acrylic acid)/ammonium molybdophosphate composite exchanger. Toxin Rev. 2020, 1, 1–13. [Google Scholar] [CrossRef]
- Sun, J. Removal of Mercury (Hg(II)) from Seaweed Extracts by Electrodialysis and Process Optimization Using Response Surface Methodology. J. Ocean Univ. China 2020, 19, 135–142. [Google Scholar] [CrossRef]
- Abdel-Aal, S.E.; Gad, Y.H.; Dessouki, A.M. The use of wood pulp and radiation-modified starch in wastewater treatment. J. Appl. Polym. Sci. 2010, 99, 2460–2469. [Google Scholar] [CrossRef]
- Igura, M.; Okazaki, M. Selective sorption of heavy metal on phosphorylated sago starch-extraction residue. J. Appl. Polym. Sci. 2012, 124, 549–559. [Google Scholar] [CrossRef]
- Feng, K.; Wen, G. Absorbed Pb2+ and Cd2+ Ions in Water by Cross-Linked Starch Xanthate. Int. J. Ploym. Sci. 2017, 58, 275–276. [Google Scholar] [CrossRef]
- Shahzamani, M.; Taheri, S.; Roghanizad, A.; Naseri, N.; Dinari, M. Preparation and characterization of hydrogel nanocomposite based on nanocellulose and acrylic acid in the presence of urea. Int. J. Biol. Macromol. 2020, 147, 187–193. [Google Scholar] [CrossRef]
- Saberi, A.; Sadeghi, M.; Alipour, E. Design of AgNPs -Base Starch/PEG-Poly (Acrylic Acid) Hydrogel for Removal of Mercury (II). J. Polym. Environ. 2020, 28, 303–315. [Google Scholar] [CrossRef]
- Hadavifar, M.; Bahramifar, N.; Younesi, H.; Qin, L. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem. Eng. J. 2014, 237, 217–228. [Google Scholar] [CrossRef]
- Peng, C.; He, M.; Chen, B.; Huang, L.; Hu, B. Magnetic sulfur-doped porous carbon for preconcentration of trace mercury in environmental water prior to ICP-MS detection. Analyst 2017, 142, 4570–4583. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, M.K.; Anirudhan, T.S. Preparation of an adsorbent by graft polymerization of acrylamide onto coconut husk for mercury(II) removal from aqueous solution and chloralkali industry wastewater. J. Appl. Polym. Sci. 2000, 75, 1261–1269. [Google Scholar] [CrossRef]
- Fatiha, B.; Hayet, B.; Zohra, F.; Radjaa, B.; Hfz, B. Journal of Drug Delivery and Therapeutics Toxicity of mercury on the brain: Ability of extract of Pistacia atlantica regulated effect. J. Drug Deliv. Ther. 2020, 10, 17–25. [Google Scholar] [CrossRef]
- Teixeira, G.; Raimundo, J.; Goulart, J.; Costa, V.; Martins, I. Hg and Se composition in demersal deep-sea fish from the North-East Atlantic. Environ. Sci. Pollut. Res. 2020, 27, 24–36. [Google Scholar] [CrossRef]
- Amani, V.; Sharafie, D.; Hamedani, N.F.; Naseh, M. Zinc(II) and mercury(II) iodide complexes containing 2-pyridinealdoxime compound: Synthesis, characterization, crystal structure determination and DFT study. J. Iran. Chem. Soc. 2020, 17, 441–451. [Google Scholar] [CrossRef]
- Tripathi, N.K. Equilibrium studies of Mercury (II), Lead (II) and Cadmium (II) ions involving Aspartic acid and Thymine. Int. J. Res. Pharm. Chem. 2020, 10, 314–317. [Google Scholar] [CrossRef]
- Feng, C.; Pedrero, Z.; Lima, L.; Olivares, S.; de la Rosa, D.; Berail, S.; Tessier, E.; Pannier, F.; Amouroux, D. Assessment of Hg contamination by a Chlor-Alkali Plant in riverine and coastal sites combining Hg speciation and isotopic signature (Sagua la Grande River, Cuba). J. Hazard. Mater. 2019, 371, 558–565. [Google Scholar] [CrossRef]
- Muhammad, Y.A.; Sunaryono; Nenohai, A.; Mufti, N.; Taufiq, A. Adsorption Properties of Magnetic Sorbent Mn0. 25Fe2. 75O4@ SiO2 for Mercury Removal. Key Eng. Mater. 2020, 851, 197–204. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Wang, P.; Yang, Z.; Yasin, A.; Zhang, L. Preparation and Applications of Salt-Resistant Superabsorbent Poly (Acrylic Acid-Acrylamide/Fly Ash) Composite. Materials 2019, 12, 596. [Google Scholar] [CrossRef]
- Xu, X.; Schierz, A.; Xu, N.; Cao, X. Comparison of the characteristics and mechanisms of Hg(II) sorption by biochars and activated carbon. J. Colloid Interface Sci. 2016, 463, 55–60. [Google Scholar] [CrossRef]
- Srikhaow, A.; Butburee, T.; Pon-On, W.; Srikhirin, T.; Smith, S.M. Efficient Mercury Removal at Ultralow Metal Concentrations by Cysteine Functionalized Carbon-Coated Magnetite. Appl. Sci. 2020, 10, 262. [Google Scholar] [CrossRef]
- Long, C.; Li, X.; Jiang, Z.; Zhang, P.; Feng, B. Adsorption-improved MoSe2 nanosheet by heteroatom doping and its application for simultaneous detection and removal of mercury (II). J. Hazard. Mater. 2021, 413, 125470–125487. [Google Scholar] [CrossRef]
- Qasim, G.H.; Lee, S.; Lee, W.; Han, S. Reduction and removal of aqueous Hg(II) using indium-modified zero-valent iron particles. Appl. Catal. B Environ. 2020, 277, 119198–119218. [Google Scholar] [CrossRef]
- Cheng, H.; Yu, J.; Zeng, K.; Hou, G. Application of Poly(Acrylic Acid)-Multiwalled Carbon Nanotube Composite for Enrichment of Rrace Hg(II). Plasma Process. Polym. 2013, 10, 931–937. [Google Scholar] [CrossRef]
- Udomkitpanya, T.; Srikulkit, K. Properties of Poly(Lactic Acid) Blended with Natural Rubber-Graft- Poly(Acrylic Acid). Key Eng. Mater. 2020, 845, 45–50. [Google Scholar] [CrossRef]
- Kolya, H.; Das, S.; Tripathy, T. Synthesis of Starch-g-Poly-(N-methylacrylamide-co-acrylic acid) and its application for the removal of mercury (II) from aqueous solution by adsorption. Eur. Polym. J. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T. Hydroxyethyl Starch-g-Poly-(N,N-dimethylacrylamide-co-acrylic acid): An efficient dye removing agent. Eur. Polym. J. 2013, 49, 4265–4275. [Google Scholar] [CrossRef]
- Perfetti, G.; Jansen, K.; Wildeboer, W.J.; Hee, P.V.; Meesters, G. Characterization of physical and viscoelastic properties of polymer films for coating applications under different temperature of drying and storage. Int. J. Pharm. 2010, 384, 109–119. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Suchithra, P.S.; Divya, L. Adsorptive Potential of 2-Mercaptobenzimidazole-Immobilized Organophilic Hydrotalcite for Mercury(II) Ions from Aqueous Phase and Its Kinetic and Equilibrium Profiles. Water Air Soil Pollut. 2009, 196, 127–139. [Google Scholar] [CrossRef]
- Yan, D.; Dong, H.; Ye, P.; Müller, A. Kinetic Analysis of “Living” Polymerization Processes Exhibiting Slow Equilibria. 6. Cationic Polymerization Involving Covalent Species, Ion Pairs, and Free Cations. Macromolecules 1996, 29, 8057–8063. [Google Scholar] [CrossRef]
- Ling-Yun, Y.U.; Rui-Li, L.I.; Hai-Lun, W.U.; Zhang, S.F.; Lin, H. Selective Removal of Cu2+ Ion in Aqueous Solution by Poly (Acrylic Acid/Acrylamide) Hydrogel. Chin. J. Anal. Chem. 2020, 48, 20098–20106. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Forouzandehdel, S.; Meskini, M.; Rami, M.R. Design and application of (Fe3O4)-GOTfOH based AgNPs doped starch/PEG-poly (acrylic acid) nanocomposite as the magnetic nanocatalyst and the wound dress. J. Mol. Struct. 2020, 1214, 128–142. [Google Scholar] [CrossRef]
- Pereira, A.G.; Rodrigues, F.H.; Paulino, A.T.; Martins, A.F.; Fajardo, A.R. Recent advances on composite hydrogels designed for the remediation of dye-contaminated water and wastewater: A review. J. Clean. Prod. 2020, 284, 124703–124730. [Google Scholar] [CrossRef]
- Cai, L.; Liu, C.; Fan, G.; Liu, C.; Sun, X. Preventing viral disease by ZnONPs through directly deactivating TMV and activating the plant immunity in Nicotiana benthamiana. Environ. Sci. Nano 2019, 6, 3653–3669. [Google Scholar] [CrossRef]
- Awad, F.S.; AbouZied, K.M.; Bakry, A.M.; Abou El-Maaty, W.M.; El-Wakil, A.M.; El-Shall, M.S. Polyacrylonitrile modified partially reduced graphene oxide composites for the extraction of Hg(II) ions from polluted water. J. Mater. Sci. 2021, 56, 7982–7999. [Google Scholar] [CrossRef]
- Elkhaleefa, A.; Ali, I.H.; Brima, E.I.; Shigidi, I.; Karama, B. Evaluation of the Adsorption Efficiency on the Removal of Lead(II) Ions from Aqueous Solutions Using Azadirachta indica Leaves as an Adsorbent. Processes 2021, 9, 559. [Google Scholar] [CrossRef]
- Kuncoro, E.P.; Roussy, J.; Guibal, E. Mercury recovery by polymer-enhanced ultrafiltration: Comparison of chitosan and poly (ethylenimine) used as macroligand. Sep. Sci. Technol. 2005, 40, 659–684. [Google Scholar] [CrossRef]
- Mi, Y.K.; Seo, H.; Tai, G.L. Removal of Hg(II) ions from aqueous solution by poly(allylamine-co-methacrylamide-co-dimethylthiourea)—ScienceDirect. J. Ind. Eng. Chem. 2020, 84, 82–86. [Google Scholar] [CrossRef]
- Li, H.P.; Huang, Y.Y.; Yuan, J.W.; Yang, G.W.; Lv, H.Q.; Yang, S.J. Absorption of Hg (II) Form Aqueous Solution onto Double Crosslinked Amphoteric Cassava Starch Resin. Adv. Mater. Res. 2014, 886, 253–256. [Google Scholar] [CrossRef]
- Chattoraj, D.K.; Im Ae, T.; Mitra, A. A generalized scale of free energy of excess adsorption of solute and absolute composition of the interfacial phase. Langmuir 2004, 20, 4903–4915. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, P.; Lan, G.; Liu, Y.; Cai, Q.; Xi, J. High crosslinked sodium carboxyl methylstarch- g -poly (acrylic acid- co -acrylamide) resin for heavy metal adsorption: Its characteristics and mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 38617–38630. [Google Scholar] [CrossRef]
- Wang, X.; Wang, A. Adsorption characteristics of chitosan-g-poly (acrylic acid)/attapulgite hydrogel composite for Hg (II) ions from aqueous solution. Sep. Sci. Technol. 2010, 45, 2086–2094. [Google Scholar] [CrossRef]
- Singh, V.; Singh, S.K.; Maurya, S. Microwave induced poly(acrylic acid) modification of Cassia javanica seed gum for efficient Hg(II) removal from solution. Chem. Eng. J. Lausanne 2010, 160, 129–137. [Google Scholar] [CrossRef]





| Sample | Mn | RSD | Mp | RSD | Mw | RSD | Mz | RSD | Mz+1 | RSD |
|---|---|---|---|---|---|---|---|---|---|---|
| Starch | 876,032 | 4.7% | 906,360 | 4.0% | 933,126 | 2.3% | 936,921 | 0.9% | 967,686 | 0.7% |
| PAA-AM/St | 10,096,811 | 4.5% | 10,546,420 | 3.0% | 10,848,525 | 2.1% | 11,602,465 | 1.0% | 12,353,127 | 0.9% |
| Initial Concentration of Hg(II) (mg·L−1) | Pseudo First Order Model | Pseudo Second Order Model | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (g·mg−1·min−1) | R2 | |
| 3.0 | 2.3 × 10−2 | 0.325 | 3.3 × 10−3 | 0.999 |
| 7.0 | 3.1 × 10−3 | 0.103 | 8.9 × 10−4 | 0.999 |
| 9.0 | 3.3 × 10−3 | 0.386 | 8.1 × 10−4 | 0.999 |
| 15.0 | 4.1 × 10−3 | 0.468 | 6.4 × 10−4 | 0.999 |
| 26.5 | 4.3 × 10−3 | 0.369 | 5.2 × 10−4 | 0.999 |
| 30 | 4.2 × 10−3 | 0.412 | 4.9 × 10−4 | 0.999 |
| Composites Used as Adsorbent | Initial Concentration of Hg(II) ion (mg·L−1) | Qt (mg·g−1) | Reference |
|---|---|---|---|
| PAN-AA/AMP | 200 | 221.23 | [1] |
| PAN-AA | 200 | 202.43 | [1] |
| St-PEG-PAA | 300 | 158.21 | [7] |
| AgNPs-St-PEG-PAA | 300 | 182.53 | [7] |
| P3HT-CNT/Ti | 200 | 164 | [20] |
| PAA-MWCNTs | 18 | 5.64 | [22] |
| PNMA-AA/St | 27.5 | 7.9 | [24] |
| PAN-PRGO | 150 | 164.79 | [34] |
| P(AA-co-MA-co-DMTU) | 400 | 198.23 | [37] |
| PAA-DMDAAC/St | 4000 | 191.97 | [38] |
| CTS-g-PAA | 3669 | 798.92 | [40] |
| CTS-g-PAA/50%APT | 3669 | 503.21 | [41] |
| CJ-g-PAA | 500 | 135 | [42] |
| Cys-C@Fe3O4 | 100 | 94.33 | [19] |
| MBI-OHTC | 25 | 11.63 | [27] |
| MBI-OHTC | 50 | 21.52 | [27] |
| PAA-AM/St | 15 | 19.23 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Yang, Z.; Yasin, A.; Liu, Y.; Zhang, L. Preparation of Poly(acrylic acid-acrylamide/starch) Composite and Its Adsorption Properties for Mercury (II). Materials 2021, 14, 3277. https://doi.org/10.3390/ma14123277
Zhu W, Yang Z, Yasin A, Liu Y, Zhang L. Preparation of Poly(acrylic acid-acrylamide/starch) Composite and Its Adsorption Properties for Mercury (II). Materials. 2021; 14(12):3277. https://doi.org/10.3390/ma14123277
Chicago/Turabian StyleZhu, Wenjuan, Zhiyong Yang, Akram Yasin, Yanxia Liu, and Letao Zhang. 2021. "Preparation of Poly(acrylic acid-acrylamide/starch) Composite and Its Adsorption Properties for Mercury (II)" Materials 14, no. 12: 3277. https://doi.org/10.3390/ma14123277
APA StyleZhu, W., Yang, Z., Yasin, A., Liu, Y., & Zhang, L. (2021). Preparation of Poly(acrylic acid-acrylamide/starch) Composite and Its Adsorption Properties for Mercury (II). Materials, 14(12), 3277. https://doi.org/10.3390/ma14123277





